Abstract
Hygroscopicity and phase transitions were measured with deposited particles of ammonium sulfate (AS), ammonium nitrate (AN), malonic acid (MA), glutaric acid (GA), glyoxylic acid (GlyA), as well as two mixed particle systems AS-MA and AS-GA using micro-Raman spectroscopy. Hygroscopicity was presented in terms of water-to-solute mass ratios, which were obtained from the integrated area ratios of the Raman water band to a distinct solute peak. Deliquescence and crystallization were confirmed by abrupt changes in the Raman peak positions and the full-width-half-heights of distinct solute peaks. The results for AS, AN, MA, and GA agreed well with literature reports and model predictions. For GlyA, we detected the Raman water band at near 0% RH, indicating that the spectral technique is sensitive for hygroscopic measurements at very low RH. Additional spectral feature at ∼ 0% RH was also observed. In the case of more complicated AS-dicarboxylic acid mixed systems, the partial phase transitions of the organic components were identified using the intensity ratios of aqueous to solid C = O peaks. AS-MA particles did not completely crystallize and gradual water uptake with increasing RH from 3% was observed. Moreover, it was found that AS-GA particles showed step-wise crystallization in which the AS fraction crystallized prior to the GA fraction. The measured water content and complete DRH of both mixed systems were consistent with the published values. The results show the utility of micro-Raman spectroscopic analysis in studying hygroscopicity and phase characterizations of the chemical species in mixed particles.
1. INTRODUCTION
The role of atmospheric particles in global climate change has been recognized due to their effects on radiative forcing (CitationCharlson et al. 1992; CitationHaywood and Boucher 2000; CitationPenner et al. 2001; CitationKaufman et al. 2002; CitationForster et al. 2007). The forcing mechanisms always depend on the physical properties of the particles, such as their hygroscopic properties and phase states. As humidity cycles, hygroscopic particles can interact with water vapor and change in size, concentration and even chemical composition, all of which can influence light absorption and scattering (CitationHorvath 1993; CitationNemesure et al. 1995; CitationPilinis et al. 1995), cloud condensation nuclei (CCN) activity (CitationSvenningsson et al. 1997; CitationKreidenweis et al. 2005) as well as chemical reactivity (CitationHerrmann 2003; CitationSeinfeld and Pandis 2006). The transitions between the solid and aqueous phases at certain points (or in certain ranges) of relative humidity (RH) are described by the deliquescence relative humidity (DRH) where dry particles absorb water and dissolve and the crystallization relative humidity (CRH) at which solution droplets crystallize. Such transitions are important in determining optical properties, CCN activity, and chemical reactivity (CitationMartin 2000). In depth investigation of the phase behaviors and hygroscopicity of atmospheric aerosols is therefore necessary to elucidate their impacts on global climate and tropospheric chemistry.
Various techniques have been applied to studying the hygroscopicity and phase transitions of levitated particles. The electrodynamic balance (EDB) (CitationRichardson and Spann 1984; CitationCohen et al. 1987; CitationTang et al. 1995) and humidified tandem differential mobility analyzer (HTDMA) (CitationMcMurry and Stolzenburg 1989; CitationSvenningsson et al. 1997; CitationChan and Chan 2005; CitationSwietlicki et al. 2008) have been widely used to study the hygroscopic growth of suspended particles. However, these techniques give no direct information of the phase state of the particles' components. In-situ spectroscopic analysis is well known to be useful for phase characterization of chemical species. An aerosol flow tube coupled with a Fourier transform infrared (FTIR) spectrometer has been used to study phase transitions of suspended particles (CitationCziczo et al. 1997; CitationOnasch et al. 1999). In addition, a combined EDB/Raman spectrometer (CitationFung and Tang 1988) has been demonstrated to provide solid spectral evidence of the phase state of a particle simultaneously with hygroscopicity measurements (CitationZhang and Chan 2002; CitationJordanov and Zellner 2006; CitationLee et al. 2008; CitationLing and Chan 2008). More recently, micro-Raman spectroscopic systems have been employed to investigate the molecular interactions in supersaturated droplets of different inorganic species (CitationWang et al. 2005; CitationLi et al. 2006; CitationDong et al. 2007) and the polymorphic transformation of NH4NO3 particles deposited on a hydrophobic substrate (CitationWu and Chan 2008). Such micro-Raman systems have also been used to study hygroscopicity and phase transitions of Ca(NO3)2 and Ca(NO3)2/CaCO3 (CitationLiu et al. 2008a), and ammonium sulfate–adipic acid mixtures (CitationYeung et al. 2009). Moreover, spatial spectroscopic imagining has proved useful for the phase and chemical characterization of heterogeneous particles (CitationLiu et al. 2008a; CitationYeung et al. 2009). A similar technique that utilizes micro-FTIR to study the hygroscopic behavior of pure inorganic and sea-salt particles has also been reported (CitationLiu et al. 2008b). Compared to EDB and TDMA, systems for the microscopic spectral analysis of deposited particles are rather simple, making this a potentially useful approach for studying the phase transitions and hygroscopic properties of atmospheric aerosols.
Organic components are the major constituent of atmospheric aerosols (CitationNovakov and Penner 1993; CitationRogge et al. 1993; CitationSaxena and Hildemann 1996) but they are often mixed with inorganic species (CitationSaxena et al. 1995; CitationZappoli et al. 1999). Micro-Raman spectroscopic systems have previously been applied to investigate hygroscopicity and phase transitions of inorganic droplets, but they have not previously been used for studying organic or mixed inorganic–organic droplets. To evaluate the capability of the micro-Raman technique for studying particles involving inorganic and organic mixtures, we examined the phase and hygroscopic properties of droplets of pure ammonium sulfate (AS) and ammonium nitrate (AN), the pure organics malonic acid (MA), glutaric acid (GA), and glyoxylic acid (GlyA), and two inorganic–organic mixtures AS-MA and AS-GA. Changes in peak area, peak position and full-width-at-half-height (fwhh) were quantified to determine hygroscopicity in terms of water-to-solute ratios, the phase states of the pure and mixed particles, and the transition relative humidities. The results for pure particles of the inorganic salts and dicarboxylic acids agreed well with literature reports and model predictions. Furthermore, organic compounds are known to frequently exhibit non-deliquescent behaviours upon evaporation of water. By size and mass measurements, it is not easy to confirm the existence of water at low RH or not. In this study, we confirmed the existence of supersaturated GlyA droplets at near 0% RH based on the detection of the Raman water band. Additional spectral feature of GlyA at ∼ 0% RH was also observed. The partial phase transitions and water contents of the AS-MA and AS-GA systems were determined under both aqueous and mixed phase conditions using in-situ Raman analyses. For the organic components in the mixtures, the intensity ratio of the C = O double-peak at about 1700 cm− 1 was used as a spectral indicator for the partial phase transitions. Agreement between the results and published data collected using other techniques, and also agreement with modelling predictions, give us confidence in using micro-Raman spectroscopy for studying more complicated mixed systems involving inorganics and organics.
2. EXPERIMENTAL
2.1. Air-Flow Observation Cell and Sample Preparation
An air-flow observation cell (CitationYeung et al. 2009) was used to maintain stable humidity conditions for examining the particles. The cell was made of stainless steel with a quartz window in the top to allow micro-Raman observations. A smooth fluorocarbon film (1.0 mil, available from YSI Inc.) was used as the hydrophobic substrate for the deposited particles. The substrate was supported by a glass plate in the cell. The environment inside the cell was monitored by a humidity sensor (HIH-3610 Series, Honeywell) and a temperature sensor (LM35CZ, RS Components) accurate to ± 2% RH and ± 1°C, respectively.
The RH inside the cell was controlled by a filtered airflow feed. The RH of the airflow was adjusted by mixing dry air with an air stream humidified with a water bubbler. Pure AS particles reach equilibrium within 15 min (CitationYeung et al. 2009). To ensure that equilibrium was reached in these experiments, a minimum of an hour was allowed. Typically, the experiments were performed at ambient temperatures of 24 ± 2°C. Another observation cell with a heat plate (TCM-Series, Electron dynamics), which can control the temperature of the substrate from 10 to 100°C, was used for high temperature measurements of GlyA particles.
To prepare the aqueous solutions, pure AS, AN, MA, GA, and GlyA were dissolved in 18.0 MΩ purified water. The AS-MA and AS-GA mixed solutions were prepared at a 1:1 mole ratio. All the compounds were purchased from Sigma-Aldrich and had purity better than 99.0%. A pre-washed spray bottle was used to deposit droplets directly on the hydrophobic substrate in the observation cell. Droplets with diameters less than 40 μ m were chosen by an optical microscope for investigation. The diameters of the dry particles obtained from evaporation of droplets ranged from 10 to 30 μ m.
2.2. Raman Spectral Measurements
Raman spectra were acquired under different RH conditions using a Renishaw RM series micro-Raman spectrometer. This was the same Raman system used by CitationWu and Chan (2008). A 20 mW Argon ion laser (514.5 nm) was applied for sample excitation. Single particles were located with an optical microscope (Leica DMLM) with a reflected light illumination attached to the Raman system. The laser beam was focused on the center of a particle through a 50× objective lens, which provided spatial resolution of about 6 μ m. An 1800 g mm− 1 grating was used to form the spectra in the range from 200 to 4000 cm− 1 with a resolution of 1.4 cm− 1.
For all the pure compounds and mixtures, duplicate particles were monitored through a full humidity cycle. The humidity cycle started from evaporation at high RH (> 80%) to the dry state (near 0% RH). The particles were then allowed to grow in gradually increasing RH. Additional evaporation experiments were performed to obtain more hygroscopic data.
2.3. Hygroscopicity Determination Based on Raman Spectra
The integrated area of certain Raman peaks was used to quantify the hygroscopic properties of the particles. CitationYeung et al. (2009) has described a similar analysis. A big envelope of overlapping Raman peaks containing two O-H bands (3240 and 3430 cm− 1), three NH4 + bands (2880, 3080, and 3220 cm− 1, for ammonium-containing particles) and two C-H peaks (near 3000 cm− 1, for organic particles) was recorded between 2700 and 3800 cm− 1. Gaussian peak fitting was used to extract individual water peaks at 3430 cm− 1. To compare the water peaks measured at different RHs with different particles, normalization was performed by dividing the water peak area by the area of a distinct solute peak. The sulfate stretching peak (980 cm− 1) was chosen for all AS-containing particles, while the nitrate (1048 cm− 1) and C = O (∼ 1700 cm− 1) peaks were used for AN and organic acid particles, respectively.
The peak area ratios, A(water)/A(solute) were converted to water-to-solute mass ratios (WSR). The conversion was based on the assumption that the peak area was directly proportional to the species concentration. WSR values in the aqueous phase (> 80% RH) were predicted using the Extended-Aerosol Inorganic Model (E-AIM). The principle of the model to calculating solute and solvent activities in aqueous solution was described by CitationClegg et al. (1992). Inorganic Model II (available at http://www.aim.env.uea.ac.uk/aim/aim.php) was used to calculate the water activities of AN and AS (CitationClegg et al. 1998) and the UNIFAC model was used for the organic compounds. For the AS-organic mixed systems, the water activities were estimated using the method described by CitationClegg et al. (2001). The predicted WSR values were used as reference points to calculate the WSR at other RH values. In the growth experiments, no water signal was detected with the solid particles until they dissolved. The WSR was therefore set to zero for solid particles.
3. RESULTS AND DISCUSSION
3.1. Hygroscopicity
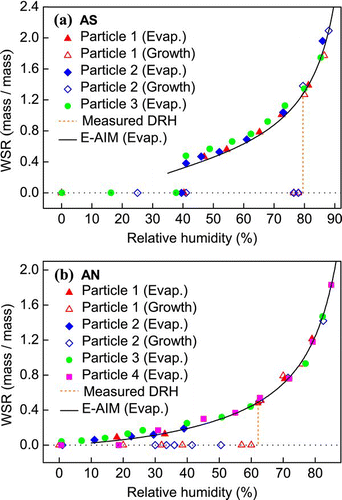
AS and AN have been recognized as the predominant inorganic constituents in atmospheric aerosols. Their hygroscopic properties have been modelled and are well understood. So their hygroscopicity as determined through the Raman spectral analyses was first compared with the modelling results to validate the Raman method for quantitative hygroscopic measurements. and illustrate the changes in the water content of pure AS and AN particles, respectively. The WSRs of aqueous AS and AN calculated from the E-AIM model are also included in the figure for comparison. The water contents of both pure inorganic particles determined by Raman spectroscopy agreed well with those predicted by the model. Aqueous AS particles became more saturated with decreasing RH and finally lost all water below 40% RH. In contrast, AN either formed dry particles below 30% RH or persisted as supersaturated droplets even at ∼ 0% RH.
FIG. 1 Hygroscopicity (WSR, mass of water/mass of solute) of (a) AS and (b) AN particles as a function of RH obtained by micro-Raman spectroscopy. Predictions from the E-AIM model are also included. (Figure provided in color online.)
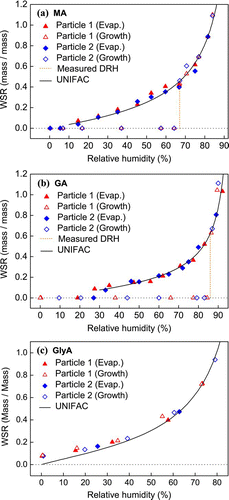
The hygroscopic results for the organics (MA, GA, and GlyA) are shown in . The calculated WSRs were compared with the predictions of the UNIFAC model using Peng's modified interaction parameters (CitationPeng et al. 2001). The hygroscopic properties of pure MA agreed well with the modified UNIFAC predictions. The continuous curve down to about 10% RH was generally consistent with Peng's observation that water was retained by MA particles at decreasing RH, but no water was detected at RH less than 15% (CitationPeng et al. 2001). The GA results were also in excellent agreement with modelling predictions. With decreasing RH, water in the GA particles gradually evaporated until the RH reached about 30%, when the WSR dropped to zero, indicating crystallization. The hygroscopicity of GlyA was recently studied by CitationChan et al. (2008), who found that GlyA was non-deliquescent and followed the trend of UNIFAC predictions. In this study, the results also indicates the presence of water at RH down to 0%, but the water contents were slightly higher than the predicted values at low RHs. For the most part the observations were generally in line with those of CitationChan et al. (2008), but in their measurements the mass ratios (m/m o ) in response to the changing RH were reported and the particle mass at the lowest RH (6%) was used as the reference mass (m o ). The mass ratio was unity at that RH and it did not necessarily represent the anhydrous state.
FIG. 2 Hygroscopicity (WSR, mass of water/mass of solute) of (a) MA, (b) GA, and (c) GlyA particles as a function of RH obtained by micro-Raman spectroscopy. Predictions from the UNIFAC model are also included. (Figure provided in color online.)
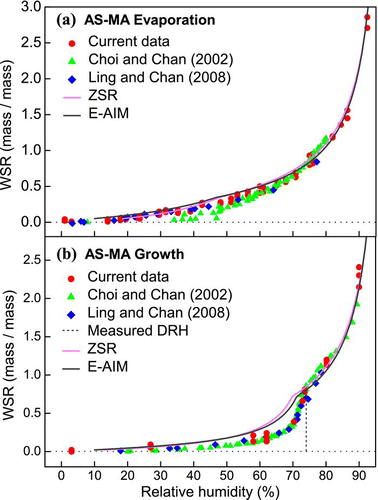
Variation in the water content of the AS-MA particles in the course of a humidity cycle is shown in along with data from previous measurements using the EDB techniques (CitationChoi and Chan 2002; CitationLing and Chan 2008). The AS-MA particles in this study are of the same composition (1:1 mole ratio) with those studied in the literature. The spectral results were consistent with the hygroscopic properties described by CitationLing and Chan (2008). However, a difference between these measurements and those of CitationChoi and Chan (2002) was seen in the evaporation measurements. In that study, partial crystallization occurred at about 46% RH, as indicated by the small decrease in particle mass, and the mixture became anhydrous at RH below 20%. In the present study, water signals were detected even at 3% RH, indicating incomplete crystallization. In the growth experiments, gradual water uptake with increasing RH from 3% was observed. This also indicates that the AS-MA particles did not completely crystallize. Moreover, a notable increase in the water content at about 74% RH indicates complete deliquescence. The black curves in present the E-AIM model's predictions (the solute activity was based on UNIFAC calculations). Assuming that the system partially crystallized, with only AS existing as a solid, water uptake would gradually start from the lowest RH of 10% (the lowest operating RH of the model). The growth curve overlapped the evaporation curve at 72% RH, the predicted DRH, which was close to the 74% DRH measured in the experiments. When compared with the predictions, all the laboratory studies measured lower WSR values under conditions below 70% RH. The discrepancy reveals over-prediction by the model of water uptake in supersaturated environments (CitationLing and Chan 2008). The water content of AS-MA system was also estimated based on the Zdanovskii-Stokes-Robinson (ZSR) relationship (CitationStokes and Robinson 1966; CitationClegg and Seinfeld 2004). The ZSR estimations are included in the figure for comparison with the E-AIM model. The E-AIM predictions were very close to the ZSR estimations for the AS-MA system.
FIG. 3 Hygroscopicity (WSR, mass of water/mass of solute) of AS-MA mixed particles (in 1:1 mole ratio) undergoing (a) evaporation and (b) growth processes obtained by micro-Raman spectroscopy. Data from CitationChoi and Chan (2002) and CitationLing and Chan (2008) and predictions from the ZSR and E-AIM models are also included. (Figure provided in color online.)
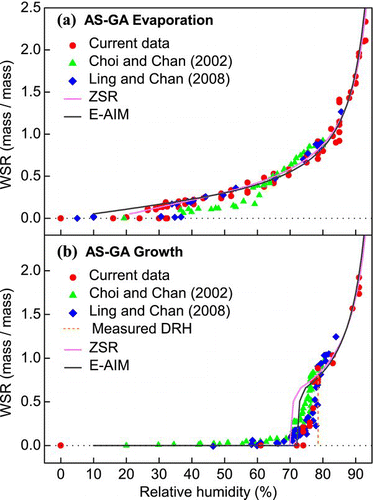
The hygroscopicity of the AS-GA system (1:1 mole ratio) is illustrated in . The responses of the AS-GA system to water again showed trends very similar to those measured by CitationLing and Chan (2008). During evaporation, the AS-GA particles gradually lost water until, at an RH of 30% RH or less, the anhydrous state was reached. The CRH of the AS-GA system, as inferred from the zero WSR, ranged from 16 to 32.5% RH. CitationChoi and Chan (2002) reported a clear mass reduction at about 60% RH, meaning that partial crystallization took place. In their study, early crystallization at significantly higher RH relative to other studies was observed with both AS-MA and AS-GA. This might have been due to heterogeneous nucleation initiated by trace amounts of contaminants. In the growth experiments, significant water uptake occurred at 78.5% RH, as indicated in . Hence, the DRH of the system was slightly lowered relative to the pure components. The decreased DRH has also been confirmed by studies which observed values ranging from 77 to 78.5% RH (CitationPrenni et al. 2003; CitationPant et al. 2004; CitationZardini et al. 2008). Moreover, water absorption started at about 70% RH. The pre-deliquescence water uptake of the AS-GA system has previously been observed in EDB measurements (CitationChoi and Chan 2002; CitationLing and Chan 2008). Early water uptake was also predicted by the E-AIM and the ZSR methods. However, both methods again overpredicted the water uptake during partial deliquescence. The DRH estimated by ZSR method is about 70% RH, which is even lower than that predicted by the E-AIM model (74.5% RH). CitationLing and Chan (2008) suggested that the higher water content was due to the over-prediction of the extent of dissolution of the organic acids during partial deliquescence.
FIG. 4 Hygroscopicity (WSR, mass of water/mass of solute) of AS-GA mixed particles (in 1:1 mole ratio) undergoing (a) evaporation and (b) growth processes obtained by micro-Raman spectroscopy. Data from CitationChoi and Chan (2002) and CitationLing and Chan (2008) and predictions from the ZSR and E-AIM models are also included. (Figure provided in color online.)
3.2. Phase Transitions of Single-Component Systems
Phase transitions involve the interruption of molecular interactions, leading to Raman spectral variations such as abrupt changes in the solute peak's position and shape. These can provide further evidence of phase transitions.
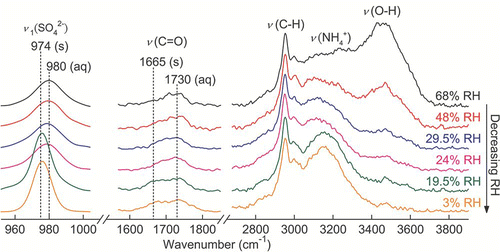
The Raman spectra of a single AS and AN droplets undergoing evaporation with the occurrence of crystallization are presented in and . From the Raman spectra, the phase transitions of particles can be directly recognized from particular spectral changes. The presence of water bands can distinguish between aqueous and dry states. Changes in the Raman shift magnitude during phase transitions of AS and AN have been reported by a few investigators (CitationMusick et al. 2000; CitationZhang and Chan 2002; CitationJordanov and Zellner 2006). The Raman peaks of solid particles are more intense and sharper relative to aqueous particles, so a sudden change in the fwhh (normalized to that at the lowest RH) of a distinct peak is also useful for phase identification (CitationLee et al. 2008; CitationLing and Chan 2008). In this study, the changes in the fwhh and the positions of the ν 1 (SO4 2 −) and ν 1 (NO3 −) peaks were observed through a humidity cycle. The results are depicted in and . During evaporation, the sudden drops in the fwhh at 39.5% RH (AS) and between 0 and 10% RH (AN) indicate crystallization. Clear steps in the red shift from 980 to 974 cm− 1 for AS and 1048 to 1043 cm− 1 for AN were also observed, consistent with literature reports. Conversely, deliquescence of dry particles was confirmed by an abrupt increase in fwhh due to peak broadening and blue shifting of the ν 1(SO4 2 −) and ν 1(NO3 −) peaks. According to the parallel trend of peak position and fwhh, the DRHs measured for AS and AN particles were 79.5% and 63%, respectively.
FIG. 5 Raman spectra of a single (a) AS and (b) AN particles upon evaporation. (Figure provided in color online.)
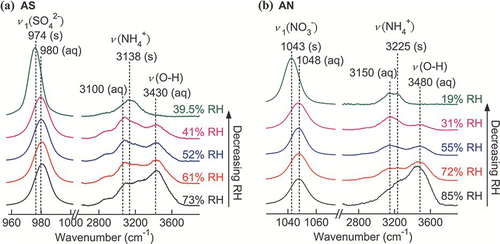
FIG. 6 Changes in normalized fwhh and Raman shift of the (a) sulfate peak (980 cm− 1) of a single AS particle and (b) nitrate peak (1048 cm− 1) of a single AN particle through a humidity cycle, and (c) changes in the Raman shift of the nitrate peak (1048 cm− 1) of three individual AN particles upon crystallization. (Figure provided in color online.)
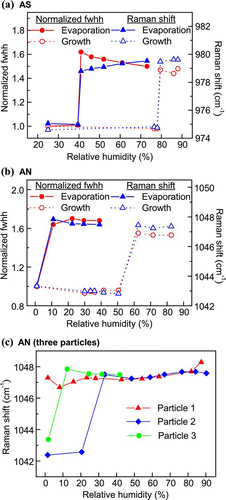
The crystallization properties of AN particles have been reported in several studies, and relatively large deviations have been reported. CitationTang (1996) and CitationChan et al. (1992) both observed solid formation near 30%. However, CitationDougle et al. (1998) and CitationCziczo and Abbatt (2000) did not detect crystallization in submicron AN particles below 10% RH. Furthermore, CitationLightstone et al. (2000) detected a wide range of CRH from 0 to 30% RH for the AN microparticles generated from aqueous solution. presents the results of three evaporation experiments. These experiments also produced evidence of supersaturated particles even at 0% RH and of crystallization between 0% and 30% RH. CitationLightstone et al. (2000) suggested the large deviations were due to the crystallization of larger AN particles promoted by the presence of impurities via heterogeneous nucleation. CitationSchlenker et al. (2004) also suggested heterogeneous nucleation as the primary crystallization pathway of aqueous AN particles. They observed the noncrystallite AN particles at 1% RH. However, formation of AN solids from the initially aqueous particles via heterogeneous nucleation in the presence of sulfate crystals was evident.
and illustrate the Raman spectra of MA and GA droplets undergoing evaporation. Changes in the fwhh and in the position of the ν (C = O) of MA and GA are shown in and . A sharp shift in the peak position and a decrease in fwhh indicate that crystallization of MA and GA particles occurred at RHs below 10% and 30%, respectively. Although CitationPeng et al. (2001) reported that MA particles did not crystallize at low RH of ∼ 5%, such crystallization events were observed by CitationBraban et al. (2003) at 6% RH. During the growth process, the abrupt increase in both the ν(C = O) peak's position and fwhh at 67% RH indicates deliquescence, consistent with Peng's bulk measurement of 65.3% RH and Braban's determination of 69% RH (CitationPeng et al. 2001; CitationBraban et al. 2003). For GA, the CRH has been reported as 29–33% (CitationPeng et al. 2001) and 22.5–36% (CitationPant et al. 2004). Deliquescence has been observed between 83 and 90% RH (CitationZardini et al. 2008 and the references therein). The results of this study with GA agreed with those literature reports.
FIG. 7 Raman spectra of a single (a) MA and (b) GA particles upon evaporation. (Figure provided in color online.)
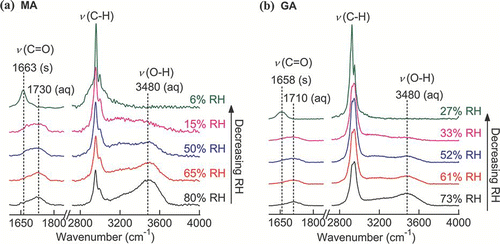
FIG. 8 Changes in normalized fwhh and Raman shift of the C = O peak of single (a) MA and (b) GA particles through a humidity cycle, and (c) a GlyA particle upon evaporation. (Figure provided in color online.)
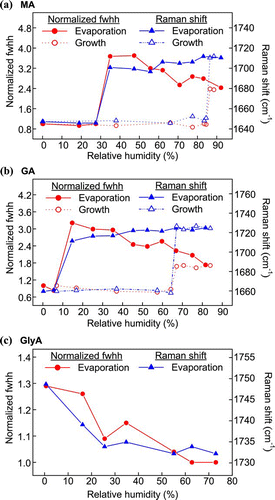
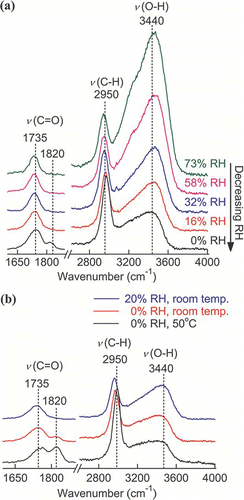
No phase transition was found for GlyA, as there was no abrupt change in the position or fwhh of the ν(C = O) peak (see ). However, additional spectral feature of GlyA particles were observed at 0% RH. The Raman spectra of a GlyA particle undergoing evaporation are presented in . A new small peak at 1820 cm− 1 appeared to be a new C = O peak together with the aqueous C = O peak at 1735 cm− 1. Due to its polar nature, GlyA dissolves in water. If the water content is greatly reduced, GlyA will form metastable solution droplets, and interactions between solutes will be enhanced. In this study, the measured WSR of GlyA near 0% RH was 0.1, at which point the GlyA molecules would be 40 times more abundant than the water molecules. Additional measurements of GlyA particles were made at 0% RH and at a temperature of 50°C to see if GlyA could retain water under dry and hot conditions. The Raman signal from the water was still detected, as seen in , albeit at a reduced intensity. At the same time, the C = O stretching at 1820 cm− 1 intensified as the temperature increased. A possible reason for the C = O stretching at the higher shift position (> 1800 cm− 1) is the presence of some anhydride (CitationSocrates 2001). However, the data were not sufficient to confirm the formation of anhydride.
3.3. Partial Phase Transitions of AS-Organic Mixed Systems
We have observed the gradual change of water content in AS-MA and AS-GA mixed systems, which suggests the presence of mixed-phase particles under certain RH conditions. However, WSR ratio measurements cannot provide direct information on the phase state of each species. This must be obtained through additional spectroscopic analysis.
includes plots of the changing fwhh of the ν 1(SO4 2 −) peaks of two AS-MA particles, showing the two different behaviors of the AS fraction. For particle 1, crystallization was evident in the sharp decrease in the fwhh at 19.5% RH, consistent with the work of CitationLing and Chan (2008) in which partial crystallization for the AS-MA system was observed between 16 and 20% RH. However, no complete crystallization was observed in particle 2 down to 1% RH. The observations generally agreed with the results by CitationBraban et al. (2004) and CitationParsons et al. (2004) who detected no crystallization of submicron particles and observed CRHs between 0 and 20% RH. It is clear that MA could significantly suppress or even inhibit the crystallization of AS in this system. In the growth measurements, obvious increases in the fwhh at 70% RH imply that the crystalline AS fraction absorbed water below the DRH of pure AS. The further increase in fwhh at 74% suggests that the AS fraction completely deliquesced at this RH. The complete DRH found in this study also agreed well with the 73.4% RH reported by CitationParsons et al. (2004).
FIG. 10 Changes in (a) the normalized fwhh of the sulfate peak (980 cm− 1) and (b) the intensity ratio of the C = O double-peak (I1730/I1665) of AS-MA mixed particles through a humidity cycle. (Figure provided in color online.)
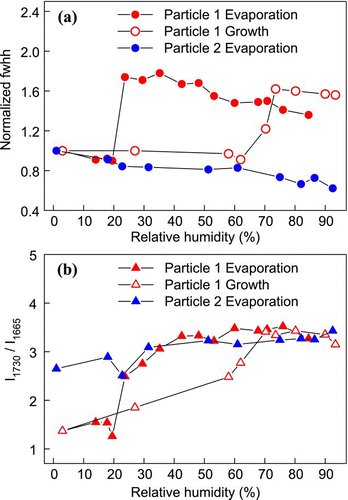
There was no distinct change in either the shifting or fwhh of the ν(C = O) peak (figures not shown) to indicate any phase transition in the MA fraction. However, a gradual development of a shoulder was noticeable at 1665 cm− 1 which gave a ν(C = O) double-peak with decreasing RH (see ). The 1665 cm− 1 shoulder, which was also observed by CitationLing and Chan (2008), arises from crystalline MA. Since the features of the peak at 1730 cm− 1 were affected by the shoulder, the peak position and fwhh of the ν(C = O) peak might not be accurate for phase characterization. Instead, the intensity ratio of the double-peak I1730/I1665 was used to identify the phase state of MA in the system. The intensity ratios at different RHs are shown in . The ratio suddenly dropped at 19.5% RH (Particle 1) due to the significant contribution of the 1680 cm− 1 peak. This large change serves as a good indication of the partial crystallization of the MA at about 20% RH, and it is probably induced by the AS crystals. CitationLing and Chan (2008) also postulated from the presence of the shoulder peak that MA was partially crystallized. Further reduction of RH to 3% did not cause significant decrease in the ratio, meaning that aqueous and solid MA coexisted at RHs below 19.5%. As the droplet grew, the ratio gradually increased from 3% to 70% RH, which may be explained by water uptake due to the presence of aqueous MA. The ratio reached its maximum when the RH increased to 70%, indicating that the MA fraction completely dissolved below the DRH at 74%. On the other hand, the MA fraction in particle 2 did not crystallize, as indicated by the absence of any abrupt change in the ratio. The MA fraction remained as a supersaturated solution, presumably because no AS crystal was present to serve as a heterogeneous nucleus.
FIG. 11 Raman spectra of a single AS-MA mixed particle upon evaporation. (Figure provided in color online.)
The results with the AS-GA system are shown in . Again, the phase of the AS fraction in AS-GA mixture was inferred from the changing fwhh of the ν 1(SO4 2 −) peak shown in . AS crystallization took place at 32.5–36% RH, which was confirmed by the abrupt decrease in fwhh. This observed RH value was higher than the complete CRH of the system (16 to 32.5%). This implies that partially crystallized metastable AS-GA particles were present. During the growth experiments, partial dissolution of AS starting at about 70% RH was confirmed by the gradually increasing fwhh, followed by the sharply increased fwhh at 78.5% RH that confirmed complete deliquescence, consistent with the DRH inferred from the hygroscopic observations.
FIG. 12 Changes in (a) the normalized fwhh of the sulfate peak (980 cm− 1) and (b) the intensity ratio of the C = O double-peak (I1708/I1658) of AS-GA through a humidity cycle. (Figure provided in color online.)
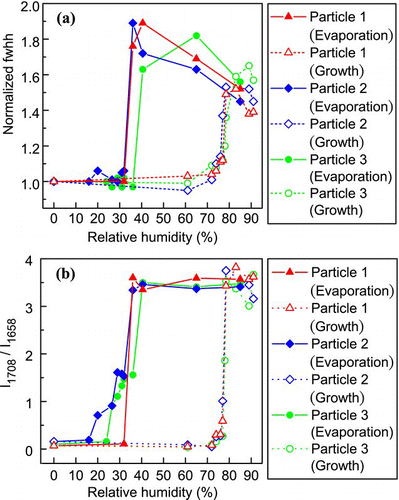
As with the AS-MA system, the intensity ratio of the C = O stretching peaks was used to correlate the phase properties of GA in the AS-GA system. Two peaks were observed at 1708 cm− 1 and 1658 cm− 1, corresponding to the aqueous phase and the solid form of GA, respectively. The variation in the intensity ratio I1705/I1658 at different RHs is illustrated in . For Particle 1, a “single-step” complete crystallization was confirmed at 32.5% RH by the sudden drops in both the fwhh of the ν 1(SO4 2 −) peak and the intensity ratio of the C = O stretching peaks. The results were consistent with the observations of CitationLing and Chan (2008) and CitationZardini et al. (2008), who reported the single-step crystallization of AS-GA between 31 and 36% RH. On the other hand, we observed gradual reductions in the intensity ratio in Particles 2 and 3. When the AS fractions first crystallized at 32.5 and 36% RH, respectively, the C = O ratio dropped from 3.5 to 1.5. The ratio further gradually decreased and reached near zero upon complete crystallization at 16 and 24% RH. shows the Raman spectra of the gradual crystallization of Particle 3. It is apparent that AS has crystallized at 36% RH while GA remained in the aqueous state until it crystallized at 24% RH. The gradual reduction of the intensity ratio suggests that GA partially formed along with the crystalline AS prior to complete crystallization. The CRH of pure GA is generally lower than that of pure AS. This suggests that the AS fraction in the system might have crystallized first followed by that of the GA fraction, leading to partial crystallization. When the RH increased to about 70% in the growth measurement, the aqueous peak was detectable and the peak ratio increased slightly. A small and gradual increase in the ratio was observed roughly between 70% and 78% RH. More significant enhancement in the ratio was noted at 78.5% RH due to the full development of the aqueous peak and the disappearance of the solid peak. The gradual dissolution of GA below the DRH may explain the overpredicted results by the E-AIM model, in which GA was assumed to fully deliquesce at 72% RH.
4. CONCLUSIONS
The application of micro-Raman spectroscopy to studying the hygroscopicity and phase properties of particles incorporating inorganic species, organic species, and their mixtures has been demonstrated here. Excellent agreement between the hygroscopicity measured in this study and previously published results confirms the possibility of accurate quantitative measurements with Raman spectroscopy. Spectroscopic measurements also enable the characterization of particle phases. Changes in peak positions and fwhh serve as good indicators to identify phase transitions with both inorganic and organic solutes, especially with species that can retain water at very low RH. For example, malonic acid and glyoxylic acid remain as supersaturated droplets at low RH and exhibited continuous hygroscopicity. For mixed particles, it is not easy to infer the occurrence of the phase transition from the continuous curves of water content over the whole range of RH. Instead, spectral analysis can provide direct evidence of the phase states.
EDB and HTDMA techniques are widely used to investigate the hygroscopic properties and phase transitions of levitated particles. However, without also calling on spectroscopy, these techniques cannot give direct information about the phase state of the components in mixed particles. The conventional EDB technique can be combined with spontaneous Raman spectroscopy (CitationFung and Tang 1988). Such a coupled system is able to measure particle mass and spectra at the same time. On the other hand, micro-Raman spectroscopy is a stand-alone technique for characterization which facilitates in situ measurements of hygroscopicity and phase transition. Sampling and operation are relatively straightforward. Data generated by EDB are limited because only one particle can be examined at a time. In micro-Raman measurements, individual particles are firmly held on the substrate and can be easily located with a microscope, enabling multiple individual particles to be analyzed in a single measurement. Some limitations of the HTDMA technique can also be avoided using micro-Raman analysis. Size correction is essential when using HTDMA with non-spherical particles to obtain accurate measurements. Moreover, mass transfer delay effects can be an issue in HTDMA measurements in which only a short period of time is allowed for humidity equilibration (CitationChan and Chan 2005).
Micro-Raman spectroscopy is particularly valuable for elucidating gradual water uptake behavior and partial phase changes, which are usually expected to occur in multi-component systems. We successfully quantified the water content of mixed-phase AS-MA and AS-GA particles during the course of partial deliquescence. As with pure AS, the phase properties of the AS fractions in the mixtures were easily characterized by the changes in position and fwhh of the sulfate peak. For the organic acid components, the utility of the C = O stretching intensity ratio for identifying phase states during the partial crystallization and deliquescence has been amply demonstrated. Our measurements illustrate the complicated hygroscopic behavior and phase transitions in such mixed systems, which can be untangled by comprehensive spectral analyses. However, it should be noted that sample fluorescence is the major limitation of Raman spectroscopic studies. Relatively weak Raman signals will be overwhelmed by any broadband fluorescence background. Moreover, some species such as NaCl do not give Raman signals in solution for quantitative measurement of the water content, and external calibration is necessary. Finally, the size of particles examined by this technique is comparable with those of EDB but much larger than those of HTDMA and of ambient particles.
This work was funded by Earmarked Grant 600208 from the Hong Kong's Research Grants Council and HKUST internal grant HKUST 1/CRF-SF/08.
REFERENCES
- Braban , C. F. and Abbatt , J. P. D. 2004 . A Study of the Phase Transition Behavior of Internally Mixed Ammonium Sulfate-Malonic Acid Aerosols . Atmo. Chem. Phys. , 4 : 1451 – 1459 .
- Braban , C. F. , Carroll , M. F. , Styler , S. A. and Abbatt , J. P. D. 2003 . Phase Transitions of Malonic and Oxalic Acid Aerosols . J. Phy. Chem. A , 107 : 6594 – 6602 .
- Chan , C. K. , Flagan , R. C. and Seinfeld , J. H. 1992 . Water Activities of NH4NO3/(NH4)2SO4 Solutions . Atmo. Env. Part A , 26 : 1661 – 1673 .
- Chan , M. N. and Chan , C. K. 2005 . Mass Transfer Effects in Hygroscopic Measurements of Aerosol Particles . Atmo. Chem. Phys. , 5 : 2703 – 2712 .
- Chan , M. N. , Kreidenweis , S. M. and Chan , C. K. 2008 . Measurements of the Hygroscopic and Deliquescence Properties of Organic Compounds of Different Solubilities in Water and Their Relationship with Cloud Condensation Nuclei Activities . Environ. Sci. Technol. , 42 : 3602 – 3608 .
- Charlson , R. J. , Schwartz , S. E. , Hales , J. M. , Cess , R. D. , Coakley , J. A. , Hansen , J. E. and Hofmann , D. J. 1992 . Climate Forcing by Anthropogenic Aerosols . Science , 255 : 423 – 430 .
- Choi , M. Y. and Chan , C. K. 2002 . The Effects of Organic Species on the Hygroscopic Behaviors of Inorganic Aerosols . Environ. Sci. Technol. , 36 : 2422 – 2428 .
- Clegg , S. L. , Brimblecombe , P. and Wexler , A. S. 1998 . Thermodynamic Model of the System H+-NH4 +-SO4 2 −-NO3 −-H2O at Tropospheric Temperatures . J. Phy. Chem. A , 102 : 2137 – 2154 .
- Clegg , S. L. , Pitzer , K. S. and Brimblecombe , P. 1992 . Thermodynamics of Multicomponent, Miscible, Ionic Solutions. II. Mixtures Including Unsymmetrical Electrolytes . J. Phys. Chem. , 96 : 9470 – 9479 .
- Clegg , S. L. and Seinfeld , J. H. 2004 . Improvement of the Zdanovskii- Stokes-Robinson Model for Mixtures Containing Solutes of Different Charge Types . J. Phys. Chem. A , 108 : 1008 – 1017 .
- Clegg , S. L. , Seinfeld , J. H. and Brimblecombe , P. 2001 . Thermodynamic Modelling of Aqueous Aerosols Containing Electrolytes and Dissolved Organic Compounds . J. Aerosol Sci. , 32 : 713 – 738 .
- Cohen , M. D. , Flagan , R. C. and Seinfeld , J. H. 1987 . Studies of Concentrated Electrolyte Solutions Using the Electrodynamic Balance. 1. Water Activities for Single Electrolyte Solutions . J. Phy. Chem. , 91 : 4563 – 4574 .
- Cziczo , D. J. and Abbatt , J. P. D. 2000 . Infrared Observations of the Response of NaCl, MgCl2, NH4HSO4, and NH4NO3 Aerosols to Changes in Relative Humidity from 298 to 238 K . J. Phy. Chem. A , 104 : 2038 – 2047 .
- Cziczo , D. J. , Nowak , J. B. , Hu , J. H. and Abbatt , J. P. D. 1997 . Infrared Spectroscopy of Model Tropospheric Aerosols as a Function of Relative Humidity: Observation of Deliquescence and Crystallization . J. Geophys. Res. , 102 : 18843 – 18850 .
- Dong , J. L. , Li , X. H. , Zhao , L. J. , Xiao , H. S. , Wang , F. , Guo , X. and Zhang , Y. H. 2007 . Raman Observation of the Interactions between NH4 +, SO4 2 −, and H2O in Supersaturated (NH4)2SO4 droplets . J. Phy. Chem. B , 111 : 12170 – 12176 .
- Dougle , P. G. , Veefkind , J. P. and ten Brink , H. M. 1998 . Crystallisation of Mixtures of Ammonium Nitrate, Ammonium Sulphate and Soot . J. Aerosol Sci. , 29 : 375 – 386 .
- Forster , P. , Ramaswamy , V. , Artaxo , P. , Berntsen , T. B. R. , Fahey , D. W. , Haywood , J. , Lean , J. , Lowe , D. C. , Myhre , G. , Nganga , J. , Prinn , R. , Raga , G. , Schulz , M. and Van Dorland , R. 2007 . “ Changes in Atmospheric Constituents and in Radiative Forcing ” . In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Edited by: Solomon , S. , Qin , D. , Manning , M. , Chen , Z. , Marquis , M. , Averyt , K. B. , Tignor , M. and Miller , H. L. 129 – 234 . New York
- Fung , K. H. and Tang , I. N. 1988 . Raman Scattering from Single Solution Droplets . Appl. Opt. , 27 : 206 – 208 .
- Haywood , J. and Boucher , O. 2000 . Estimates of the Direct and Indirect Radiative Forcing due to Tropospheric Aerosols: A Review . Rev. Geophys. , 38 : 513 – 543 .
- Herrmann , H. 2003 . Kinetics of Aqueous Phase Reactions Relevant for Atmospheric Chemistry . Chem. Rev. , 103 : 4691 – 4716 .
- Horvath , H. 1993 . Atmospheric Light Absorption—A Review . Atmo. Env. Part A , 27 : 293 – 317 .
- Jordanov , N. and Zellner , R. 2006 . Investigations of the Hygroscopic Properties of Ammonium Sulfate and Mixed Ammonium Sulfate and Glutaric Acid Micro Droplets by Means of Optical Levitation and Raman Spectroscopy . Phys. Chem. Chem. Phys. , 8 : 2759 – 2764 .
- Kaufman , Y. J. , Tanre , D. and Boucher , O. 2002 . A Satellite View of Aerosols in the Climate System . Nature , 419 : 215 – 223 .
- Kreidenweis , S. M. , Koehler , K. , DeMott , P. J. , Prenni , A. J. , Carrico , C. and Ervens , B. 2005 . Water Activity and Activation Diameters from Hygroscopicity Data—Part I: Theory and Application to Inorganic Salts . Atmo. Chem. Phys. , 5 : 1357 – 1370 .
- Lee , A. K. Y. , Ling , T. Y. and Chan , C. K. 2008 . Understanding Hygroscopic Growth and Phase Transformation of Aerosols Using Single Particle Raman Spectroscopy in an Electrodynamic Balance . Faraday Discuss. , 137 : 245 – 263 .
- Li , X. H. , Wang , F. , Lu , P. D. , Dong , J. L. , Wang , L. Y. and Zhang , Y. H. 2006 . Confocal Raman Observation of the Efflorescence/Deliquescence Processes of Individual NaNO3 Particles on Qartz . J. Phy. Chem. B , 110 : 24993 – 24998 .
- Lightstone , J. M. , Onasch , T. B. , Imre , D. and Oatis , S. 2000 . Deliquescence, Efflorescence, and Water Activity in Ammonium Nitrate and Mixed Ammonium Nitrate/Succinic Acid Microparticles . J. Phy. Chem. A , 104 : 9337 – 9346 .
- Ling , T. Y. and Chan , C. K. 2008 . Partial Crystallization and Deliquescence of Particles Containing Ammonium Sulfate and Dicarboxylic Acids . J. Geophys. Res. , 113 : D14205 doi:10.1029/2008JD009779
- Liu , Y. J. , Zhu , T. , Zhao , D. F. and Zhang , Z. F. 2008a . Investigation of the Hygroscopic Properties of Ca(NO3)2 and Internally Mixed Ca(NO3)2/CaCO3 Particles by Micro-Raman Spectrometry . Atmo. Chem. Phys. , 8 : 7205 – 7215 .
- Liu , Y. , Yang , Z. , Desyaterik , Y. , Gassman , P. L. , Wang , H. and Laskin , A. 2008b . Hygroscopic Behavior of Substrate-Deposited Particles Studied by Micro-FT-IR Spectroscopy and Complementary Methods of Particle Analysis . Anal. Chem. , 80 : 633 – 642 .
- Martin , S. T. 2000 . Phase Transitions of Aqueous Atmospheric Particles . Chem. Rev. , 100 : 3403 – 3453 .
- McMurry , P. H. and Stolzenburg , M. R. 1989 . On the Sensitivity of Particle Size to Relative Humidity for Los Angeles Aerosols . Atmo. Env. , 23 : 497 – 507 .
- Musick , J. , Popp , J. and Kiefer , W. 2000 . Observation of a Phase Transition in an Electrodynamically Levitated NH4NO3 Microparticle by Mie and Raman Scattering . J. Raman Spectrosc. , 31 : 217 – 219 .
- Nemesure , S. , Wagener , R. and Schwartz , S. E. 1995 . Direct Shortwave Forcing of Climate by the Anthropogenic Sulfate Aerosol: Sensitivity to Particle Size, Composition, and Relative Humidity . J. Geophys. Res. , 100 : 26105 – 26116 .
- Novakov , T. and Penner , J. E. 1993 . Large Contribution of Organic Aerosols to Cloud Condensation Nuclei Concentrations . Nature , 365 : 823 – 826 .
- Onasch , T. B. , Siefert , R. L. , Brooks , S. D. , Prenni , A. J. , Murray , B. , Wilson , M. A. and Tolbert , M. A. 1999 . Infrared Spectroscopic Study of the Deliquescence and Efflorescence of Ammonium Sulfate Aerosol as a Function of Temperature . J. Geophys. Res. , 104 : 21317 – 21326 .
- Pant , A. , Fok , A. , Parsons , M. T. , Mak , J. and Bertram , A. K. 2004 . Deliquescence and Crystallization of Ammonium Sulfate-Glutaric Acid and Sodium Chloride-Glutaric Acid Particles . Geophys. Res. Lett. , 31 : L12111 doi:10.1029/2004GL020025
- Parsons , M. T. , Knopf , D. A. and Bertram , A. K. 2004 . Deliquescence and Crystallization of Ammonium Sulfate Particles Internally Mixed with Water–Soluble Organic Compounds . J. Phy. Chem. A , 108 : 11600 – 11608 .
- Peng , C. , Chan , M. N. and Chan , C. K. 2001 . The Hygroscopic Properties of Dicarboxylic and Multifunctional Acids: Measurements and UNIFAC Predictions . Environ. Sci. Technol. , 35 : 4495 – 4501 .
- Penner , J. E. , Andreae , M. , Annegarn , H. , Barrie , L. , Feichter , J. , Hegg , D. , Jayaraman , A. , Leaitch , R. , Murphy , D. , Nganga , J. and Pitari , G. 2001 . “ Aerosols, Their Direct and Indirect Effects ” . In Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change Edited by: Houghton , J. T. , Ding , Y. , Griggs , D. J. , Noguer , M. , van der Linden , P. J. , Dai , X. , Maskell , K. and Johnson , C. A. 289 – 348 . New York
- Pilinis , C. , Pandis , S. N. and Seinfeld , J. H. 1995 . Sensitivity of Direct Climate Forcing by Atmospheric Aerosols to Aerosols Size and Composition . J. Geophys. Res. , 100 : 18739 – 18754 .
- Prenni , A. J. , De Mott , P. J. and Kreidenweis , S. M. 2003 . Water Uptake of Internally Mixed Particles Containing Ammonium Sulfate and Dicarboxylic Acids . Atmo. Env. , 37 : 4243 – 4251 .
- Richardson , C. B. and Spann , J. F. 1984 . Measurement of the Water Cycle in a Levitated Ammonium Sulfate Particle . J. Aerosol Sci. , 15 : 563 – 571 .
- Rogge , W. F. , Mazurek , M. A. , Hildemann , L. M. , Cass , G. R. and Simoneit , B. R. T. 1993 . Quantification of Urban Organic Aerosols at a Molecular Level Identification, Abundance and Seasonal Variation, 1309–1330 . Atmo. Env. Part A , 27 : 1309 – 1330 .
- Saxena , P. and Hildemann , L. M. 1996 . Water-Soluble Organics in Atmospheric Particles: A Critical Review of the Literature and Application of Thermodynamics to Identify Candidate Compounds . J. Atmo. Chem. , 24 : 57 – 109 .
- Saxena , P. , Hildemann , L. M. , McMurry , P. H. and Seinfeld , J. H. 1995 . Organics Alter Hygroscopic Behavior of Atmospheric Particles . J. Geophys. Res. , 100 : 18755 – 18770 .
- Schlenker , J. C. , Malinowski , A. , Martin , S. T. , Hung , H. -M. and Rudich , Y. 2004 . Crystals Formed at 293 K by Aqueous Sulfate-Nitrate-Ammonium-Proton Aerosol Particles . J. Phy. Chem. A , 108 : 9375 – 9383 .
- Seinfeld , J. H. and Pandis , S. N. 2006 . Atmospheric Chemistry and Physics: From Air Pollution to Climate Change , New Jersey : John Wiley & Sons .
- Socrates , G. 2001 . Infrared and Raman Charactristic Group Frequencies: Tables and Charts , Chichester : John Wiley & Sons .
- Stokes , R. H. and Robinson , R. A. 1966 . Interactions in Aqueous Nonelectrolyte Solutions. I. Solute-Solvent Equilibria . J. Phys. Chem. , 70 : 2126 – 2130 .
- Svenningsson , B. , Hansson , H. C. , Martinsson , B. , Wiedensohler , A. , Swietlicki , E. , Cederfelt , S. I. , Wendisch , M. , Bower , K. N. , Choularton , T. W. and Colvile , R. N. 1997 . Cloud Droplet Nucleation Scavenging in Relation to the Size and Hygroscopic Behaviour of Aerosol Particles . Atmo. Env. , 31 : 2463 – 2475 .
- Swietlicki , E. , Hansson , H. C. , Hameri , K. , Svenningsson , B. , Massling , A. , McFiggans , G. , McMurry , P. H. , Petaja , T. , Tunved , P. , Gysel , M. , Topping , D. , Weingartner , E. , Baltensperger , U. , Rissler , J. , Wiedensohler , A. and Kulmala , M. 2008 . Hygroscopic Properties of Submicrometer Atmospheric Aerosol Particles Measured with H-TDMA Instruments in Various Environments—A Review . Tellus Series B , 60 : 432 – 469 .
- Tang , I. N. 1996 . Chemical and Size Effects of Hygroscopic Aerosols on Light Scattering Coefficients . J. Geophys. Res. , 101 : 19245 – 19250 .
- Tang , I. N. , Fung , K. H. , Imre , D. G. and Munkelwitz , H. R. 1995 . Phase Transformation and Metastability of Hygroscopic Microparticles . Aerosol Sci. Technol. , 23 : 443 – 453 .
- Wang , F. , Zhang , Y. H. , Li , S. H. , Wang , L. Y. and Zhao , L. J. 2005 . A Strategy for Single Supersaturated Droplet Analysis: Confocal Raman Investigations on the Complicated Hygroscopic Properties of Individual MgSO4 Droplets on the Quartz Substrate . Anal. Chem. , 77 : 7148 – 7155 .
- Wu , H. B. and Chan , C. K. 2008 . Effects of Potassium Nitrate on the Solid Phase Transitions of Ammonium Nitrate Particles . Atmo. Env. , 42 : 313 – 322 .
- Yeung , M. C. Y. L. A. K. and Chan , C. K. 2009 . Phase Transition and Hygroscopic Properties of Internally Mixed Ammonium Sulfate and Adipic Acid (AS-AA) Particles by Optical Microscopic Imaging and Raman Spectroscopy . Aerosol Sci. Technol. , 43 : 387 – 399 .
- Zappoli , S. , Andracchio , A. , Fuzzi , S. , Facchini , M. C. , Gelencser , A. , Kiss , G. , Krivacsy , Z. , Molnar , A. , Meszaros , E. , Hansson , H. C. , Rosman , K. and Zebuhr , Y. 1999 . Inorganic, Organic and Macromolecular Components of Fine Aerosol in Different Areas of Europe in Relation to Their Water Solubility . Atmo. Env. , 33 : 2733 – 2743 .
- Zardini , A. A. , Sjogren , S. , Marcolli , C. , Krieger , U. K. , Gysel , M. , Weingartner , E. , Baltensperger , U. and Peter , T. 2008 . A Combined Particle Trap/HTDMA Hygroscopicity Study of Mixed Inorganic/Organic Aerosol Particles . Atmo. Chem. Phys. , 8 : 5589 – 5601 .
- Zhang , Y. H. and Chan , C. K. 2002 . Understanding the Hygroscopic Properties of Supersaturated Droplets of Metal and Ammonium Sulfate Solutions Using Raman Spectroscopy . J. Phy. Chem. A , 106 : 285 – 292 .