Abstract
The transport and deposition of polydispersed expiratory aerosols in an aircraft cabin were simulated using a Lagrangian-based model validated by experiments conducted in an aircraft cabin mockup. Infection risk by inhalation was estimated using the aerosol dispersion data and a model was developed to estimate the risk of infection by contact. The environmental control system (ECS) in a cabin creates air circulation mainly in the lateral direction, making lateral dispersions of aerosols much faster than longitudinal dispersions. Aerosols with initial sizes under 28 μm in diameter can stay airborne for comparatively long periods and are favorable for airborne transport. Using influenza data as an example, the estimated risk of infection by inhalation are at least two orders of magnitude higher than the risk of infection by contact. An increase in the supply airflow rate enhances ventilation removal and the dispersion of these aerosols. It reduces the risk of infection by inhalation for passengers seated within one row and one column from the index patient but it increases the risk for passengers seated further away. The deposition fraction increases with aerosol size. The ECS supply airflow rate has insignificant impact on the deposition behavior of these large aerosols, making the impact on the risk of infection by contact insignificant. Comparatively, the contact behavior of passengers is highly influential to the contact infection risk. Passengers seated within one row from the index patient are subject to contact risks that are one to two orders of magnitude higher than are passengers seated further away.
NOMENCLATURE
| A | = |
Area [m2] |
| C c | = |
Cunningham correction factor |
| D p | = |
droplet or droplet nuclei diameter [μ m] |
| F adh | = |
adhesive force [dyn] |
| f D | = |
Stoke's drag modification function |
| f h | = |
hand touching frequency on contaminated surfaces[hr− 1] |
| f m | = |
hand touching frequency on mucous membrane [hr− 1] |
| f s | = |
coughing frequency [hr− 1] |
| g | = |
gravitational acceleration [m/s2] |
| k | = |
turbulence kinetic energy [m2/s2] |
| N | = |
number of droplets |
| q | = |
supply airflow rate [m3/s] |
| t | = |
time [s] |
| u | = |
instantaneous velocity of air [m/s] |
| u' | = |
fluctuation velocity component of air [m/s] |
| u p | = |
instantaneous velocity of aerosols [m/s] |
| v d | = |
deposition velocity [m/s] |
| y | = |
distance from wall [m] |
| y + | = |
wall unit, y + = ρ u∗ y/μ |
| Greek and other symbols | ||
| μ | = |
molecular viscosity of air [g/ms] |
| ρ | = |
density of air [g/m3] |
| ρ p | = |
density of aerosols [g/m3] |
| ζ | = |
random number obeying the Gaussian distribution |
| ∀ | = |
volume of a droplet [mL] |
| Subscript | ||
| i, j | = |
Cartesian coordinates |
| k | = |
surface |
| m | = |
mesh |
| p | = |
measurement position |
1. INTRODUCTION
The aircraft cabin environment is unique compared with other types of transportation vehicles and built environments in many aspects. The relatively high occupant density in a typical commercial aircraft cabin, especially in the economy compartment, leads to the fact that passengers sit in close proximity to each other. Passengers in aircraft cabins have access to lower per-person outside air supply rates than in normal built environment, as shown by the higher CO2 level measured in cabins (CitationHaghighat et al. 1999). Compared with other modes of transport, air travel is relatively long and passengers are unable to leave the cabin. This environment has been the focus of health concerns. The low per-person ventilation rate may lead to a reduction in the dilution of human-exhaled infectious pathogens. The high occupant density and long exposure time may provide a favorable environment for airborne and contact modes of infectious disease transmission. These observations are supported by numerous outbreaks of respiratory diseases among passengers in aircraft cabins, as reviewed by CitationMangili and Gendreau (2005). They suggested that the airborne transmission and the large droplet transmission are of the highest risk for disease transmission in the aircraft cabin environment. A number of epidemiological investigations, e.g., CitationKenyon et al. (1996) and CitationMiller et al. (1996), suggested that some tuberculosis outbreaks were very likely caused by airborne transmission among passengers in aircraft cabins. In-flight transmission of diseases that are believed to be transmitted by large droplets, such as severe acute respiratory syndrome (SARS) (e.g., CitationOlsen et al. 2003), and influenza (e.g., CitationMoser et al. 1979; CitationKlontz et al. 1989), are also well documented.
For airborne and large droplet transmission to occur, infectious agents are aerosolized in the form of expiratory droplets by an infected person. In the droplet size spectrum of human expiratories, such as coughing (CitationNicas et al. 2005), the majority of droplets becomes residue within a second due to evaporation (CitationMorawska 2006). The residues of expiratory droplets are small enough to remain suspended in the air and to be dispersed widely on the airflow. Airborne transmission is transmission of an infection by inhalation of pathogen-laden aerosols. In an aircraft cabin, the expiratory droplets, especially the large ones, from an infected person may directly impact onto other passengers. In addition, pathogen-laden expiratory droplets may also deposit on surfaces and subsequently infect susceptible people by contact, leading to contact transmission.
Previous studies conducted in other types of indoor environments, including idealized rooms (CitationChao and Wan 2006), office settings (CitationGao and Niu 2007), and health care settings (CitationZhu et al. 2006; CitationWan et al. 2007, CitationChao et al. 2008a), indicate that the ventilation airflow impacts the transport and deposition characteristics of expiratory aerosols. These studies also revealed that polydispersed expiratory aerosols behave very differently depending on their size. A recent study by CitationSze To et al. (2008a) suggested that the size not only affects droplet transport and exposure to pathogenic aerosols by occupants but also the risk of infection because small aerosols can penetrate deep into the respiratory system. However, the size effect has not been widely investigated until recently since gaseous surrogates were commonly used as proxies for expiratory aerosols in earlier studies, as reviewed by CitationChao and Wan (2006). The investigation of the transport and deposition of expiratory aerosols in aircraft cabins follows a similar trend. Some works focused on the airflow and ventilation characteristics created by the ECS in aircraft cabins (CitationLin et al. 2005a; CitationWang et al. 2008), which can be the basis for understanding the effects of ventilation airflow on the behaviors of expiratory aerosols. In a majority of existing studies on the dispersion of expiratory pollutants, gaseous surrogates were commonly used to simulate airborne pathogenic aerosols (CitationRydock 2004; CitationZhang and Chen 2007a; CitationLin et al. 2005b; CitationYan et al. 2008). CitationZhang and Chen (2007b) used single-sized particles 0.31 μ m in diameter in their numerical simulations of an aircraft cabin and did not simulate particle deposition. A detailed investigation of the fate of expiratory aerosols in aircraft cabins that also considers the size effect is still lacking. Recently, CitationSze To et al. (2008b) conducted experiments in a three-row aircraft cabin mockup to study the effects of the seating location of the index patient and the ECS supply airflow rate on the transport of polydispersed aerosols. Aerosol deposition was measured only under one supply airflow rate but with different index patient seating locations. In this study, the transport and deposition of expiratory droplets were simulated using the Lagrangian modeling approach for an aircraft cabin geometry having the same dimensions as the mockup used by CitationSze To et al. (2008b). The numerical results were validated by the results obtained from the cabin mockup experiments. The modeling was then extended to cover more cases not covered in the experiments. The risk of infection by inhalation of pathogenic aerosols was estimated using the model proposed by CitationSze To et al. (2008a). A model for estimating the risk of infection by hand contact with surfaces contaminated by deposited pathogens was also developed here. The effect of different ECS supply airflow rates on the transport and deposition characteristics of expiratory aerosols and the associated infection risks were studied using influenza data as an example.
2. METHODS
2.1. The Numerical Cabin Geometry
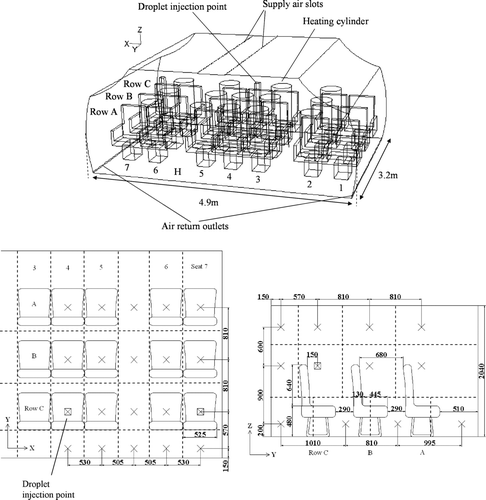
The numerical cabin geometry had internal dimension of 4.9 m × 3.2 m × 2.1 m (W × L × H). The dimensions of the numerical geometry were adopted from the cabin mockup that was used for the experimental validation. A detailed description of the validation experiment will be given in later sections. There were three rows of seven seats inside the cabin, arranged in the two-aisle configuration. Ventilation air was supplied from two longitudinal overhead slots (12 mm W × 3200 mm L each) located at the middle part of the ceiling. The return air was extracted through two perforated ducts installed along the lower longitudinal edge of the floor, one on each side. Heat release by passengers was simulated by fifteen heating cylinders (or manikins, 0.8 m high and 0.5 m in diameter) releasing a maximum of 60 W of heat each. Detailed dimensions of the numerical cabin geometry are shown in . The numerical geometry was created using a commercial mesh generator, GAMBIT. A grid system containing 1374k meshes was chosen from three grid systems (the other two contained 568k (coarsest) and 1936k (finest) meshes, respectively) based on the grid convergence index (GCI) analysis (CitationRoache 1998) on the computed velocity magnitudes with these grid systems at 800 selected points. With the coarsest grid system as the reference, the finest grid system did not have much improvement in GCI compared to the selected grid system (GCI1936k = 4.27%, GCI1374k = 3.98%). Therefore, the medium grid system was selected. Wall unit adaptation was applied to wall-adjacent cells to ensure that the wall y + values were in the range of 1–4 when creating the meshes.
2.2. Numerical Model and Boundary Conditions
A multiphase numerical model similar to that employed by CitationChao et al. (2008a) was adopted in this study. A detailed description of the model is presented in their study. Briefly, the governing equations of the air phase were considered in the Eulerian frame (Navier-Stokes equations). A transient Eulerian species transport equation for water vapor was also used to model the humidity in the air. The density of the air followed a second-order polynomial function determined by fitting the thermodynamic density/temperature data of air to simulate the buoyancy effect. Turbulence closure was achieved by adopting the renormalization group (RNG) k-ε turbulence model. The motion of each expiratory droplet (or the discrete phase) was tracked by solving the force balance equation in a Lagrangian frame of reference,
Aerodynamic drag (the first term), gravity force (the second term), thermophoretic force and Brownian diffusion (denoted as F other ) were modeled in Equation (Equation1). Evaporation was also considered by solving the classical gradient of diffusion equation for water vapor. The governing equations for the air and the droplet phases were solved using a finite-volume based code, Fluent, with the second-order upwind solution scheme and the SIMPLE algorithm for pressure and velocity coupling in the air phase. Since the injected droplets occupy an extremely small portion of the volume compared with that of the entire geometry, the influence of the droplets on the surrounding airflows was assumed to be negligible.
As discussed by CitationChao et al. (2008a), accurate modeling of the near-wall turbulence is crucial for aerosol deposition prediction in Lagrangian-based models. In the current simulations, an enhanced two-layer wall treatment was also employed instead of the standard wall function used by CitationWan et al. (2007). The turbulence anisotropy in the near-wall region was modeled by adopting the near-wall correction functions proposed by CitationWang and James (1999) to the fluctuating velocity component normal to the wall,
The correction was incorporated into the code by modifying the turbulence kinetic energy to k′,
The correction was applied to the first two layers of the meshes from the walls and f v was set to 1 elsewhere. A similar boundary treatment was adopted in a number of studies using Lagrangian-based models, e.g., CitationChao et al. (2008a). The wall boundary effect of solid surfaces on expiratory aerosols was set as “trap,” which implies that re-suspension was not considered in the simulations. In aircraft cabins, airflow near the walls can be quite high sometimes under the influence of the supply air jet. A simple order of magnitude analysis was performed to examine the validity of this assumption using the particle adhesive force relation (CitationHinds 1999) for smooth surfaces: F adh ≅ 126 D p [0.5 + 0.0045(%RH)] where the unit of D p is in cm. For a 100 μ m aerosol under 5% RH, the estimated adhesive force is 0.66 dyn which is much larger than the detachment forces of 6 × 10− 2 dyn by air current of 10 m/s and 5 × 10− 4 dyn by the aerosol's own weight under gravity. The difference in the adhesive force compared to the detachment forces is even larger for smaller aerosols.
Three cases were considered in the current study. The first two cases simulated the cruising condition in the cabin with the ECS operating properly. The supply airflow rates in these two cases were 200 and 100 L/s, which corresponded to 9.5 and 4.8 L/s/person or 19.5 and 9.8 air changes per hour, respectively. The cabin temperature and relative humidity (RH) were maintained at 24°C and 5% to simulate cruising conditions. The internal wall surface temperature was set at 18°C. The third case simulated a major failure of the ECS when the plane is grounded. This case was added to simulate the widely referred influenza outbreak incident aboard an aircraft which aborted takeoff due to an engine failure (CitationMoser et al. 1979). Most of the passengers were kept in the aircraft with a failed engine (so as the ECS) for 4.5 h before a replacement aircraft came. Detailed air conditions in the cabin during this 4.5 h grounded period were not reported but CitationMoser et al. (1979) suggested that the cabin should be warm and the air change was poor during this period. In the current simulations, a supply airflow rate of only 20 L/s (corresponding to 0.95 L/s/person) was assumed for this case. The supply air and the internal wall surface temperatures were set at 22°C. Since the heating cylinders were not releasing any water vapor, the RH of the supply air was set at 99% to simulate a humid environment during ECS failure. Under these settings, the simulated average air temperature and RH in the cabin were about 28°C and 70%, respectively. The passenger seated in the middle of the last row was chosen as the injection source of the expiratory aerosols for all cases. A circular droplet injection (cough) outlet was created on the face of that passenger, 1.1 m above the floor. The air volume of each injection was 400 mL and the duration was 1 s. This setting corresponded to exhaling a lung tidal volume of a normal adult in the injection process. The velocity of air leaving the injection outlet was set at 10.6 m/s to match the velocity measured at the outlet of the droplet generator used in the validation experiments. According to these settings, the injection outlet had a diameter of 7.1 mm.
Nine different sizes of droplets were simulated, including 2, 6, 12, 20, 28, 45, 87.5, 137.5, and 225 μ m in diameter, covering the majority of the expiratory droplet size spectrum during coughing reported by CitationDuguid (1946) and CitationLoudon and Roberts (1967). These two studies employed collection media for measuring the droplet size. Several studies employing sampling-based optical particle counts reported that the majority of exhaled particles were in the submicron size range, e.g., CitationPapineni and Rosenthal (1997), CitationFabian et al. (2008), etc. CitationNicas et al. (2005) reviewed and compared the studies by CitationDuguid (1946), CitationLoudon and Roberts (1967), and CitationPapineni and Rosenthal (1997). They suggested that the limitations of sampling associated with optical particle counts could have been the reason for the deficit of large particles in the study by CitationPapineni and Rosenthal (1997). CitationNicas et al. (2005) also compared the three sets of data with a bioaerosol study. They found that the results by CitationDuguid (1946) and CitationLoudon and Roberts (1967) are consistent with the bioaerosol study but not for the study by CitationPapineni and Rosenthal (1997). Another recent human-subject study employing a collection of different measurement methods also revealed a similar trend. Optical particle counter measurements showed major size modes at about 0.8 μ m (CitationMorawska et al. 2008) while the IMI measurements showed size modes at about 12–16 μ m (CitationChao et al. 2008b). Based on the analysis by CitationNicas et al. (2005), the size ranges reported by CitationDuguid (1946) were referred when designing the size of tracer droplets to be studied. The range of aerosol size studied coved both “droplet” and “droplet nuclei” sizes as indicated by CitationSehulster et al. (2004) and they are shown to have very different transport behaviors (CitationChao et al. 2008a). In the 200 and 100 L/s cases, the relative humidity was very low. The expiratory droplets were assumed to be completely desiccated after evaporation in the air (losing 94% of their volume in the volatile fraction). The corresponding residue (nuclei) sizes after evaporation are listed in . This assumption was made with reference to the saliva non-volatile content as reported by CitationEffros et al. (2002) and the fact that the cabin RH was much lower than the crystallization RH of saline solution (around 40% as indicated by CitationNicas et al. 2005). In the case of ECS failure, the equilibrium droplet residue sizes were assumed to be 61% of their original size (as suggested by CitationNicas et al. 2005) and the corresponding residue sizes are also listed in . This was achieved by adjusting the volatile fraction to 77% in the simulations. 10,000 tracer droplets of each size were injected in the simulations. This injection number was selected based on a numerical test on the total number of droplets deposited after the injection of 1,000, 10,000, and 50,000 droplets. The test showed that injecting 10,000 and 50,000 tracer droplets gave similar predictions while the results differed significantly when 1,000 droplets were injected for each size. After balancing considerations of computational time and statistical stability, the injection number of 10,000 tracer droplets was selected for each droplet size. The simulations were run until all the injected droplets were removed by air extraction or by deposition. Other boundary conditions and simulation inputs are summarized in . Since there are two different sets of residue sizes for different cases, to avoid confusion, the initial droplet size is referred in the following discussions unless otherwise specified.
TABLE 1 Measured size distribution of injected droplets in the validation experiments and the factorization of each tracer droplet in the simulations
TABLE 2 Boundary conditions and inputs for numerical simulations
2.3. Experimental Validation
Experiments were conducted in an aircraft cabin mockup having the same dimensions as the numerical cabin geometry to validate the numerical results. The experimental details are presented in a companion paper (CitationSze To et al. 2008b). Here, a brief description is given. Airflows at selected locations in the cabin mockup were characterized by particle image velocimetry (PIV) (LaVision FlowMaster) measurements. Dispersion of expiratory aerosols was also measured. An in-house-built droplet generator was installed to produce droplets of a “simulated saliva” solution. The “simulated saliva” solution was prepared by mixing 76 g of glycerin and 12 g of salt (NaCl) in 1 liter of distilled water. The glycerin and salt content was added to simulate the non-volatile content in human respiratory fluid. With this composition, the residues were about 6% in of the droplets' original volumes. To simulate coughs, each droplet injection lasted for one second and 0.075 ml of “simulated saliva” solution were injected into the cabin with 0.4 liter of air. This amount of solution roughly corresponded to the amount of expiratory fluid expelled by 10 coughs (CitationZhu et al. 2006). A detailed description of the droplet generator can be found in CitationWan et al. (2007). The size distribution of the droplets injected by the droplet generator was characterized by the interferometric Mie imaging (IMI) (LaVision SizingMaster) technique. The measured size distribution is shown in . The droplet generator was mounted at the middle seat of the last row, producing droplet injections in the forward direction. The outlet of the droplet injector was 1.1 m above the floor, about the breathing height of a seating passenger. The dispersion of the droplets in the cabin mockup was measured by the IMI system and an aerosol spectrometer (GRIMM model 1.108) in a measurement grid covering half of the cabin, which is shown in . When measuring the aerosol dispersion, the aerosol measurement instruments were set at one of the measurement points in the grid. The aerosol spectrometer measured the background aerosol level at that point for 1 min, which would later be subtracted from the aerosol counting results obtained after the injection. A droplet injection would then be produced. The aerosol measurement instruments counted the aerosols every second for 6 min after the injection. The instruments were then moved to another measurement location and the above process was repeated until all the measurement points were covered. The mean positions for aerosols of each size relative to the position of the droplet injection point were then calculated. A similar experimental validation method was adopted in other studies, e.g., CitationChao et al. (2008a) in a hospital ward and CitationChao and Wan (2006) in a clean room. The airflow and aerosol dispersion measurements were conducted under the 100 L/s and 200 L/s supply airflow rate conditions.
Another set of experiments was performed to measure the amounts of aerosols deposited on surfaces in the cabin. In the deposition measurements, the surfaces inside the cabin were covered with sheets of polyethylene film. The heated surfaces of the heating cylinders were covered with aluminum foil. Two grams of water-soluble fluorescent dye (Kingscote Chemicals, Bright Dyes FLT Yellow/Green) was added to 10 liters of the “simulated saliva” solution. Droplets with fluorescent dye were then produced by the droplet generator using the same injection procedure as described above. After each injection, the aerosols were allowed to deposit for one hour before making another injection. A total of five injections were made before the films/foils were carefully detached. The deposited fluorescent droplets were extracted by soaking the film/foil into a known volume of distilled water. A sample of the water containing the extracted fluorescent dye was then analyzed in a spectrophotometer (Hach, DR/2500) to determine the amount of fluorescent dye deposited on that sheet. Deposition measurements were performed under the 200 L/s supply airflow rate condition.
3. RESULTS
3.1. Airflow Patterns
shows the numerically predicted lateral airflow pattern on half of the XZ plane cutting through the center of the cabin geometry for the 200 L/s and ECS failure cases. In the 200 L/s case, a high-velocity supply air jet of up to nearly 3 m/s was produced laterally (X-direction) at the supply air slot outlet. The air jet attached to the wall as it continued to flow downward, creating a strong downward airflow along the side wall. When the downward airflow reached the floor, it turned towards the center of the cabin and became an up-rising air current following the heat plumes of the passengers. Downward airflows of around 0.2–0.6 m/s were created near the head region of the passenger sitting next to the sidewall. Airflows at the head regions of other passengers were mainly up-rising. The up-rising airflows near the head region of the middle seat passenger were about 0.2–0.3 m/s. The airflow pattern of the 100 L/s condition was similar to the airflow pattern in the 200 L/s condition but the magnitudes of the velocity were lower. The supply air jet at the slot outlet was reduced to about 1.5–2 m/s. The airflow pattern of the ECS failure condition was more irregular compared to the other two conditions since the heat plumes of the passengers became the major driving force of the airflow in the cabin. Downward airflows near the side wall and up-rising heat plumes around the heating cylinders were also found. However, the strong air jets attached to the walls as observed in the 100 and 200 L/s cases were replaced by a much weaker downward airflow induced by the cooler wall surface. Under-seat airflows towards the cabin center were also not observed in the ECS failure case.
FIG. 2 (a) Predicted lateral airflow velocity profile on the XZ plane cutting through the center of the cabin. (b) Predicted longitudinal airflow velocity profile on the YZ plane cutting through the center of the cabin.
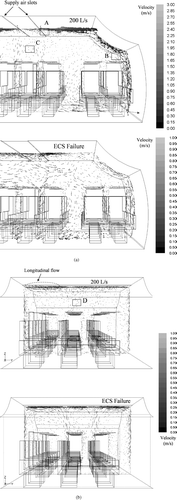
The predicted longitudinal airflow patterns on the YZ plane cutting through the center of the cabin under the 200 L/s supply airflow rate and ECS failure conditions are shown in . Airflows in this plane were mainly up-rising. No major longitudinal (Y-direction) airflow was observed in the occupied space (below 1.7 m height) but some longitudinal airflows of around 0.3–0.4 m/s were observed along the ceiling. Again, a similar airflow pattern was obtained in the 100 L/s condition. The airflow pattern became more random in the ECS failure case but the longitudinal airflow was still found along the ceiling. The magnitudes of airflow velocities measured by PIV at selected locations (marked A, B, C, and D in ) are compared with those predicted by simulations in . The PIV flow fields at these locations are also shown in so that the measured flow directions can be compared with those predicted. The two sets of results agree to each other fairly well.
FIG. 3 (A) PIV measurement results at location A indicated in . (B) PIV measurement results at location B indicated in . (C) PIV measurement results at location C indicated in . (D) PIV measurement results at location D indicated in .
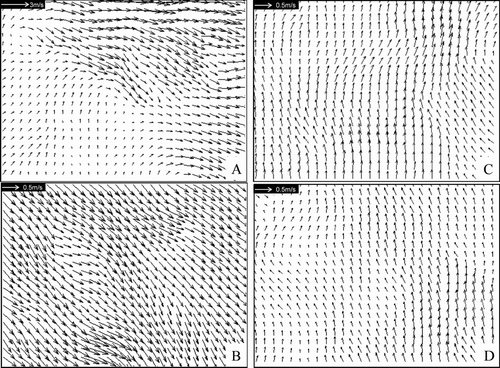
TABLE 3 Comparisons between numerically predicted and measured airflow velocity magnitudes at selected locations
3.2. Dispersion of Expiratory Aerosols
– show the mean lateral positions for different sizes of expiratory aerosols under the three airflow conditions. The aerosol sizes shown in the figure refer to the initial droplet size immediately after the droplets were produced. For the 200 and 100 L/s conditions ( and ), the numerical predictions and the experimental results are plotted in the same figure for comparisons. The numerical mean lateral position is the mean of absolute lateral positions, x, of all airborne expiratory aerosols of a certain size at time t obtained from the numerical particle tracking data. The lines in the figure are fitting curves of the predicted results (denoted as “Num”). The experimental mean lateral position was determined by the following method. In each second, the measured number of expiratory aerosols of a certain size was multiplied by the lateral position, x, at that measurement point. The products of every measurement point were summed and the sum was divided by the total number of expiratory aerosols counted to obtain the mean lateral position at time t for that size of expiratory aerosol. The experimental results are indicated by the symbols in and . Since the aerosol measurement lasted for 360 s in the validation experiment, results are shown up to 360 s. Numerical results are also shown up to 360 s because the number of aerosols remaining airborne became very small at that time in the 100 and 200 L/s conditions. The predicted mean lateral position started to become highly scattered starting from this time. The “0” mean lateral position is the position of the droplet injector located at the middle seat of the last row.
The effect of aerosol size on the dispersion is revealed in the figure. Smaller aerosols dispersed faster under all conditions. For instance, under the 200 L/s supply airflow rate, the 2 μ m aerosols reached a mean lateral position of around 0.9 m at 10 s. In the same amount of time, the mean lateral position of the 28 μ m aerosols was around 0.5 m. The line for the 45 μ m aerosols terminated at around 35 s, 60 s, and 130 s for the 200, 100 L/s, and ECS failure cases, respectively, in . This was because none of the 45 μ m aerosols remained airborne by that time. The line terminated earlier at a higher supply airflow rate since the loss rate of airborne aerosols by ventilation was higher. Similar results were also obtained in the experiments as shown in the figure indicating that no more data points were shown for the 45 μ m aerosols after a time close to the time when the line representing numerical results terminated. Results for larger aerosols, i.e., 87.5 μ m and above, are not shown since the loss rates of these aerosols were very high. The airborne time of these very large aerosols was less than 20 s in all cases. This observation was similar to that reported in studies in other indoor environments (e.g., CitationChao et al. 2008a). The high loss rate of these very large airborne aerosols was mainly due to high deposition rate. More deposition results are discussed below.
FIG. 5 (a) Mean lateral positions for aerosols of 2 to 45 μ m in size for the 200 L/s case. (b) Mean lateral positions for aerosols of 2 to 45 μ m in size for the 100 L/s case. (c) Mean lateral positions for aerosols of 2 to 45 μ m in size for the ECS failure case.
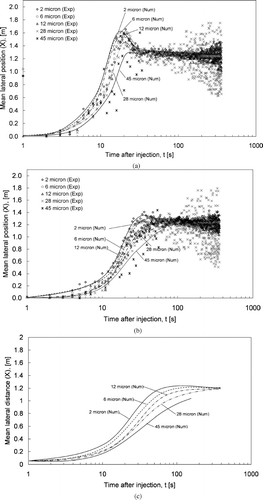
The effect of the supply airflow rate on aerosol dispersion can also be seen in the figures. The mean lateral position increased more rapidly at higher supply airflow rates. For example, the mean lateral position of the 2 μ m aerosols reached about 0.9 m under the 200 L/s supply airflow rate at 10 s. It was about 0.4 m in the 100 L/s case and about 0.3 m in the ECS failure case. also shows that the mean lateral positions of the 2–28 μ m aerosols became relatively steady at around 1.2–1.3 m at about 30 s. This suggests that the distribution of aerosols of these sizes became “evenly distributed” along the lateral direction after about 30s at the 200 L/s supply airflow rate. The time for achieving the “evenly distributed” state in the lateral direction was about 60 s in the 100 L/s case and about 200 s in the ECS failure case. These results suggest that setting a higher supply airflow rate increases the dispersion of expiratory droplets and enhances their mixing. – show the mean longitudinal positions for different sizes of expiratory aerosols under the three airflow conditions. The “0” mean longitudinal position is the position of the droplet generator. It can be seen that the expiratory aerosols started at a mean longitudinal position of around 1 m. This is because the initial cough air jet moved many of the aerosols forward for about 1 m, reaching one row of seat ahead. In the subsequent seconds, the change in the mean longitudinal position was within 0.2 m. The mean longitudinal position decreased at 5–15 s as the aerosols continued to disperse, depending on the airflow conditions. In contrast to the lateral direction, an “evenly distributed” condition was not observed in any of the three testing conditions within the 360 s period. This suggests that the dispersion and mixing of the aerosols were slower in the longitudinal direction compared to the lateral direction. This observation is in line with the airflow patterns described in the previous section where the air circulations created by the cabin ECS were described as mainly in the lateral direction.
FIG. 5 (a) Mean longitudinal positions for aerosols of 2 to 45 μ m in size for the 200 L/s case. (b) Mean longitudinal positions for aerosols of 2 to 45 μ m in size for the 100 L/s case. (c) Mean longitudinal positions for aerosols of 2 to 45 μ m in size for the ECS failure case.
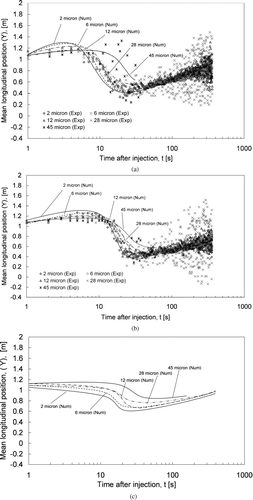
Readers are advised to be cautious to some measurement uncertainties in the validation experiments. As shown in , aerosol measurements were performed in a predefined grid of points. This grid had spatial resolution of 505–530 mm (lateral), 570–810 mm (longitudinal), and 600–900 mm (vertical). When the number of aerosols remaining in the cabin was small, e.g., only a few detected in each point, the calculated mean position would start to fluctuate significantly. This is realized in and , . Starting from 60 s after the injection, the experimental results for 28 μ m aerosols began to fluctuate. The fluctuation increased with time since there were less and less aerosols left in the cabin as time went on. Fluctuation of experimental results began even earlier (around 20 s after the injection) for 45 μ m aerosols since their deposition losing rate was higher than 28 μ m aerosols (to be discussed in the following section). This made the number of 45 μ m decrease even faster. Validation of aerosol dispersion results at the time after the fluctuation has occurred remained questionable.
3.3. Deposition of Expiratory Aerosols
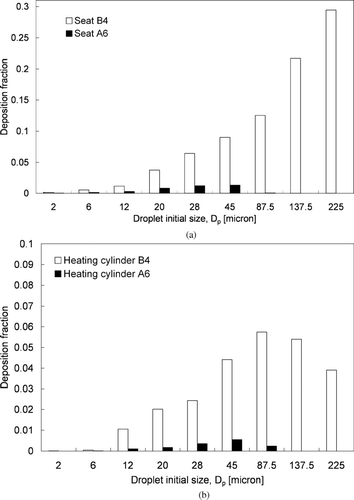
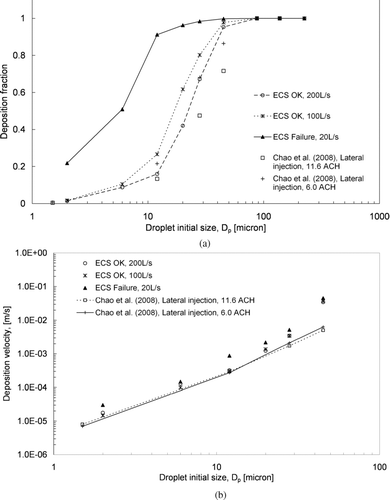
shows the predicted deposition fractions for different sizes of expiratory aerosols. The deposition fraction is the ratio of the number of deposited aerosols to the total number of injected aerosols for each size. When the ECS was working properly (100 and 200 L/s), the deposition fraction for the 2 μ m aerosols was less than 0.02. The deposition fraction increased to over 0.6 for the 28 μ m aerosols. The increase in the deposition fraction with an increase in aerosol size indicates that the significance of deposition increased with the aerosol size. Considering the fact that deposition and ventilation were the only two aerosol loss mechanisms in the cabin, the significance of ventilation loss decreased correspondingly as the deposition fraction increased. This is because the gravitational settling effect was more dominant for larger aerosols, which increased the deposition on upward-facing surfaces. Another reason is that the higher inertia of the larger aerosols led to more deposition on the seat in front of the droplet injection point by direct impaction of the cough jet. Another observation is that the deposition fraction decreased with the supply airflow rate. For instance, over 95% of the 28 μ m aerosols were lost by deposition in the ECS failure case. The deposition fraction decreased to below 75% when an airflow rate of 100L/s was supplied. About 60% of the 28 μ m aerosols were lost by deposition by increasing the supply airflow rate to 200 L/s. A similar observation can be made for other aerosol sizes below 45 μ m. This suggests that setting a higher supply airflow rate promotes aerosol removal by air extraction for smaller aerosols. The supply airflow rate effect become less significant for aerosols of 45 μ m and above in size. All aerosols 87.5 μ m and larger were lost by deposition. The predicted removal number fractions (similar to the deposition fraction in the current study) in a hospital ward reported by CitationChao et al. (2008a) are plotted in the figure for comparison. Considering the fact that the surface-to-volume ratio of the cabin (4.4 m− 1) was much higher than that of the hospital ward (1.92 m− 1) examined by CitationChao et al. (2008a), we expect that the deposition fraction in the cabin would also be higher than those in the hospital ward. The effect of the supply airflow rate on the deposition behavior is shown in for aerosols up to 45 μ m in terms of overall deposition velocity. The overall deposition velocity for each size of the expiratory aerosol was estimated using the following relation:
FIG. 6 (a) Deposition fractions for different sizes of aerosols. (b) Deposition velocities for different sizes of aerosols.
4. DISCUSSION
From the physical dispersion and deposition characteristics of expiratory aerosols shown above, several issues in relation to the risk of infectious disease are discussed here. The first issue is that it is commonly believed that increasing the ventilation rate can reduce the risk of infection, as indicated in many well-known infection risk models (CitationBeggs et al. 2003). The current results support this belief in one way that a higher ventilation rate is shown to enhance the ventilation dilution and reduce the deposition of pathogenic aerosols. In another way, a higher ventilation rate may also enhance the dispersion and mixing of the pathogenic aerosols, increasing the exposure of passengers seated further away from the index patient. Another issue is that a significant amount of the expiratory aerosols was deposited on surfaces inside the cabin. This brings up the concern of the associated risk of infection transmission via physical contact with contaminated surfaces. In order to provide more insights on these issues, an infection risk analysis was conducted using the current numerical data and some epidemiological data from the literature. Influenza was chosen as the model infectious disease in this analysis since the epidemiological data and the in-flight transmission cases of influenza are rather well documented.
4.1. Infection Risk via Inhalation of Airborne Pathogenic Aerosols
The infection risk caused by the inhalation of virus-laden aerosols was estimated using the model proposed by CitationSze To et al. (2008a). Exposure to airborne influenza viruses during an exposure time interval, t 0, at any spatial location, x i , was calculated by
TABLE 4 Literature data used for infection risk analysis
TABLE 5 Estimated exposure to airborne expiratory aerosols for the case of 200 L/s when 1 droplet injection was made
TABLE 6 Estimated infection risks by inhalation of pathogenic aerosols
TABLE 7 Estimated infection risk via airborne (aerosol size < 5 μ m) and droplet (aerosol size > 5 μ m) transmission modes for the 200 L/s case
4.2. Infection Risk via Hand Contact with Contaminated Surfaces
A model was developed to estimate the exposure to the virus through contact with contaminated surfaces. We consider that the index patient coughs f s times per hour and an amount of N k viruses was deposited on the kth surface after each cough, where N k is determined from the concentration of the virus per unit volume of the saliva of the index patient. A fraction, c h , of the virus deposited on that surface will be transferred to the hand after a contact. Transfer of virus from hand to mucus membranes after a contact was assumed 100%. The frequencies of contact with the contaminated surface and with the mucous membranes by the hand are f h and f m , respectively. Pathogens can survive on surfaces for days or even weeks (CitationWalther and Ewald 2004) but the exposure time during air travel is usually for a few hours. Therefore the decay of pathogens on the contaminated surface due to virus survival was neglected in this analysis. The total dose of viruses left on the contaminated surface after the n th contact with the surface would be
At the same time, the total dose of viable virus transmitted to the hand after the nth contact with the surface would be
Normally, a passenger in an aircraft cabin may contact their clothes, the seat he/she is sitting in, and the seat back immediately in front much more frequently than the ceiling and the floor of the cabin. Therefore, the seat surface, the seat back in front of the passenger and the manikin surface were considered in the exposure estimation. The transfer efficiency of influenza virus to the finger pad was taken as 0.251% () when the hand touched these surfaces. An area of 3.5 cm2 was assumed for the finger pad. c h was estimated as 3.5 cm2/area of the contaminated surface × 0.251%. Since no observational study on the hand contact rate of passengers in aircraft cabin was found in the literature, a rate of three contacts per hour (1 contact each to the seat, seat back and manikin surface per hour) was assumed. No study reporting the transfer efficiency of pathogen from the hand to the mucous membrane was found but one study reported that about 34% of virus on the finger pad will transfer to the lips after a contact (CitationRusin et al. 2002). Transfer efficiency of microorganism between surfaces with moisture was 1 to 2 orders higher than without moisture (CitationSatter et al. 2001). Mucous membranes in the nostrils and eyes are generally much moister than in the lips. Based on these considerations, the transfer efficiency from the finger pad to the mucous membranes was assumed to be 1. Other data used for the estimation contact exposure are listed in . After obtaining the exposure, the infection risk by contact with contaminated surfaces was estimated using a dose-response model similar to Equation (Equation6)
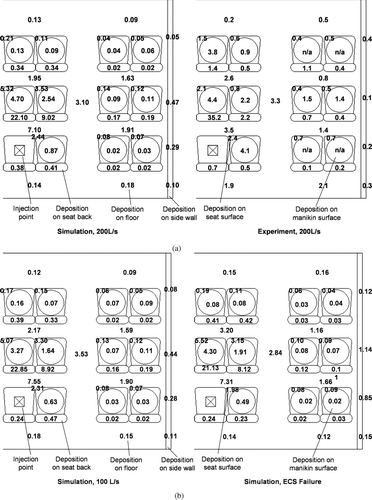
shows the estimated risk of infection transmission by hand contact with contaminated surfaces for a 3 hour exposure period. Compared with , the estimated infection risk by hand contact was lower than the estimated risk by inhalation of aerosolized pathogens by at least four orders of magnitude, under the assumptions listed in . This means that the transmission of disease by hand contact with contaminated surfaces is not as efficient as by inhalation. The passengers in close proximity to the index patient, including in seats B3, B4, and C3, had contact infection risks about 1–2 orders higher than those of the other passengers. This is because more expiratory aerosols were deposited on the surfaces close to the source, as shown in . The virus exposure and, thus, the risk of infection were highly dependent on the deposited mass. As discussed earlier, the change in the supply airflow rate had an insignificant effect on the distribution of the deposited mass fraction. The distribution of the estimated contact infection risk was similar in the three cases. This suggests that the distribution of contact infection risk is more dependent on the coughing habits of the index patient, e.g., if the index patient covers his/her mouth during coughing, than on the airflow conditions in the cabin.
TABLE 8 Estimated infection risk by hand contact with surfaces contaminated by deposited pathogenic aerosols
Cases shown in assumed a contact rate of three per hour to the seat, seat back and manikin surface. Higher contact rates could be expected for surfaces such as tray tables or laptop keyboards. The deposition results also suggest that horizontal surfaces are favorable sites for deposition since gravitational settling is the major deposition mechanism for large aerosols. To test the sensitivities of these parameters, two extra sets of risk assessment were performed for the case of 200 L/s with an additional laptop keyboard surface in each seat. Each keyboard surface was assumed to have a horizontal surface area of 330 cm2. The aerosol deposition on the keyboard surface was projected from the aerosol deposition on the floor right below each seat. The risk assessment results are shown in . One set of assessment was performed with a contact frequency of three per hour (same frequency for cases shown in ) on the keyboard surface only. This case is denoted as “keyboard” in . Another set of assessment was performed with a contact frequency of thirty per hour on the keyboard in addition to the original contact behavior (). This case is denoted as “30/hr keyboard + original.” The contribution of the keyboard surface to infection risk compared to surfaces considered in cases shown in can be seen by comparing the “keyboard” case with . The infection risks in Row C for the “keyboard” case are about an order of magnitude higher when compared with , while the infection risks contributed by the keyboard are in the same order of magnitude for other seats when the two cases are compared. For the “30/hr keyboard + original” case, risks of infection increased by about 2 orders of magnitude compared to the original contact behavior. The result suggests that these behavioral factors have more significant impact than the ventilation rate on disease transmission via hand contact. With the extra horizontal surface and the higher contact frequency, the infection risks via hand contact for the “30/hr keyboard + original” case are still 2–4 orders of magnitude lower than inhalation risks.
TABLE 9 Estimated infection risks by hand contact with an extra laptop keyboard surface in each seat for the 200 L/s case
5. CONCLUSIONS
New insights on the transport and deposition characteristics of polydispersed expiratory aerosols in aircraft cabins and the relation with the risk of infection transmission can be provided.
The ECS in the cabin created air circulation mainly in the lateral direction. No major longitudinal airflow was created in the occupied region (below 1.7 m height) but some longitudinal airflow was created along the ceiling. Downward airflows were created at the head region of the passengers seated next to the side wall but upward airflows were created near the head regions of other passengers. In the case that simulated the failure of the ECS, the flow pattern in the cabin became more irregular.
Aerosols less than 28 μ m in size (corresponding residue size: 11.0 μ m at 5% RH, 17.1 μ m at 70% RH) exhibited behaviors favorable to airborne transport. Aerosols 45 μ m and above in size could not stay airborne for more than 60 s when the ECS was working properly and not more than 130 s in the ECS failure case. Dispersion of expiratory aerosols was enhanced by increasing the supply airflow rate. For small aerosols (less than 28 μ m in size), an even aerosol distribution was attained in 30 s at the 200 L/s supply airflow rate in the lateral direction. The time was delayed to 60 s and 200 s for the 100L/s and ECS failure conditions, respectively. The “evenly distributed” condition was not achieved in a 360 s period in all the three cases in the longitudinal direction, suggesting that the dispersion (and mixing) of expiratory aerosols in the longitudinal direction was much slower than in the lateral direction.
The deposition behavior was highly dependent on aerosol size. Nearly all of the aerosols larger than 45 μ m in diameter (corresponding residue size: 17.6 μ m at 5% RH, 27.5 μ m at 70% RH) were lost by deposition regardless of the supply airflow rate. The deposition fraction (in terms of number) decreased with the aerosol size. For the 2 μ m aerosols (corresponding residue size: 0.6 μ m at 5% RH, 1.2 μ m at 70% RH), the deposition fractions were reduced to below 0.02 in the 100 and 200 L/s cases. An increase in the supply airflow rate reduced the deposition fraction for aerosols less than 45 μ m in size and, thus, increased the fraction of aerosols removed by ventilation. However, the effect of airflow on the deposition behavior was limited to small aerosols only. The deposition behavior of aerosols larger than 45 μ m in size was mainly affected by the initial cough jet and the gravitational effect while the effect of the ventilation airflow was insignificant. The change in supply airflow rate had an insignificant effect on the aerosol deposition in the cabin in terms of the mass fraction.
The estimated infection risk for influenza due to inhalation of pathogenic aerosols was at least two orders of magnitude higher than the risk due to hand contact with surfaces contaminated by deposition of pathogenic aerosols. The passenger seated in front of the index patient had the highest infection risk due to inhalation of pathogenic aerosols. This risk was at least five times higher than the risk to the other passengers. An increase in the supply airflow rate reduced the infection risk of passengers seated close (within 1 row and 1 column) to the index patient but increased the infection risk of other passengers seated further. Passengers seated within 1 row and 1 column had contact risks of about 1–2 orders of magnitude higher than the other passengers had. The change in the supply airflow rate had insignificant impact on the distribution of risk of infection. Comparatively, the hand contact behavior played a more significant role on infection risk via hand contact.
Several factors including the movements of passengers and the health condition of susceptible passengers were not considered in the current study. Some assumptions made for the infection risk analysis were without adequate support, such as the finger pad size and the hand touching frequency, due to the lack of related studies in the literature. The current study provided a first attempt to perform infection risk analysis (for inhalation and hand contact transmission) based on the predicted aerosol transport and deposition results. More studies are needed to address these issues further.
This study was financially supported by the Research Grants Council of the Government of Hong Kong S.A.R. through grant no. 611505. Technical and partial financial support from the International Centre for Indoor Environment and Energy, Technical University of Denmark is acknowledged.
REFERENCES
- Alford , R. H. , Kasel , J. A. , Gerone , P. J. and Knight , V. 1966 . Human Influenza Resulting from Aerosol Inhalation . Proc. Soc. Exp. Biol. Med. , 122 : 800 – 804 .
- Bean , B. , Moore , B. M. , Sterner , B. , Peterson , L. R. , Gerding , D. N. and Balfour , H. H. Jr. 1982 . Survival of Influenza Viruses on Environmental Surfaces . J. Infect. Dis. , 146 ( 1 ) : 47 – 51 .
- Beggs , C. B. , Noakes , C. J. , Sleigh , P. A. , Fletcher , L. A. and Siddiqi , K. 2003 . The Transmission of Tuberculosis in Confined Spaces: An Analytical Review of Alternative Epidemiological Models . Int. J. Lung Dis. , 7 ( 11 ) : 1015 – 1026 .
- Chao , C. Y. H. and Wan , M. P. 2006 . A Study of the Dispersion of Expiratory Aerosols in Uni-Directional Downward and Ceiling-Return Type Airflows Using Multiphase Approach . Indoor Air , 16 ( 4 ) : 296 – 312 .
- Chao , C. Y. H. , Wan , M. P. and Sze To , G. N. 2008a . Transport and Removal of Expiratory Droplets in Hospital Ward Environment . Aerosol Sci. Technol. , 42 : 377 – 394 .
- Chao , C. Y. H. , Wan , M. P. , Morawska , L. , Johnson , G. R. , Ristovski , Z. D. , Hargreaves , M. , Mengersen , K. , Corbett , S. , Li , Y. , Xie , X. and Katoshevski , D. 2008b . Characterization of Expiration Air Jets and Droplet Size Distributions Immediately at the Mouth Opening . J. Aerosol Sci. , doi:10.1016/j.jaerosci.2008.10.003.
- Douglas , R. G. 1975 . “ Influenza in Man ” . In The Influenza Viruses and Influenza , Edited by: Kilbourne , E. D. 375 – 447 . New York : Academic Press .
- Duguid , J. P. 1946 . The Size and the Duration of Air-Carriage of Respiratory Droplets and Droplet-Nuclei . J. Hyg. , 44 : 471 – 479 .
- Effros , R. M. , Wahlen , K. and Bosbous , M. 2002 . Dilution of Respiratory Solutes in Exhaled Condensates . Am. J. Resp. Crit. Care Med. , 165 : 663 – 669 .
- Fabian , P. , McDevitt , J. J. , DeHaan , W. H. , Fung , R. O. P. , Cowling , B. J. , Chan , K. H. , Leung , G. M. and Milton , D. K. 2008 . Influenza Virus in Human Exhaled Breath: An Observational Study . Plos One , 3 ( 7 ) : e2691
- Gao , N. P. and Niu , J. L. 2007 . Modeling Particle Dispersion and Deposition in Indoor Environments . Atmos. Environ. , 41 : 3862 – 3876 .
- Haghighat , F. , Allard , F. , Megri , A. C. , Blondeau , P. and Shimotakahara , R. 1999 . Measurements of Thermal Comfort and Indoor Air Quality Aboard 43 Flights on Commercial Airlines . Indoor Built Environ. , 8 : 58 – 66 .
- Hendley , J. O. , Wenzel , R. P. and Gwaltney , J. M. Jr. 1973 . Transmission of Rhinovirus by Self-Inoculation . New Engl. J. Med. , 288 : 1361 – 1364 .
- Hinds , W. C. 1999 . Aerosol Technology , New York : John Wiley & Sons, Inc. .
- Kenyon , T. A. , Valway , S. E. , Ihle , W. W. , Onorato , I. M. and Castro , K. G. 1996 . Transmission of Multidrug-Resistant Mycobacterium Tuberculosis During a Long Airplane Flight . N. Engl. J. Med. , 334 ( 15 ) : 933 – 938 .
- Klontz , K. C. , Hynes , N. A. , Gunn , R. A. , Wilder , M. H. , Harmon , M. W. and Kendalan , A. P. 1989 . Outbreak of Influenza A/Taiwan/1/86 (H1N1) Infections at a Naval Base and its Association with Airplane Travel . Am. J. Epidemio. , 129 ( 2 ) : 341 – 348 .
- Lin , C. H. , Horstman , R. H. , Ahlers , M. F. , Sedgwick , P. E. , Dunn , K. H. , Topmiller , J. L. , Bennett , J. S. and Wirogo , S. 2005a . Numerical Simulation of Airflow and Airborne Pathogen Transport in Aircraft Cabins—Part I: Numerical Simulation of the Airflow Field . ASHRAE Trans. , 111 : 755 – 764 .
- Lin , C. H. , Horstman , R. H. , Ahlers , M. F. , Sedgwick , P. E. , Dunn , K. H. , Topmiller , J. L. , Bennett , J. S. and Wirogo , S. 2005b . Numerical Simulation of Airflow and Airborne Pathogen Transport in Aircraft Cabins—Part II: numerical simulation of airborne pathogen transport . ASHRAE Trans , 111 : 764 – 768 .
- Loudon , R. G. and Brown , L. C. 1967 . Cough Frequency in Patients with Respiratory Disease . Am. Rev. Respir. Dis. , 96 ( 6 ) : 1137 – 1143 .
- Loudon , R. G. and Roberts , R. M. 1967 . Droplet Expulsion from the Respiratory Tract . Am. Rev. Resp. Dis. , 95 : 435 – 442 .
- Mangili , A. and Gendreau , M. A. 2005 . Transmission of Infectious Diseases During Commercial Air Travel . Lancet , 365 : 989 – 996 .
- Miller , M. A. , Valway , S. and Onorato , M. I. 1996 . Tuberculosis Risk after Exposure on Airplanes . Tubercle and Lung Dis. , 77 : 414 – 419 .
- Morawska , L. 2006 . Droplet Fate in Indoor Environments, or can We Prevent the Spread of Infection? . Indoor Air , 16 : 335 – 347 .
- Morawska , L. , Johnson , G. R. , Ristovski , Z. , Hargreaves , M. , Mengersen , K. , Chao , C. Y. H. , Li , Y. and Katoshevski , D. 2008 . Size Distribution and Sites of Origins of Droplets Expelled from the Human Respiratory Tract During Expiratory Activities . J. Aerosol Sci. , doi: 10.1016/b.jaerosci.2008.11.002
- Moser , M. R. , Bender , T. R. , Margolis , H. S. , Noble , G. R. , Kendal , A. P. and Ritter , D. G. 1979 . An Outbreak of Influenza aboard a Commercial Airline . Am. J. Epidemio. , 110 : 1 – 6 .
- Murphy , B. R. , Chalhub , E. G. , Nusinoff , S. R. , Kasel , J. and Chanock , R. M. 1973 . Temperature-Sensitive Mutants of Influenza Virus. 3. Further Characterization of the ts–1 Influenza A Recombinant (H3N2) Virus in Man . J. Infect. Dis. , 128 : 479 – 487 .
- Nicas , M. , Nazaroff , W. W. and Hubbard , A. 2005 . Toward Understanding the Risk of Secondary Airborne Infection: Emission of Respirable Pathogens . J. Occup. Environ. Hyg. , 2 : 143 – 154 .
- Nicas , M. and Sun , G. 2006 . An Integrated Model of Infection Risk in a Health-Care Environment . Risk Anal. , 26 ( 4 ) : 1085 – 1096 .
- Olsen , S. J. , Chang , H. L. , Cheung , T. Y. Y. , Tang , A. F. Y. , Fisk , T. L. , Ooi , S. P. L. , Kuo , H. W. , Jiang , D. D. S. , Chen , K. T. , Lando , J. , Hsu , K. H. , Chen , T. J. and Dowell , S. F. 2003 . Transmission of Severe Acute Respiratory Syndrome on Aircraft . N. Engl. J. Med. , 349 ( 25 ) : 2416 – 2422 .
- Papineni , R. S. and Rosenthal , F. S. 1997 . The Size Distribution of Droplets in the Exhaled Breath of Healthy Human Subjects . J. Aerosol Med. , 10 : 105 – 161 .
- Roache , P. J. 1998 . Verification of Codes and Calculations . AIAA J. , 36 ( 5 ) : 696 – 702 .
- Rusin , P. , Maxwell , S. and Gerba , C. 2002 . Comparative Surface-to-Hand and Fingertip-to-Mouth Transfer Efficiency of Gram-Positive Bacteria, Gram-Negative Bacteria, and Phage . J. Applied Microbiol. , 93 : 585 – 592 .
- Rydock , J. P. 2004 . Tracer Study of Proximity and Recirculation Effects on Exposure Risk in an Airliner Cabin . Aviat. Space Environ. Med. , 75 ( 2 ) : 168 – 171 .
- Satter , S. A. , Springthorpe , S. , Mani , S. , Gallant , M. , Nair , R. C. , Scott , E. and Kain , J. 2001 . Transfer of Bacteria from Fabrics to Hands and Other Fabrics: Development and Application of a Quantitative Method Using Staphylococcus Aureus as a Model . J. Applied Microbiol. , 90 : 962 – 970 .
- Schaffer , F. L. , Soergel , M. E. and Straube , D. C. 1976 . Survival of Airborne Influenza Virus: Effects of Propagating Host, Relative Humidity, and Composition of Spray Fluids . Arch. Virol. , 51 ( 4 ) : 263 – 273 .
- Sehulster , L. M. , Chinn , R. Y. W. , Arduino , M. J. , Carpenter , J. , Donlan , R. , Ashford , D. , Besser , R. , Fields , B. , McNeil , M. M. , Whitney , C. , Wong , S. , Juranek , D. and Cleveland , J. 2004 . “ Guidelines for Environmental Infection Control in Health-Care Facilities. Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) ” . Chicago, IL : American Society for Healthcare Engineering/American Hospital Association .
- Sze To , G. N. , Wan , M. P. , Chao , C. Y. H. , Wei , F. , Yu , S. C. T. and Kwan , J. K. C. 2008a . A Methodology for Estimating Airborne Virus Exposures in Indoor Environments Using the Spatial Distribution of Expiratory Aerosols and Virus Viability Characteristics . Indoor Air , 18 : 425 – 438 .
- Sze To , G. N. , Wan , M. P. , Chao , C. Y. H. , Fang , L. and Melikov , A. 2008b . Experimental Study of Dispersion and Deposition of Expiratory Aerosols in Aircraft Cabins and Impact on Infectious Disease Transmission . Aerosol Sci. Technol. , Submitted.
- Tellier , R. 2006 . Review of Aerosol Transmission of Influenza A Virus . Emerg. Infect. Dis. , 12 ( 11 ) : 1657 – 1662 .
- Wan , M. P. , Chao , C. Y. H , Ng , Y. D. , Sze To , G. N. and Yu , W. C. 2007 . Dispersion of Expiratory Aerosols in a General Hospital Ward with Ceiling Mixing Type Mechanical Ventilation System . Aerosol Sci. Technol. , 41 : 244 – 258 .
- Walther , B. A. and Ewald , P. W. 2004 . Pathogen Survival in the External Environment and the Evolution of Virulence . Biol. Rev. , 79 ( 4 ) : 849 – 869 .
- Wang , A. , Zhang , Y. , Sun , Y. and Wang , X. 2008 . Experimental Study of Ventilation Effectiveness and Air Velocity Distribution in an Aircraft Cabin Mockup . Build. Environ. , 43 : 337 – 343 .
- Wang , Y. and James , P. W. 1999 . On the Effect of Anisotropy on the Turbulent Dispersion and Disposition of Small Particles. . Int. J. Multiphase Flow , 25 : 551 – 558 .
- Yan , W. , Zhang , Y. , Sun , Y. and Li , D. 2008 . Experimental and CFD Study of Unsteady Airborne Pollutant Transport within an Aircraft Cabin Mockup . Build. Environ. , doi:10.1016/j.buildenv.2008.01.010.
- Zhang , T. and Chen , Q. 2007a . Novel Air Distribution Systems for Commercial Aircraft Cabins . Build. Environ. , 42 : 1675 – 1684 .
- Zhang , T. and Chen , Q. 2007b . Comparison of the Eulerian and Lagrangian Methods for Predicting Particle Transport in Enclosed Spaces . Atmos. Environ. , 41 : 5236 – 5248 .
- Zhu , S. , Kato , S. and Yang , J. H. 2006 . Study on Transport Characteristics of Saliva Droplets Produced by Coughing in a Calm Indoor Environment . Build. Environ. , 41 : 1691 – 1702 .