Abstract
Daily mass concentrations of water-soluble inorganic (WS-i) ions, organic carbon (OC), and elemental carbon (EC) were determined for fine particulate matter (PM1, particles < 1.0 μm in diameter) collected at Xi'an, China. The annual mean PM1 mass concentration was 127.3 ± 62.1 μg m–3: WS-i ions accounted for ∼38% of the PM1 mass; carbonaceous aerosol was ∼30%; and an unidentified fraction, probably mostly mineral dust, was ∼32%. WS-i ions and carbonaceous aerosol were the dominant species in winter and autumn, whereas the unidentified fraction had stronger influences in spring and summer. Ion balance calculations indicate that PM1 was more acidic than PM2.5 from the same site. PM1 mass, sulfate and nitrate concentrations followed the order winter > spring > autumn > summer, but OC and EC levels were higher in autumn than spring. Annual mean OC and EC concentrations were 21.0 ± 12.0 μg m−3 and 5.1 ± 2.7 μg m–3 with high OC/EC ratios, presumably reflecting emissions from coal combustion and biomass burning. Secondary organic carbon, estimated from the minimum OC/EC ratios, comprised 28.9% of the OC. Positive matrix factorization (PMF) analysis indicates that secondary aerosol and combustion emissions were the major sources for PM1.
1. Introduction
Fine aerosol particles can affect human health and degrade visibility in urban areas (CitationDockery et al. 1993; CitationSchwartz et al. 1996; CitationWilson and Suh 1997; CitationTsai and Cheng 1999; Pope 2000; CitationSchichtel et al. 2001; CitationTsai et al. 2003; CitationYadav et al. 2003). Finer particles can penetrate more deeply into the respiratory tract than coarser ones (CitationJames et al. 1991; CitationOwen and Ensor 1992; CitationSeaton et al. 1995), and they also typically contain higher levels of harmful substances, such as polycyclic aromatic hydrocarbons and chemical mutagens (CitationAndo et al. 1996; CitationKiss et al. 1998). The major components of urban ambient TSP (total suspended particulates), PM10 (particulate matter, PM, less than 10 μm in diameter), and PM2.5 (particles less than 2.5 μm in diameter) have been extensively investigated with many studies focusing on sulfate (SO2– 4), nitrate (NO– 3), OC, and EC. In comparison, much less is known about PM1, and even less has been done to control PM1 pollution.
Several studies (CitationVallius et al. 2000; CitationCabada et al. 2004) have indicated that the major components of PM1 and PM2.5 originate from the same sources, and those investigations of PM1 yield little new information when compared with what is obtained from studies of PM2.5. Nevertheless, one could argue that PM1 may be a better indicator of anthropogenic sources than PM2.5, because natural sources have less of an impact on the smaller sized particles than pollution emissions (CitationLundgren et al. 1996; CitationLee et al. 2006). For example, results from recent studies (CitationLin 2002; CitationLin and Lee 2004) at Kaohsiung city led to the conclusion that combustion emissions and the formation of secondary aerosols were the most important sources for ambient PM1 in that urban area.
With reference to the health effects of fine particles, epidemiological studies by CitationDockery et al. (1993), CitationSchwartz et al. (1996), CitationWilson and Suh (1997), and CitationPope (2000) have suggested a statistical association between health effects and aerosol loadings, especially the submicron fraction (CitationHuang et al. 2003). The relationship to particle size is presumably due to the fact that fine particles can penetrate into the alveolar region of the lungs. Along these lines, CitationHuang et al. (2003) demonstrated that the particle-induced cytokine production by epithelial cells was affected by particle size; that is, submicron particles were more effective in causing cytokine secretion and lipid peroxidation than larger ones.
Aerosol particle toxicity may be related to the presence of transition metals, organic compounds, biological compounds, and acidic pollutants, especially nitrate and sulfate. The finding that elemental carbon (EC) and organic carbon (OC) were positively associated with the induction of lipid peroxidation suggests that some organic components of the aerosol may elicit biological responses (CitationHuang et al. 2003). Because of the strong influence of PM1 on ambient visibility and human health, the U.S. Environmental Protection Agency (US EPA) has considered using PM1, instead of PM2.5, as the particle-size cut-point for monitoring air quality (US EPA 2004). As previously mentioned, there is limited data available on the concentrations and chemical composition of submicron particles in urban areas in China, thus, one of the main reasons for our study.
Air quality in Xi'an is a public health concern due to the persistently high loadings of PM (CitationCao et al. 2005a; CitationShen et al. 2008). Some actions taken by the local government, beginning around 1998, have led to improvements in air quality, but PM pollution in Xi'an continues to be a serious problem. For example, whereas clean fuels, such as natural gas, have progressively replaced coal for residential heating and cooking inside Xi'an's Second Ring Road, coal remains the main fuel used for energy production in many Chinese cities, including Xi'an. In addition, the number of motor vehicles in Xi'an has grown rapidly, increasing from ∼180,000 in 1997 to ∼820,000 in 2008.
Prior aerosol studies in Xi'an have focused mainly on the chemical composition of TSP, PM10, and PM2.5 (CitationZhang et al. 2002; CitationCao et al. 2005a; CitationShen et al. 2008, Citation2009). To the best of our knowledge, no data for PM1 over Xi'an has yet been reported. The objectives of the present study were to evaluate PM1 pollution at Xi'an and to compare the results with those from other studies. Here we present data for the PM1 mass concentrations, as well as the concentrations of water-soluble inorganic (WS-i) ions, organic carbon (OC), and elemental carbon (EC). The data was obtained over a period of one year.
2. Methodology
2.1. Aerosol Sample Collection
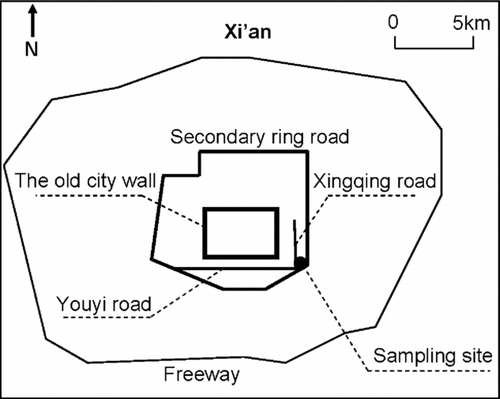
The PM1 samples were collected on 47 mm quartz microfiber filters (Whatman Ltd, Maidstone, UK) from the roof surface of a 15-m high building on the campus of Xi'an Jiaotong University (XJU). The sampling site is located in the southeastern part of downtown Xi'an, about 100 m from the South Second Ring Road (). Residential areas and the XJU campus are to the north and east of the sampling site, the South Second Ring road is to the south, and the Xingqin and Youyi Roads are to the west; all of these roads are heavily travelled.
Daily 24 h PM1 (10:00 am to 10:00 am, local time) filter samples were collected in four seasons in 2008: summer (from July 1 to July 31), autumn (from October 15 to November 14), winter (from December 15 to January 19) in 2007 and spring (from March 16 to April 15). A mini-volume sampler (BGI Inc, Waltham, MA, USA) operating at a flow rate of 5 L min–1 was used to collect a total of 123 aerosol samples. The 47 mm diameter quartz filters were pre-combusted at 800°C for 3 h before use to remove any carbonaceous contaminants. One field-blank filter for each sampling season was also collected by exposing filters in the sampler but without drawing air through them. The four blanks were analyzed to account for any artefacts introduced in each season sampling procedure individually.
Aerosol mass loadings were determined gravimetrically, using an electronic microbalance (Sartorius MC5, Göttingen, Germany, ±1 μg sensitivity). Before weighing, the filters were equilibrated for 24 h in a controlled environment between 20°C and 23°C with relative humidity between 35% and 45%. Each filter was weighed at least three times before and after sampling following the 24 h equilibration period. The mean net mass for each filter was obtained by subtracting the average pre-deployment weight from the average of the post-sampling readings. The difference among the three repeated weightings was less than 10 μg for a blank filter and less than 20 μg for a sampled filter. The uncertainty of quartz mass concentrations was less than 10% in this study.
2.2. Water-Soluble Inorganic Ion Analyses
One-fourth of each filter sample was used to determine the mass concentrations of selected WS-i ions, following procedures described in detail in CitationShen et al. (2008). The concentrations of four anions (SO2– 4, NO– 3, Cl–, and F–) and five cations (Na+, NH+ 4, K+, Mg2+, and Ca2+) were determined in aqueous extracts of the filters prepared in three steps using ultrapure (18 MΩ) water. A DX 500 ion chromatograph (IC, Dionex Corp, Sunnyvale, CA) was used for the analysis. Standard reference materials produced by the National Research Center for Certified Reference Materials, China, were analyzed for quality assurance purposes. The correlation coefficient between ion concentrations (at 0.06 μg ml–1, 0.18 μg ml–1, 0.45 μg ml–1, 0.9 μg ml–1, 1.8 μg ml–1, 3.6 μg ml–1, and 6 μg ml–1) with their peak intensity exceeds 99.9% for all ions. The blank values in each season were subtracted from sample concentrations. One sample in each group of ten samples was analyzed twice for quality control purposes. Typical precisions (percent relative standard deviation) for the 12 pairs of samples were calculated by the equation: X i = (C i1–C i2)/C ia . Here C i1 and C i2 are the routine and duplicate concentrations; C ia is the mean concentration for measurement pair i; Xi is the relative differences. The maximum relative precisions were: 1.8% for Na+, 0.9% for NH+ 4, 0.6% for K+, 4.0% for Ca2+, 1.0% for Mg2+, 1.2% for SO2– 4, 2.6% for NO– 3, 0.3% for Cl–, and 1.4% for F–.
2.3. Carbonaceous Aerosol Analyses
The quartz PM1 sample filters were analyzed for EC and OC with the use of a DRI Model 2001 Thermal and Optical Carbon Analyzer (Atmoslytic Inc., Calabasas, CA, USA). A 0.5 cm2 aliquot from each quartz filter was analyzed for eight carbonaceous fractions following the Interagency Monitoring to Protect Visual Environments total organic carbon protocol (IMPROVE TOC, Chow et al. 1993, 2001; Fung et al. 2002). This method produces data for four OC fractions (OC1, OC2, OC3, and OC4), which are released in a helium atmosphere at temperatures of 140°C, 280°C, 480°C, and 580°C, respectively, a pyrolyzed carbon fraction (OPC), determined when reflected laser light returns to its original intensity after oxygen is added to the combustion atmosphere, and three EC fractions (EC1, EC2, and EC3), which are generated in a 2% oxygen/98% helium atmosphere at temperatures of 580°C, 740°C, and 840°C, respectively.
The IMPROVE protocol defines OC as OC1 + OC2 + OC3 + OC4 + OPC and EC as EC1 + EC2 + EC3 – OPC. Replicate analyses were performed at the rate of one per group of ten samples. Blank samples were also analyzed, and the sample results were corrected for the seasonal blank concentration individually. Additional quality assurance and quality control procedures have been described in detail in CitationCao et al. (2003).
2.4. Source Assessment
Positive matrix factorization (PMF), a statistical technique developed by Paatero and colleagues (CitationPaatero and Tapper 1993 1994; Paatero 1997), provides a flexible modeling approach that can use the information contained in aerosol concentration data to infer sources. PMF has been widely used for receptor modeling, and it is most often applied when source profiles are unknown (such as CitationHwang and Hopke 2007; CitationLee and Hopke 2006; CitationLestari et al. 2009; CitationMiller et al. 2002; CitationShrivastava et al. 2007; CitationSong et al. 2007; CitationUchimiya et al. 2008). Here, PMF was applied as a way to identify and evaluate the origins of the PM1.
PMF analyses were conducted using the concentration data for nine water-soluble ions and eight carbon fractions that we determined for the complete ensemble of PM1 samples. The PMF model was run multiple times, extracting three to eight factors, and each run was initialized with different starting points (i.e., changing the seed value from 1 to 20). The discussion that follows is based on the six source-factor model resulting from the PMF analyses.
3. Results and Discussion
3.1. Seasonal Variations of PM1 Mass
The PM1 mass ranged from 27.2 μg m–3 to 373.3 μg m–3 with an annual arithmetic mean value of 127.3 μg m–3 (); the latter exceeding the U.S. National Ambient Air Quality Standards (NAAQS) for annual PM2.5 (15 μg m–3) by more than a factor of 8. Moreover, 122 PM1 of the total of 123 samples analyzed exceeded the 24 h NAAQS standard for PM2.5 of 35 μg m−3, further illustrating the severity of the PM pollution in Xi'an. Seasonal variations of PM1 mass were distinct, as shown in and ; the mass concentrations followed the sequence of winter (178.2 μg m−3)> spring (139.2 μg m−3)> autumn (120.8 μg m−3)> summer (72.5 μg m−3). The high PM1 mass loadings in winter are most likely due to emissions from coal and biomass, which are burned for heat in the city and surrounding suburbs.
FIG. 2 Temporal variations of PM1 mass concentrations and selected chemical species in four seasons (2007–2008).
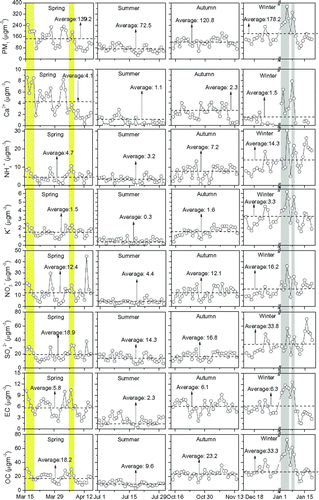
TABLE 1 Mean mass concentration and standard deviation (SD) of PM1 and its chemical species in four seasons
Daily variations of PM1 mass are plotted in . During winter, two strong pollution episodes were found, the first from January 2 to 6 and the second from January 8 to 9; the mean PM1 mass loadings during these events were 285.1 μg m−3 and 308.1 μg m−3, respectively. Four high PM1 events, which included two pollution episodes and two dust storms, occurred in spring. The two dust events occurred from March 16 to 19 and from April 5 to 7, when the mean PM1 mass values reached 208.7 μg m−3and 152.5 μg m−3, respectively. Numerous studies have shown that dust storms can lead to heavy PM loadings in urban and rural regions of China (CitationCao et al. 2005b; CitationZhang et al. 2005; CitationWang et al. 2006; CitationShen et al. 2009). However, the PM1 mass concentrations during the two pollution episodes, which occurred on March 27 and from April 1 to 3, were higher than those in dust storms (PM1= 236.4 and 225.2 μg m−3, respectively. See ). This can be explained by the fact that dust storms generally have large proportions of coarse particles. For example, CitationShen et al. (2009) reported the PM2.5/TSP mass ratios during dust storm events averaged only 21% in spring in Xi'an. This is much lower than the ratios observed during hazy conditions or during pollution episodes caused by biomass burning.
For further perspective, the PM1 measured in this study can be compared with the loadings in other cities: These comparisons indicate that the PM1 levels in Xi'an are much higher than in other urban areas and that controls are needed to alleviate the fine particle pollution in Xi'an. For example, CitationLee et al. (2006) sampled PM1 alongside a road in Hong Kong and reported PM1 levels of 40.9 μg m–3 in winter and 33.8 μg m–3 in spring. A study by CitationLi and Lin (2002) investigated the characteristics of PM1, PM2.5, and PM10 at an ambient general site (Chung-Shan) and a traffic monitoring station (Da-Tung) in Taipei. The PM1 mass concentrations at Chung-Shan were 17.1, 13.1, and 9.7 μg m–3 in spring, autumn, and winter, respectively. In comparison, the PM1 masses at the traffic monitoring station (45.6 μg m–3 in summer and 29.5 μg m–3 in autumn) were much higher than those at the other urban site. Their study also revealed that fine particles originating from combustion sources play an important role in urban atmosphere.
In a related study, CitationLin and Lee (2004) reported the concentrations and seasonal variations of urban PM1 at Kaohsiung, Taiwan, where the PM1 mass varied from 16 to 108 μg m–3 with a mean value of 52 μg m–3. Seasonal variations of PM1 in that study followed the sequence of winter (67.1 μg m–3)> autumn (52.2 μg m–3)> summer (24.5 μg m–3). Another study by Gomišèek et al. (2004) showed that the mean PM1 levels at four sites in Austria ranged from 1.2 μg m–3 to 75.1 μg m–3 and averaged 16.0 μg m–3.
3.2. Water-Soluble Inorganic Ions
The annually averaged total measured WS-i ion concentration was 48.1 ± 26.4 μg m–3, and that accounted for about 38.8% of the measured PM1 mass, thus, these ions are major components of the submicron aerosol in Xi'an. In general, sulfate, nitrate, and ammonium dominated the WS-i species studied, combining to account for 77.1%, 85.2%, 81.9%, and 83.0% of the total measured mass from spring to winter, respectively. Previous studies have suggested that gas-to-particle conversion is important, relative to the formation of these three ions, that is, SO2 to SO2– 4, NO2 to NO– 3 and NH3 to NH+ 4 (CitationLin 2002; CitationWang et al. 2005; CitationShen et al. 2008); and therefore, much of the PM1 in our study apparently results from heterogeneous processes.
Strong seasonal variations in the PM1WS-i ion loadings were observed. For example, the total measured WS-i ion mass concentrations decreased in the order winter (77.1 ± 29.5 μg m–3)> spring (46.4 ± 18.4 μg m–3)> autumn (44.0 ± 14.0 μg m–3)> summer (25.8 ± 9.0 μg m–3). In terms of their relative contributions to the WS-i ion loadings, the annual mean concentrations for four anions ranked in the order SO2– 4> NO3–> Cl–> F–, whereas the annual mean concentration of the five cations followed the sequence of NH+ 4> Ca2+> K+> Na+> Mg2+. Sulfate, nitrate, and chloride all exhibited the same seasonal pattern in concentrations, following the order winter > spring > autumn > summer.
The cations exhibited the same relative abundances in spring, summer, and autumn as for the entire year; however, in winter, the order was different: NH+ 4> K+> Na+> Ca2+>Mg2+. Soluble K+ is often enriched in the aerosol as a result of biomass burning (Andreae 1983; Watson et al. 2001), and the enrichments we observed in winter were more than likely caused by the burning of wheat straw and maize stalks for heat in suburban areas around Xi'an. Two other ions, NH+ 4 and Na+, showed the same seasonal patterns as K+ (i.e., winter > autumn > spring > summer).
The water soluble Ca2+ concentrations in PM1 were much lower than those reported for TSP or PM2.5 in a prior study at Xi'an (CitationShen et al. 2008). This ion has been used as an indicator of Asian dust, which typically exhibits its highest concentrations in spring (CitationChoi et al. 2001; CitationCao et al. 2005b; CitationShen et al. 2007). Prior studies revealed that fugitive dust in the downtown (i.e., dust from road traffic, construction activities, and traditional street sweeping) and soil dust from outside of downtown caused high dust loading in spring in Xi'an, and these lead to high Ca2+ concentrations (CitationZhang et al. 2002; CitationShen et al. 2008). Indeed, our Ca2+ PM1 loadings ranked in the order spring > autumn > winter > summer. In the two dust events that occurred during our study, the Ca2+ concentrations were 7.7 and 3.1 μg m–3, respectively. In addition, two Ca2+ peaks were found in pollution episodes in winter, and the most likely source for these is fugitive dust (CitationZhang et al. 2002; CitationShen et al. 2008).
Ion balance calculations can be useful for evaluating the acid–base balance of aerosol particles. The cation and anion microequivalents in our PM1 samples were calculated based on the following equations:
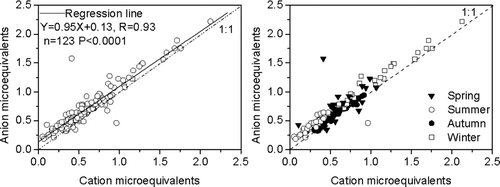
Cation and anion equivalents show a strong correlation (R = 0.93) as illustrated in (left), and this indicates that the nine ionic species included in Equations (Equation1) and (Equation2) are the major ions in the submicron aerosol. Ion balance calculations stratified by season ( (right)) show that most of the samples collected in autumn were positioned slightly above a 1:1 anion:cation (A:C) line, implying that they were weakly acid. The buffering of acidity in autumn, albeit incomplete, is likely due primarily to the ammonium ion. With further reference to sources, the high NH+ 4 concentrations in the Xi'an aerosol are most likely related to the use of fertilizers on the Guanzhong Plain in summer and autumn, as well as contributions from local sanitary wastes (CitationZhang et al. 2002). Lower temperatures in autumn, compared with summer, favor the conversion of NH3 to NH+ 4, and the end products would include ammonium sulfate and ammonium nitrate (CitationZhang et al. 2002; CitationShen et al. 2008). Interestingly, the NH+ 4 loadings were higher than those for Ca2+, the indicator of soil dust, and this is further evidence that anthropogenic activities cause much of the fine particle pollution.
More of the summer and winter PM1 samples plotted farther above the A:C unity line compared to those from autumn, illustrating that the former were more acidic. Spring samples were more scattered, indicating greater variability in acid-base balance. We noted that six springtime PM1 samples that plotted below the equiline were rich in Ca2+, consistent with previous studies that have shown that high mineral dust loadings in spring can buffer aerosol acidity (CitationZhang et al. 2002; CitationCao et al. 2005b). The remaining twenty-five spring samples plotted above the 1:1 line, showing they were acidic. Compared with previous studies at the same sampling site (CitationZhang et al. 2002; CitationShen et al. 2008), our results show that PM1 is more acid than either TSP or PM2.5, and this is true even during the high dust season.
The average ratio of total anion to cation microequivalents (A/C) for the complete set of PM1 samples was 1.20. In this study, the seasonally stratified A/C ratios from spring to winter were 1.19, 1.49, 1.06, and 1.16, respectively. These results suggest that on balance submicron particles from cities with high mineral dust loadings can be acidic. Moreover, the seasonal patterns, showing high A/C ratios during the polluted winter season, support the assertion that PM1 was mainly anthropogenic. Overall, the seasonal trends in ion concentrations and ratios strongly suggest that PM1 particle acidity is mainly due to SO2– 4, NO– 3, and Cl–, whereas NH+ 4 and Ca2+ tend to buffer the acidity.
3.3. Carbonaceous Aerosol
In general, PM1 OC ranged from 3.3 μg m–3 to 72.5 μg m–3, and EC from 0.3 μgm–3 to 12.6 μg m–3 with the annual mean concentrations of OC = 21.0 μg m–3 and EC = 5.1 μg m–3. Although the EC loadings were much lower than those of OC, the seasonal variations of the two carbonaceous aerosol fractions tracked one another, decreasing in the following order: winter > autumn > spring > summer (). Carbonaceous aerosol from thirteen other Chinese cities has been shown to exhibit the same seasonality (CitationCao et al. 2007).
In comparison with results from a previous study, PM1 OC concentrations in Xi'an were much higher than in roadside samples from Hong Kong (CitationLee et al. 2006). For example, spring and winter OC in Xi'an exceeded the values measured at the Hong Kong road site by factors of three and four. CitationLi and Lin (2002) reported the PM1 OC concentrations at the Taiwan urban site of Chung-Shan were 5.5 μg m–3 in spring, 6.2 μg m–3 in autumn, and 3.4 μg m–3 in winter. These are all much lower than at Xi'an. At the roadside monitoring station at Da-Tung, Taiwan, PM1 OC concentrations in summer (19.0 μg m–3) were higher, whereas those in autumn (11.5 μg m–3) were lower than the values we found at Xi'an. The OC concentrations reported by CitationLi and Lin (2002) also show different seasonality compared with our study, that is, summer, instead of winter, was the most polluted season at both sites in Taipei.
Further comparisons show that in contrast to OC, the EC concentrations during spring and winter in our study were lower than the corresponding roadside concentrations of 9.7 and 9.0 μg m–3 at Hong Kong. In addition, the seasonal trends in PM1 EC at Taipei showed a pattern different from what we observed at Xi'an and what CitationCao et al. (2007) found in their studies of other Chinese cities. That is, the PM1 EC concentrations at Chung-Shan decreased from spring (4.7 μg m–3) to autumn (3.7 μg m–3) to winter (1.3 μg m–3) (CitationLi and Lin 2002), and in general the EC values were lower than those at Xi'an. In contrast, much higher levels of EC were found at Da-Tung compared with Xi'an, and both EC and OC exhibited the same seasonal patterns: summer > autumn.
Differences in the seasonality of carbonaceous aerosol loadings at Xi'an, Hong Kong, and Taipei are best explained by the difference of source emissions, especially the emission associated with the heating season in northern Chinese cities. At present, coal still dominates in the total energy consumption in inland China (CitationChan and Yao 2008), accounting for more than 70% of the total energy consumption and about 85% of SO2 emissions in China (CitationChan and Yao 2008), and this is much different from the situations in Hong Kong and Taipei, where both climates are much warmer than Xi'an's. Moreover, in Xi'an, the heating season normally lasts from the middle of November to the middle March in the following year. High loadings of OC and sulfate from coal combustion in winter (CitationShen et al. 2008; CitationCao et al. 2005a), both of which are primarily pollution-derived, support this assumption.
The mass concentrations of organic matter (OM) in the atmosphere can be estimated by multiplying OC by 1.6 (CitationTurpin and Lim 2001). The total carbonaceous aerosol (TCA) concentration, calculated as the sum of OM and EC, had an annual mean 38.7 μg m–3, and this amounts to 30.2% of the measured PM1 mass. Although slightly less than the contribution of WS-i ions to the PM1 mass, the carbonaceous aerosol clearly is a major component of PM1. The seasonal arithmetic mean TCA loadings from spring to winter were 35.0 μg m–3, 17.6 μg m–3, 43.3 μg m–3, and 59.5 μg m–3, respectively, and these account for 26.7%, 24.8%, 36.4%, and 32.9% of the corresponding PM1 masses.
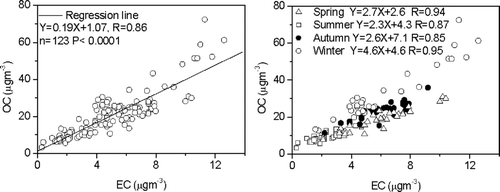
Relationships between OC and EC were used to assess the sources for the carbonaceous aerosol following the methods of Cao et al. (2003, 2004) and CitationDuan et al. (2004). A correlation coefficient of R = 0.86 (p < 0.0001) was found between OC and EC for the full set of PM1 samples ( (left)). This indicates that the two carbonaceous fractions were likely from the same sources. However, when seasonally stratified ( (right)), the results show some distinct differences; that is, strong correlations between OC and EC were found in spring and winter with R = 0.94 and 0.95, but those in summer and autumn were lower, R = 0.87 and 0.85. These results demonstrate that sources and chemistry controlling OC and EC loadings were more varied and complex in the summer and autumn. As will be discussed, estimates of the percentage of secondary organic carbon (SOC) in OC during the four seasons can shed additional light on this.
OC/EC ratios provide an indication not only of the sources for the carbonaceous aerosol but also the ways in which the particles form (CitationChow et al. 1996; CitationCao et al. 2003). As a whole, the OC/EC ratios in PM1 from Xi'an varied from 2.2 to 15.2 with an arithmetic mean value of 4.4. Seasonal changes in the OC/EC ratios decreased in the following order: winter (5.6) > summer (4.2) > autumn (3.9) > spring (3.2). The OC/EC ratios in our current study are much higher than those observed at Hong Kong or Taipei (CitationLee et al. 2006; CitationLi and Lin 2002). In addition, carbonaceous aerosol from fourteen Chinese cities also showed high OC/EC ratios; that is, the average OC/EC ratios in those cities ranged from 2.0 to 4.7 during winter and from 2.1 to 5.9 during summer (CitationCao et al. 2007).
Differences in the OC/EC ratios between the Chinese cities and Hong Kong or Taipei imply that PM from China is either relatively more abundant in OC or depleted in EC, and the geographical variations in the ratios attest to the diversity in the sources for the carbonaceous aerosol. Some studies have presented source profiles of OC/EC ratios based on speciated emission inventories and receptor models of source apportionment (CitationCao et al. 2003, Citation2005a; CitationChow et al. 2004; CitationDuan et al. 2004). Along these lines, Watson et al. (2001) found that the OC/EC ratios for coal combustion, vehicle emission, and biomass burning were 2.7, 1.1, and 9.0, respectively. Meanwhile, CitationCao et al. (2005a) reported a very high OC/EC ratio for biomass burning (60.3) in Xi'an.
Considering these characteristics of emissions, the high OC/EC ratios in winter indicate that biomass burning plays an important role in carbonaceous aerosol's origins in Xi'an. Meanwhile, coal combustion for heat should be another important emission source to carbonaceous aerosol. The high sulfate and K+ loadings observed in winter also support this contention. The OC/EC ratios in summer and autumn exceeded 2.7, reflecting mixed contributions from coal combustion, vehicle exhaust, and biomass burning. Indeed, wheat straw combustion during the summer and autumn harvests, although sporadic, may have caused the OC/EC ratios to be slightly higher than in spring. The OC/EC ratios in spring, again, are mostly indicative of emissions from coal combustion and motor vehicle exhaust.
The proportions of OC and EC in combustion emissions are influenced heavily by many factors, such as the chemical composition of the fuels, the effectiveness of pollution control devices, as well as the sampling methods used, sampling periods, analytical techniques, etc. It is worth noting that EC originates from the incomplete combustion of carbon-containing materials and does not form heterogeneously. In contrast, OC includes primary OC (POC, which originates from the combustion process directly) and secondary OC (SOC, produced by gas-to-particle conversion or chemical reactions in atmosphere). Therefore, the application of OC/EC ratios to source identification requires either an assumption that the ambient OC was primary OC or a way to account for the SOC.
In fact, favorable meteorological conditions such as sunny days in summer and autumn can lead to the production of significant proportions of SOC. Estimates of SOC for a variety of locations indicate that this material accounts for about one third of the TCA mass (CitationCao et al. 2003, Citation2007; CitationLin et al. 2002; CitationCastro et al. 1999; CitationChu 2005; CitationCabada et al. 2007). To investigate this issue further, we used the minimum OC/EC ratio method to estimate SOC concentrations based on the following equation (CitationTurpin and Huntzicker 1995; CitationCastro et al. 1999):
Results of the minimum OC/EC ratio calculations indicate that the estimated annual arithmetic mean PM1 SOC concentration was 5.5 μg m–3, which is 28.9% of the total OC (). The relative proportion of the estimated SOC in TOC was lowest in winter (22.2%) and roughly comparable in autumn (32.2%), summer (31.5%), and spring (29.7%). The lowest SOC fraction occurs when low temperatures, which averaged –1°C and ranged from –5.7 to 3.8°C, and reduced sunlight are unfavorable for the production of SOC. As mentioned earlier, contributions from coal combustion and biomass burning to primary OC are expected to be highest during this same winter heating season. In contrast, high temperatures and more abundant sunlight in autumn and summer would enhance the photochemical production of SOC, thus raising the OC/EC ratios. The seasonal variations in the estimated SOC we observed were consistent with the results for PM samples in seven northern Chinese cities (CitationCao et al. 2007) where the estimated SOC accounted for 36% to the total carbonaceous aerosol during summer and 23.7% during winter. Using this minimum OC/EC ratio method shows that nearly two thirds of the PM1 carbonaceous aerosol was from primary emission sources. We also believe that the accelerator mass spectrometry (AMS) measurements to estimate the SOC should be more accurate than other methods (CitationHildemann et al. 1994; CitationSzidat et al. 2004; CitationTanner and Miguel 1989; CitationTanner et al. 2004), but it is more expensive.
TABLE 2 Levels of estimated secondary organic carbon (estimated SOC) in PM1 estimated from minimum OC/EC ratio method
3.4. Mass Balance and Source Apportionment of Water-Soluble Ions and Carbonaceous Species in PM1
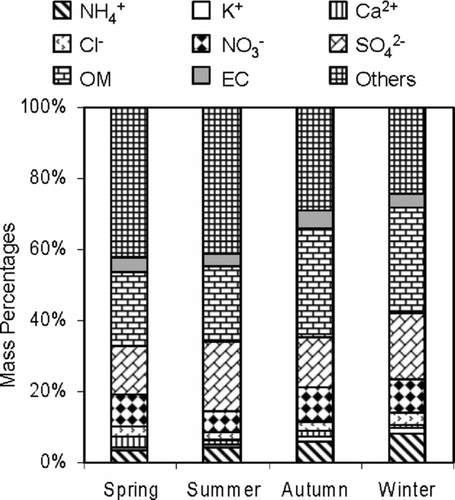
The mass balance of chemical species in PM1 is shown in . The elemental composition of the aerosol was not determined in this study, and, therefore, soil dust and other inorganic constituents are operationally defined as “other.” WS-i ions were the most abundant component of the PM1 mass on an annual basis, and “other” was the next largest. When broken down by season, the percent contribution of the “other” fraction was higher in spring (42.3%) and summer (41.5%) compared with autumn (28.9%) and winter (24.4%). High wind speeds in Xi'an during spring and summer give rise to high loadings of soil dust (CitationShen et al. 2008), and this would help explain the observed seasonality of the “other” fraction of the aerosol.
Sulfate, nitrate, and OM were the dominant PM1 species in spring and summer, combining to account for 43.4% and 46.9% of the PM1 mass for these seasons. Ammonium was more abundant in autumn and winter compared with summer and spring, and together, with the three species just discussed, accounted for over 60% of the mass in these two seasons. The relative proportions of OM were higher in autumn and winter than in spring and summer, whereas the contributions of NH+ 4 and Ca2+ to the PM1 mass were highest in winter and spring, respectively. The percent contribution of SO2– 4 was high in summer and winter, whereas the proportion of NO– 3 in PM1 was highest in autumn.
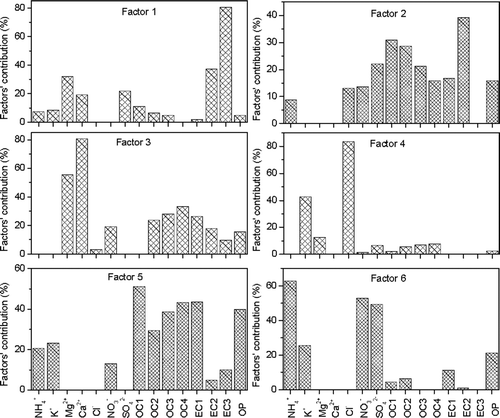
The average composition of the six factors generated by the PMF analysis was compared with known source profiles, and the results are shown in . The first factor was characterized by EC2 and EC3, which are characteristic of diesel exhaust (CitationWatson et al. 1994; Cao et al. 2006). The second factor was loaded with OC1 and OC2, representing exhaust from gasoline-powered engines (CitationWatson et al. 1994; Cao et al. 2006). Soil dust was identified as the third factor, based on the high abundances of Ca2+ and Mg2+. The fourth factor, characterized by K+ and Cl–, is consistent with biomass burning emissions (CitationAndreae 1993; CitationShen et al. 2009). The fifth factor, enriched with OC3, OC4, and EC1, is best explained by emissions from coal combustion (CitationWatson et al. 1994; Cao et al. 2006). The sixth factor was loaded with ammonium, sulfate, and nitrate, indicative of a secondary aerosol source.
FIG. 6 Factor loadings obtained from positive matrix factorization (PMF) analysis of chemical constituents of PM1 from Xi'an.
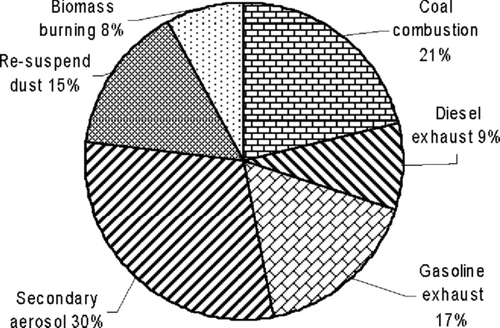
To evaluate the contributions of the presumptive sources to the PM1 mass, we used another statistical approach, multiple regression analysis (CitationPaatero and Tapper 1993 1994; Paatero 1997). This technique has also been used extensively in studies of aerosol sources (CitationLiu et al. 2005; CitationMaykut et al. 2003; CitationKim et al. 2005; CitationReff et al. 2007; CitationOgulei et al. 2005; CitationLestari and Mauliadi 2009). The contributions of the source resulting from the PMF analyses were calculated by multiple regression of the G matrix (CitationPaatero 1997) against the measured mass concentrations. Secondary aerosol contributed significantly to the detected PM1 mass (30%), followed by coal combustion (21%), gasoline exhaust (17%), soil dust (15%), diesel exhaust (9%), and biomass burning (8%) (). These results further support the notion that PM1 in Xi'an is mainly from anthropogenic sources, especially combustion sources.
4. Conclusions
The mass concentrations of PM1 observed in Xi'an were higher than those in previous studies conducted in Hong Kong and Taipei. Moreover, PM1 mass concentrations were much higher than the World Health Organization (WHO) urban ambient PM2.5 guidelines of 25 μg m–3 and 10 μg m–3 for 24 h and yearly mean mass concentration. These WHO guidelines arguably represent a significant exposure risk to public health. Seasonal variations in the mass loadings followed the sequence winter > spring > autumn > summer. High PM1 loadings associated with high concentrations of sulfate and OC in winter, which we attribute to the biomass burning and coal combustion for heating, could not only result in adverse health effects but also contribute to visibility impairments.
Total WS-i ions accounted for more than one third of the measured PM1 mass in all four seasons. Sulfate, nitrate, and ammonium, which showed different seasonal variations, were the three major WS-i ions, and ion balance calculations indicate that PM1 samples are more acidic than TSP or PM2.5, again raising the possibility of serious health effects. Seasonal variations of OC and EC followed the sequence winter > autumn > spring > summer. The PM1 OC concentrations were higher than observed in Hong Kong and Taipei, but the EC loadings were lower.
Calculations of material balances showed that carbonaceous aerosol was the largest fraction of the PM1 in autumn and winter, whereas soil dust and other unidentified materials had greater influences on submicron particles in spring and summer. Source apportionment by PMF produced six factors indicating that secondary aerosol and combustion emissions were the main sources for PM1. Therefore, pollution control methods, such as replacing coal with natural gas for heating during winter and reducing motor vehicle emissions, may be an effective means of combating the high PM1 loadings in Xi'an.
This work was supported, in part, by the Natural Science Foundation of China (grant NSFC40925009), the Knowledge Innovation Program of the Chinese Academy of Sciences (grants KZCX2-YW-BR-10 and KZCX2-YW-148), and a grant from the SKLLQG, Chinese Academy of Sciences (grant SKLLQG0905).
REFERENCES
- Andreae , M. O. 1993 . Soot Carbon and Excess Fine Potassium: Long-Range Transport of Combustion-Derived Aerosols . Science , 220 : 1148 – 1151 .
- Ando , M. , Katagiri , K. , Tamura , K. , Yamamoto , S. , Matsumoto , F. M. , Li , Y. R. , Cao , S. D. , Ji , R. K. and Liang , C. 1996 . Indoor and Outdoor Air Pollution in Tokyo and Beijing Supercities . Atmos. Environ , 30 : 695 – 702 .
- Cabada , J. C. , Rees , S. , Takahama , S. , Khlystov , A. , Pandis , S. N. , Davidson , C. I. and Robinson , A. L. 2004 . Mass Size Distributions and Size Resolved Chemical Composition of Fine Particulate Matter at the Pittsburgh Supersite . Atmos. Environ , 38 : 3127 – 3141 .
- Cabada , J. C. , Pandis , S. N. , Subramanian , Ramachandran R. , Allen , L. , Polidori , A. and Turpin , B. 2007 . Estimating the Secondary Organic Aerosol Contribution to PM2.5 Using the EC Tracer Method . Aerosol Sci. Technol , 38 : 140 – 155 .
- Cao , J. J. , Lee , S. C. , Ho , K. F. , Zhang , X. Y. , Zou , S. C. , Fung , K. , Watson , J. G. and Chow , J. C. 2003 . Characteristics of Carbonaceous Aerosol in Pearl River Delta Region, China During 2001 Winter Period . Atmos. Environ , 37 : 1451 – 1460 .
- Cao , J. J. , Wu , F. , Chow , J. C. , Lee , S. C. , Li , Y. , Chen , S. W. , An , Z. S. , Fung , K. K. , Watson , J. G. , Zhu , C. S. and Liu , S. X. 2005a . Characterization and Source Apportionment of Atmospheric Organic and Elemental Carbon During Fall and Winter of 2003 In Xi'an, China . Atmos. Chem. Phys , 5 : 3127 – 3137 .
- Cao , J. J. , Lee , S. C. , Zhang , X. Y. , Chow , J. C. , An , Z. S. , Ho , K. F. , Watson , J. G. , Fung , K. , Wang , Y. Q. and Shen , Z. X. 2005b . Characterization of Airborne Carbonate Over a Site Near Asian Dust Source Regions During Spring 2002 and Its Climatic and Environmental Significance . J. Geophys. Res , 110 : D03203 doi:10.1029/2004JD005244
- Cao , J. J. , Lee , S. C. , Chow , J. C. , Watson , J. G. , Ho , K. F. , Zhang , R. J. , Jin , Z. D. , Shen , Z. X. , Chen , G. C. , Kang , Y. M. , Zou , S. C. , Zhang , L. Z. , Qi , S. H. , Dai , M. H. , Cheng , Y. and Hu , K. 2007 . Spatial and Seasonal Distributions of Carbonaceous Aerosols Over China . J. Geophys. Res , 112 : D22S11 doi:10.1029/2006JD008205
- Castro , L. M. , Pio , C. A. , Harrison , R. M. and Smith , D. J. T. 1999 . Carbonaceous Aerosol in Urban and Rural European Atmospheres: Estimation of Secondary Organic Carbon Concentrations . Atmos. Environ , 33 : 2771 – 2781 .
- Chan , C. K. and Yao , X. 2008 . Air Pollution in Mega Cities in China . Atmos. Environ , 42 : 1 – 42 .
- Choi , J. C. , Lee , M. , Chun , Y. S. , Kim , J. Y. and Oh , S. N. 2001 . Chemical Composition and Source Signature of Spring Aerosol in Seoul, Korea . J. Geophys. Res , 106 : 18067 – 18674 .
- Chow , J. C. , Watson , J. G. , Lu , Z. , Lowenthal , D. H. , Frazier , C. A. , Solomon , P. A. , Thuillier , R. H. and Magliano , K. L. 1996 . Descriptive Analysis of PM2.5 And PM10 at Regionally Representative Locations During SJVAQS/AUSPEX . Atmos. Environ , 30 : 2079 – 2112 .
- Chow , J. C. , Watson , J. G. , Kuhns , H. D. , Etyemezian , V. , Lowenthal , D. H. , Crow , D. J. , Kohl , S. D. , Engelbrecht , J. P. and Green , M. C. 2004 . Source Profiles for Industrial, Mobile, and Area Sources in the Big Bend Regional Aerosol Visibility and Observational (BRAVO) Study . Chemospher , 54 : 185 – 208 .
- Chu , S.-H . 2005 . Stable Estimate of Primary OC/EC Ratios in the EC Tracer Method . Atmos. Environ , 39 : 1383 – 1392 .
- Dockery , D. W. , Pope , C. A. III , Xu , X. , Spengler , J. D. , Ware , J. H. , Fay , M. E. , Ferris , B. G. and Speizer , F. E. 1993 . An Association Between Air Pollution and Mortality in Six US Cities . N. Engl. J. Med , 329 : 1573 – 1759 .
- Duan , F. K. , Liu , X. D. , Yu , T. and Cachier , H. 2004 . Identification and Estimate of Biomass Burning Contribution to the Urban Aerosol Organic Carbon Concentrations in Beijing . Atmos. Environ , 38 : 1275 – 1282 .
- Gomišèek , B. , Hauck , H. , Stopper , S. and Preining , O . 2004 . Spatial and Temporal Variations of PM1, PM2.5, PM10 and Particle Number Concentration During the AUPHEP-Project . Atmos. Environ , 38 : 3917 – 3934 .
- Hildemann , L. M. , Klinedinst , D. B. , Klouda , G. A. , Currie , L. A. and Cass , G. R. 1994 . Sources of Urban Contemporary Carbon Aerosol . Environ. Sci. Technol , 28 : 1565 – 1576 .
- Huang , S. -L. , Hsu , M.-K. and Chan , C.-C . 2003 . Effects of Submicrometer Particle Compositions on Cytokine Production and Lipid Peroxidation of Human Bronchial Epithelial Cells . Environ. Health Perspect , 111 : 478 – 482 .
- Hwang , I. and Hopke , P. K. 2007 . Estimation of Source Apportionment and Potential Source Locations of PM2.5 at a West Coastal IMPROVE Site . Atmos. Environ , 41 : 506 – 518 .
- James , A. C. , Stahlhofen , W. , Rudolf , G. , Egan , M. J. , Nixon , W. , Gehr , P. and Briant , J. K. 1991 . The Respiratory Tract Deposition Model Proposed by the ICRP Task Group . Radiat. Prot. Dosim , 38 : 159 – 165 .
- Jerrett , M. 2005 . Spatial Analysis of Air Pollution and Mortality in Los Angeles . Epidemiolog , 16 : 727 – 736 .
- Kim , E. , Hopke , P. K. and Qin , Y. 2005 . Estimation of Organic Carbon Blank Values and Error Structures of the Speciation Trends Network Data for Source Apportionment . J. Air and Waste Manage. Assoc , 55 : 1190 – 1199 .
- Kiss , G. , Vargapuchony , Z. , Rohrbacher , G. and Hlavay , J. 1998 . Distribution of Polycyclic Aromatic Hydrocarbons on Atmospheric Aerosol Particles of Different Sizes . Atmos. Res , 46 : 253 – 261 .
- Lee , S. C. , Cheng , Y. , Ho , K. F. , Cao , J. J. , Louie , P. K. K. , Chow , J. C. and Watson , J. 2006 . PM1 and PM2.5 Characteristics in the Roadside Environment of Hong Kong . Aerosol Sci. Technol , 40 : 157 – 165 .
- Lee , J. H. and Hopke , P. K. 2006 . Apportioning Sources of PM2.5 in St. Louis, MO Using Speciation Trends Network Data . Atmos. Environ , 40 : 360 – 377 .
- Lestari , P. and Mauliadi , Y. D. 2009 . Source Apportionment of Particulate Matter at Urban Mixed Site in Indonesia Using PMF . Atmos. Environ , 43 : 1760 – 1770 .
- Li , C. -S. and Lin , C. -H. 2002 . PM1/PM2.5/PM10 Characteristics in the Urban Atmosphere of Taipei . Aerosol Sci. Technol , 36 : 469 – 473 .
- Lin , J. J. 2002 . Characterization of the Major Chemical Species in PM2.5 in the Kaohsiung City, Taiwan . Atmos. Environ , 36 : 1911 – 1920 .
- Lin , J. J. and Lee , L. -C . 2004 . Characterization of the Concentration and Distribution of Urban Submicron (PM1) Aerosol Particles . Atmos. Environ , 38 : 469 – 475 .
- Liu , W. , Wang , Y. , Russell , A. and Edgerton , E. S. 2005 . Atmospheric Aerosol Over Two Urban-Rural Pairs in the Southeastern United States: Chemical Composition and Possible Sources . Atmos. Environ , 39 : 4453 – 4470 .
- Lundgren , D. A. , Hlaing , D. N. , Rich , T. A. and Marple , V. A. 1996 . PM10/PM2.5/PM1 Data from a Trichotomous Sampler . Aerosol Sci. Technol , 25 : 353 – 357 .
- Maykut , N. N. , Lewtas , J. , Kim , E. and Larson , T. V. 2003 . Source Apportionment of PM2.5 at an Urban IMPROVE Site in Seattle, Washington . Environ. Sci. Technol , 37 : 5135 – 5142 .
- Miller , S. L. , Anderson , M. J. , Daly , E. P. and Milford , J. B. 2002 . Source Apportionment of Exposures to Volatile Organic Compounds. I. Evaluation of Receptor Models Using Simulated Exposure Data . Atmos. Environ , 36 : 3629 – 3641 .
- Owen , M. K. and Ensor , D. S. 1992 . Airborne Particle Sizes and Sources Found in Indoor Air . Atmos. Environ , 26 : 2149 – 2162 .
- Ogulei , D. , Hopke , P. K. , Zhou , L. , Paatero , P. , Park , S. S. and Ondov , J. M. 2005 . Receptor Modeling for Multiple Time Resolved Species: The Baltimore Supersite . Atmos. Environ , 39 : 3751 – 3762 .
- Paatero , P. and Tapper , U. 1993 . Analysis of Different Modes of Factor Analysis as Least Squares Fit Problem . Chemometrics Intell. Lab. Syst , 18 : 183 – 194 .
- Paatero , P. and Tapper , U. 1994 . Positive Matrix Factorization: A Non-Negative Factor Model with Optimal Utilization of Error Estimates of Data Values . Environmetric , 5 : 111 – 126 .
- Paatero , P. 1997 . Least Squares Formulation of Robust Nonnegative Factor Analysis . Atmos. Environ , 37 : 23 – 35 .
- Pope , C. A. III . 2000 . Review: Epidemiological Basis for Particulate Air Pollution Health Standards . Aerosol Sci. Technol , 32 : 4 – 14 .
- Reff , A. , Eberly , S. I. and Bhave , P. V. 2007 . Receptor Modeling of Ambient Particulate Matter Data Using Positive Matrix Factorization: Review of Existing Methods . J. Air and Waste Manage. Assoc , 57 : 146 – 154 .
- Seaton , A. , MacNee , W. , Donaldson , K. and Godden , D. 1995 . Particulate Air Pollution and Acute Health Effects . Lance , 345 : 176 – 178 .
- Schwartz , J. , Dockery , D. W. and Neas , L. M. 1996 . Is Daily Mortality Associated Specially with Fine Particles . J. Air & Waste Manage. Assoc , 46 : 927 – 939 .
- Schichtel , B. A. , Husar , R. B. , Falke , S. R. and Wilson , W. E. 2001 . Haze Trends Over the United States, 1980–1995 . Atmos. Environ , 35 : 5205 – 5210 .
- Shen , Z. X. , Cao , J. J. , Arimoto , R. , Zhang , R. J. , Jie , D. M. , Liu , S. X. and Zhu , C. S. 2007 . Chemical Composition and Source Characterization of Spring Aerosol Over Horqin Sand Land in Northeastern China . J. Geophys. Res , 112 : D14315 Doi:10.1029/2006JD007991
- Shen , Z. X. , Arimoto , R. , Cao , J. J. , Zhang , R. J. , Li , X. X. , Du , N. , Okuda , T. , Nakao , S. and Tanaka , S. 2008 . Seasonal Variations and Evidence for the Effectiveness of Pollution Controls on Water-Soluble Inorganic Species in Total Suspended Particulates and Fine Particulate Matter from Xi'an, China . J. Air & Waste Manage. Assoc , 58 : 1560 – 1570 .
- Shen , Z. X. , Cao , J. J. , Arimoto , R. , Han , Z. W. , Zhang , R. J. , Han , Y. M. , Liu , S. X. , Okuda , T. , Nakao , S. and Tanaka , S. 2009 . Ionic Composition of TSP and PM2.5 During Dust Storms and Air Pollution Episodes at Xi'an, China . Atmos. Environ , 43 : 2911 – 2918 .
- Shrivastava , M. K. , Subramanian , R. , Rogge , W. F. and Robinson , A. L. 2007 . Sources of Organic Aerosol: Positive Matrix Factorization of Molecular Marker Data and Comparison of Results from Different Source Apportionment Models . Atmos. Environ , 41 : 9353 – 9369 .
- Song , Y. , Shao , M. , Liu , Y. , Lu , S. , Kuster , W. , Goldan , P. and Xie , S. 2007 . Source Apportionment of Ambient Volatile Organic Compounds in Beijing . Environ. Sci. Technol , 41 : 4348 – 4353 .
- Snyder , D. C. , Rutter , A. P. , Collins , R. , Worley , C. and Schauer , J. J. 2009 . Insights into the Origin of Water Soluble Organic Carbon in Atmospheric Fine Particulate Matter . Aerosol Sci. Technol , 43 : 1099 – 1107 .
- Szidat , S. , Jenk , T. M. , Gaggeler , H. W. , Synal , H. A. , Fisseha , R. , Baltensperger , U. , Kalberer , M. , Samburova , V. , Wacker , L. , Saurer , M. , Schwikowski , M. and Hajdas , I. 2004 . Source Apportionment of Aerosols by C-14 Measurements in Different Carbonaceous Particle Fractions , 475 – 484 . Conference proceedings. Univ. Arizona Dept. Geosciences .
- Tanner , R. L. and Miguel , A. H. 1989 . Carbonaceous Aerosol Sources in Rio De Janeiro . Aerosol Sci. Technol , 10 : 213 – 223 .
- Tanner , R. L. , Parkhurst , W. J. and McNichol , A. P. 2004 . Fossil Sources of Ambient Aerosol Carbon Based on 14C Measurements . Aerosol Sci. Technol , 38 : 133 – 139 .
- Tsai , Y. I. and Cheng , M. T. 1999 . Visibility and Aerosol Chemical Compositions Near the Coastal Area in Central Taiwan . Sci. Total Environ , 231 : 37 – 51 .
- Tsai , Y. I. , Lin , Y. H. and Lee , S. Z. 2003 . Visibility Variation with Air Qualities in the Metropolitan Area in Southern Taiwan . Water Air Soil Pollut , 144 : 19 – 40 .
- Turpin , B. J. and Huntzicker , J. J. 1995 . Identification of Secondary Organic Aerosol Episodes and Quantification of Primary and Secondary Organic Aerosol Concentrations During SCAQS . Atmos. Environ , 29 : 3527 – 3544 .
- Turpin , B. J. and Lim , H. J. 2001 . Species Contributions to PM2.5 Mass Concentrations: Revisiting Common Assumptions for Estimating Organic Mass . Aerosol Sci. Technol , 35 : 602 – 610 .
- Uchimiya , M. I. , Arai , M. and Masunaga , S. 2008 . Fingerprinting Localized Dioxin Contamination: Ichihara Anchorage Case . Environ. Sci. Technol , 41 : 3864 – 3870 .
- U.S. Environmental Protection Agency . 2004 . “ Air Quality Criteria for Particulate Matter ” . Research Triangle Park, , NC : National Center for Environmental Assessment-RTP Office . Report No. EPA/600/P-99/OOa2F
- Vallius , M. J. , Ruuskanen , J. , Mirme , A. and Pekkanen , J. 2000 . Concentrations and Estimated Soot Content of PM1, PM2.5, and PM10 in a Subarctic Urban Atmosphere . Environ. Sci. Technol , 34 : 1919 – 1925 .
- Wang , Y. , Zhuang , G. S. , Tang , A. H. , Yuan , H. , Sun , Y. L. , Chen , S. and Zheng , A. H. 2005 . The Ion Chemistry and the Source of PM2.5 Aerosol in Beijing . Atmos. Environ , 39 : 3771 – 3784 .
- Wang , Y. , Zhuang , G. S. , Sun , Y. L. and An , Z. S. 2006 . The Variation of Characteristics and Formation Mechanisms of Aerosols in Dust, Haze, and Clear Days in Beijing . Atmos. Environ , 40 : 6579 – 6591 .
- Waston , J. G. , Chow , J. C. and Houck , J. E. 2001 . PM2.5 Chemical Source Profiles for Vehicle Exhaust, Vegetative Burning, Geological Materials, and Coal Burning in Northwestern Colorado During 1995 . Chemospher , 43 : 1141 – 1151 .
- Watson , J. G. , Chow , J. C. , Lowenthal , D. H. , Pritchett , L. C. , Frazier , C. A. , Neuroth , G. R. and Robbins , R. 1994 . Differences in the Carbon Composition of Source Profiles for Diesel and Gasoline Powered Vehicles . Atmos. Environ , 28 : 2493 – 2505 .
- WHO . 2005 . WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulphur Dioxide, Summary of Risk Assessment , 9 WHO .
- Wilson , W. E. and Suh , H. H. 1997 . Fine Particles and Coarse Particles: Concentration Relationships Relevant to Epidemiologic Studies . J. Air and Waste Manage. Assoc , 47 : 1238 – 1249 .
- Yadav , A. K. , Kumar , K. , Kasim , A. , Singh , M. P. , Parida , S. K. and Sharan , M. 2003 . Visibility and Incidence of Respiratory Diseases During the 1998 Haze Episode in Brunei Darussalam . Pure Appl. Geophys , 160 : 265 – 277 .
- Zhang , R. , Arimoto , R. , An , J. , Yabuki , S. and Sun , J. 2005 . Ground Observations of a Strong Dust Storm in Beijing in March 2002 . J. Geophys. Res , 110 : D18S06 doi:10.1029/2004JD004589
- Zhang , X. Y. , Cao , J. J. , Li , L. M. , Arimoto , R. , Cheng , Y. , Huebert , B. and Wang , D. 2002 . Characterization of Atmospheric Aerosol over Xi'an in the South Margin of the Loess Plateau, China . Atmos. Environ , 36 : 4189 – 4199 .