Abstract
Four commercially available batch-type bioaerosol samplers, which collect time-integrated samples in liquids, were evaluated. Sampling efficiency was characterized as a function of particle size using near-monodisperse polystyrene spheres (sizes of 1–5 μ m) and oleic acid droplets (3–10 μ m). Results show the sampling efficiency of AGI-30 impingers range from 4–67% for particle sizes of 1 to 5.1 μ m with significant variations between units; those of SKC BioSampler impingers range from 34–105% for particle sizes from 1 to 9 μ m; those of a batch-type wetted wall cyclone with compensation for evaporation (BWWC-EC) range from 5 to 65% for particle sizes 1 to 10 μ m; and, those of a batch-type wetted wall cyclone with no evaporation compensation (BWWC-NC) range of 55 to 88% for particle sizes of 1–8 μ m. Retention efficiency was measured for 1 and 10 μ m polystyrene spheres. For the AGI-30 and BWWC-EC, the retention efficiency of 1 μ m particles after 1 h was less than 30%, while that of the SKC BioSampler was 59%. Due to liquid evaporation, the BWWC-NC could not be operated for 1 h. Retention efficiencies for Bacillus atrophaeus spores and Pantoea agglomerans vegetative cells were measured for the AGI-30 and the SKC BioSampler. Results for the spores were about the same as those for 1 μ m non-viable polystyrene particles; however, the vegetative bacteria lose culturability and consequently show lower retention efficiencies. For the impingers, significant performance differences were observed in units delivered by vendors at different times.
INTRODUCTION
Aerosol sampling for bioparticles that can be hazardous to human health through the route of deposition in the thoracic region of the respiratory system (e.g., toxins and viable organisms), is usually accomplished with devices that convert the particles from the aerosol state to the hydrosol state, with the conversion process taking place in either a continuous manner or on a batch basis. The continuous approach is exemplified by wetted wall cyclones (WWCs) that collect particles onto a flowing liquid film of fresh liquid (CitationBuchanan et al. 1972; CitationErrington and Powell 1969; CitationWhite et al. 1975; CitationHu and McFarland 2007; CitationMcFarland et al. 2010). WWCs render themselves well to near-real-time detection or identification strategies where the elapsed time between aerosol collection and the start of the analysis needs to be minimal.
There are two common types of batch-type liquid-based samplers; namely, impingers (CitationMay and Harper 1957; CitationWilleke et al. 1998) and batch wetted wall cyclones (BWWCs), (CitationSaaski et al. 2003; CitationZaromb et al. 1997). For these samplers, the aerosol particles are continuously collected into a batch of liquid during a fixed sampling period. At the end of the sampling period, which is typically on the order of 10 to 100 min, the liquid is recovered. The sampling process can be automated so that a fresh batch of liquid is injected into the system to commence a new cycle, and provisions can be made to compensate for liquid evaporation when the sampling takes place over an extended period.
With respect to the particle sizes that should be collected by a bioaerosol sampler, the current range of interest is 1–10 μ m aerodynamic diameter (AD) for thoracically effective biowarfare particles (CitationU.S. National Research Council 2005). A broader range must be considered for some applications, e.g., in sampling of natural bioaerosols such as fungal spores and pollens the focus may be on particles with sizes > 10 μ m AD. However, in this study the primary goal is to examine the performance of batch-type liquid-based bioaerosol collectors for particles in the size range of 1–10 μ m AD.
Although some bioparticles, such as viruses, may have intrinsic sizes that are less than 1 μ m, typically there is no need to efficiently collect those sizes because both natural generation processes such as sneezing, and artificial processes such as atomizing a slurry will produce organisms embedded in matrices, clusters, or attached to other particles (CitationHogan et al. 2005). Also, the viability of individual viruses or vegetative cells can be significantly degraded due to environmental and mechanical stresses (CitationCox 1976; CitationStewart et al. 1995), so their collection is less important than organisms embedded in larger particles, whose viability is more likely to be retained after exposure to stresses. Particle sizes larger than 10 μ m AD do not effectively penetrate to the thoracic region of the human respiratory system, and therefore are generally considered to be of less interest than the ≤ 10 μ m size range. However, instrumental detectors and identifiers of bioaerosol particles are generally sensitive to the mass of biological particulate matter, so larger particles provide better signals than the smaller particles. For example, a 10 μ m particle may have on the order of 1000 times as many cells as a 1 μ m particle, hence, it is important that a sampler have high efficiency for the larger particles.
The focus of this study is on the sampling efficiency, η SE , and retention efficiency, η RE , of four commercially available batch type collectors. The sampling efficiency, η SE , indicates the amount of particulate matter that is recovered from the collection liquid of a batch sampler compared to the amount of particulate matter that was sampled as aerosol. Retention efficiency indicates how effectively samples can be recovered from the collection liquid as a function of time. Symbolically these efficiencies are expressed as:
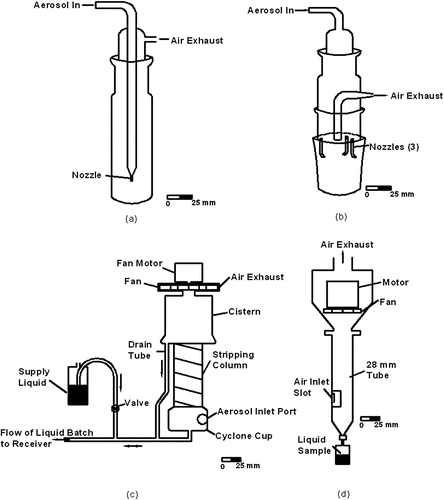
The batch type collectors, which were tested for sampling and retention efficiencies, are shown in . These devices are an All-Glass Impinger (AGI-30), a modified glass impinger (SKC BioSampler), a batch type wetted wall cyclone with provisions to compensate for liquid evaporation (BWWC-EC), and a batch type wetted wall cyclone with no provisions to compensate for liquid evaporation (BWWC-NC). In addition, tests were conducted with the AGI-30 and SKC impingers to characterize the loss of culturability of spore and vegetative cell hydrosols during extended operation of the sampler.
DESCRIPTION OF BIOAEROSOL COLLECTORS
AGI-30 Impinger
The All-Glass Impinger, AGI-30 (CitationMay and Harper 1957) is often used as a reference sampler in bioaerosol experimentation (CitationErlich et al. 1966; CitationPowitz and Balsamo 2002). The AGI-30 (Ace Glass Inc., Vineland, NJ), , has a nominal air sampling flow rate of 12.3–12.6 L/min, which is maintained by drawing a vacuum of at least 410 mm Hg to achieve sonic velocity at the exit plane of a 1.27 mm diameter acceleration nozzle, and typically the initial liquid volume is 20 mL. The jet created by the nozzle causes aerosol particles to impact on the bottom of the glass vessel.
Sampling efficiency of an impinger depends on airflow rate, distance between the exit plane of the impingement nozzle and the bottom surface of the liquid reservoir, and the properties of the collection fluid. The airflow rate is affected by the inner diameter of the impingement nozzle, which can vary from unit-to-unit (CitationLin et al. 1997). Low sampling efficiencies and high re-aerosolization losses of impingers have been reported (CitationWilleke et al. 1995; CitationGrinshpun et al. 1997; CitationLin et al. 1997). Liquid evaporation rate in an AGI-30 was measured by CitationLin et al. (1997), who observed a rate of 0.2 mL/min when an AGI-30 was operated in an environment at a temperature of 25°C and a relative humidity of 47%.
SKC BioSamplers
The SKC BioSampler impinger (SKC Inc., Eighty Four, PA), , was developed to improve the sampling and retention efficiencies of the AGI-30 (CitationLin et al. 2000). A nominal sampling flow rate of 12.5 L/min is maintained by drawing a vacuum of at least 381 mm Hg across the device. Typically, the initial volume of collection liquid is 20 mL. Instead of the aerosol being accelerated in a single jet and directed normally against an impaction surface, as is the case with the AGI-30, the airflow in the SKC impinger is accelerated through three nozzles that impinge tangentially on the cylindrical wall of the glass vessel. The BioSampler is reported to have higher sampling efficiency for smaller particles and to operate with less re-aerosolization than the AGI-30 (CitationWilleke et al. 1998).
Batch-Type Wetted Wall Cyclone with Evaporation Compensation
The BWWC-EC (CitationSaaski et al. 2003) is designed to sample at a flow rate of 265 L/min and to deposit aerosol particles into a liquid batch that is nominally maintained at 5 mL, although the device tested in this study had a set flow rate of 307 L/min. At typical indoor room air conditions, the evaporation rate would be about 1 mL/min, so the unit is fitted with a 150 mL reservoir to provide makeup collection fluid for extended operation. Air, in the form of a jet, tangentially enters a cyclone cup where the air shear force interacts with the liquid in the cup, causing the liquid to swirl around the inner surface of the cup. Liquid is atomized by the interaction of the swirling film and entering air jet, and by an auxiliary atomizer. The air jet, and entrained liquid droplets and aerosol particles, impinge on the curved inner wall of the cup, which affects aerosol particle collection onto the swirling liquid. The air stream and the hydrosol flow upward through a stripping column where further particle deposition takes place and then into a cistern where the hydrosol is recycled back into the cyclone cup while the airflow is exhausted by the fan.
Batch-Type Wetted Wall Cyclone with No Compensation for Liquid Evaporation
The BWWC-NC (CitationZaromb et al. 1997; CitationBirenzvige et al. 1998) is designed to operate at a nominal sampling flow rate of 300 L/min and to have an initial batch of 25 mL of collection fluid, although the device tested herein had a set flow rate of 306 L/min. Air enters the BWWC-NC tangentially through a port near the base of the tube, and the resulting air vortex imparts a swirling motion to the liquid. Aerosol particles are deposited onto the swirling liquid film, and the liquid subsequently moves upward in the system where it is collected and then drained back to the base of the unit. At the end of a sampling period, the air blower is turned off and the liquid drains into a collection cup where is available for analysis. There are no provisions to compensate for liquid evaporation.
METHODS
Sampling Efficiency Test Methodology
Sampling efficiency experiments were conducted in a 64-m3 aerosol chamber (CitationKesavan et al. 2008), which is equipped with HEPA filters to provide assurance that the concentration of background aerosol is low and that the test aerosols from the chamber, particularly when viable organisms are used, will not contaminate the surrounding environment. The sampling efficiency tests were conducted by releasing the aerosol in the chamber for a specified time and then mixing the aerosol with fans for approximately 45–60 s to achieve a uniform concentration. A test sampler and two reference filters were then operated simultaneously for a prescribed period of 10–20 min. Sample and reference filters were analyzed and the sampling efficiency was calculated from Equation (Equation1) based on the amounts of particulate matter collected in the liquid of the sampler and by the reference filters.
Sampling efficiency tests were conducted with near-monodisperse solid polystyrene spheres (PSL), which covered the size range of 1–5 μ m, and near-monodisperse fluorescent liquid oleic acid particles, covering the size range of 3–10 μ m. The principal test devices were two AGI-30 impingers, four SKC BioSamplers, a BWWC-EC, and a BWWC-NC. Not every sampler was tested with all particles sizes.
Tests with Solid Fluorescent Polystyrene Aerosol Particles
A 24-jet Collison nebulizer (BGI Inc., Waltham, MA) was used to generate the monodisperse fluorescent polystyrene microspheres (PSL, Duke Scientific Corp., Palo Alto, CA) with the generator placed inside the 64-m3 chamber. The PSL aerosol particles were electrically neutralized with a 10 mCi Kr-85 source (TSI Inc., St. Paul, MN) before being released into the chamber. Aerosol concentration in the chamber was monitored with an aerodynamic particle sizer APS (TSI Inc., Shoreview, MN) for assurance that the reference and test samplers would collect adequate particulate matter so the signal-to-noise ratio of fluorometer readings would be at least 10×. The desired PSL concentrations in the chamber were ≥ 29 particles/mL for the size of 1 μ m, ≥ 7 particles/mL for 3 μ m, and ≥ 0.6 particles/mL for 5 μ m. Depending on the size of the particles, aerosols were generated for the 10–20 min period to achieve the desired particle concentration in the chamber. The geometric standard deviations of the PSL aerosols produced by the Collison atomizer were checked with the APS. Results showed values of 1.14 for the 1 and 3 μ m sizes. The particle sizes reported for the experiments are those provided by the PSL vendor (Duke Scientific Corp., Palo Alto, CA).
Polycarbonate membrane filters (47 mm, Osmonics Inc., Minnetonka, Minnesota) were used to collect reference samples, with 0.2 μ m pore size filters used for 1 μ m PSL aerosol particles and 0.8 μ m pore size filters for > 1 μ m particles. At the completion of the sampling process, the microspheres were recovered by placing the filters in test tubes that contained 20 mL of filtered de-ionized water, employing five cycles of vigorously shaking the tubes by hand for 10 s, and then vortexing for 1 min with a commercially available vortexer (Model M16715, Barnstead International, Dubuque, IA). This approach has been shown to be quantitative (CitationKesavan and Doherty 1999). The hydrosols from the system under test and reference filters were analyzed with a Turner Model 450 Fluorometer (Barnstead/Thermolyne Corp., Dubuque, IA). Following the recommendations of the PSL vendor (Duke Scientific Corp.), blue fluorescent PSL microspheres (2.1 μ m diameter) were analyzed using NB 360 excitation and SC 430 emission filters in the fluorometer; and, green fluorescent PSL microspheres (1, 3, and 5 μ m diameter sizes) were analyzed using NB 460 excitation and SC 500 emission filters. Linearity of relationship between readings of the fluorometer and mass concentration of the tracer was checked for green 1 μ m PSL and the results showed linearity for PSL concentrations as high as 1 μ g of tagged PSL per mL of liquid. None of the concentrations in this study exceeded these values.
The concentration uniformity of the near-monodisperse PSL and oleic acid aerosols in the chamber was checked by simultaneously operating three filters, with two located in the usual positions for reference filters and the third situated at the test sampler location. Five particle sizes, from 1 to 8.6 μ m AD, were used in these tests and at least four replicates were run for each particle size. Temporal variations in aerosol concentration were not of consequence in sampler testing because a sampler and its reference filters were operated simultaneously. As a consequence, in checking the uniformity of aerosol concentration we normalized the measurements from the three filters in a replicate test to unity, and then analyzed the results for a given filter location over all particle sizes. The results in terms of mean values and standard deviations for Filters 1, 2, and 3 are 1.017 ± 0.047, 0.991 ± 0.040, and 0.992 ± 0.035, respectively. Thus, the average values of concentration at the three filter locations are within 3% with standard deviations for the measurements of about 4%.
Tests with Fluorescent Oleic Acid Liquid Aerosol Particles
Monodisperse fluorescent liquid oleic acid aerosol particles were generated with a vibrating orifice aerosol generator (VOAG, TSI Inc., St. Paul, MN). During the aerosol generation phase, the size constancy of the freshly generated aerosol was monitored with an APS, which showed the geometric standard deviation of the oleic acid aerosols was 1.06. The VOAG was operated for a specified period of time (10–20 min) to provide aerosol concentrations that would result in fluorometer readings for the test and reference samples of at least 100× background. After mixing the aerosol in the chamber with the fans, the samplers and the reference filters were operated simultaneously and collected the aerosol for 10 min. Because of the high signal-to-noise ratio of the fluorometric readings, the aerosol concentration was not monitored with an APS during the sampling phase. Fluorometer readings were shown by CitationRobinson et al. (1959) to be linear for sodium fluorescein concentrations as high as 1 μ g/mL. CitationKesavan and Doherty (1999) conducted tests that showed linearity slightly beyond 1 μ g/mL (however, to be conservative they suggested that an upper limit of 0.75 μ g/mL could be used). The highest value of oleic acid concentration measured in this study was 0.84 μ g/mL.
Particle size was determined by impacting the droplets onto a microscope slide and measuring their sizes with the aid of a light microscope. The measured particle diameter was converted to an aerodynamic particle diameter using the procedure of CitationOlan-Figueroa et al. (1982) together with the density of the fluorescently tagged oleic acid.
Reference samples of the fluorescent oleic acid particles were collected on glass fiber filters (Type AE, Pall Corp., Ann Arbor, MI). After exposure to the aerosol, the filters were placed in a recovery solution and shaken on a rotator table (Lab-Line Instruments, Inc., Melrose Park, IL) for 1 h. The recovery solution was 50:50 (V:V) water and alcohol with a pH between 8 and 10. The desired pH was obtained by adding a small amount of NH4OH (1 mL of 14.8 N NH4OH was mixed with 999 mL of recovery solution). Factors that affect fluorescein analysis and the removal of fluorescein from filters are described in detail by CitationKesavan et al. (2001). The samples from the test devices were pH-adjusted by adding NH4OH before measurement in the fluorometer. All analyses were performed within one day of sampling. NB 490 excitation and SC 515 emission optical filters were used in the fluorometer for the detection of sodium fluorescein.
Retention Efficiency of Polystyrene Particles
The retention efficiency, η RE , for solid non-viable test particles was measured by spiking a liquid with solid particles, inserting one appropriately sized batch of this hydrosol into the sampler, and operating the sampler for 30 s. This first batch was then removed from the sampler for analysis. A second batch was inserted into the sampler and the sampler was operated for a prescribe length of time, and that liquid was removed for analysis. This second batch was employed for all subsequent time periods. Use of the 30-s sample for the reference allows non-retention losses in the system, e.g., deposition of particles in the liquid flow components of a cyclonic collector, to be taken into account.
Particulate matter in the hydrosol could be lost by re-aerosolization or by deposition on internal walls of the sampling device (e.g., hydrosol striking an area of the wall and the particulate matter bonding to the wall so that subsequent normal liquid rinsing would not release it). Equation (Equation2) is used for calculation of retention efficiency, where the mass values are determined from the fluorescence of the samples in the case of non-viable test particles or represented by the number of colony forming units (CFU) in the case of viable particles.
Retention efficiency tests with 1 and 10 μ m PSL particles were conducted with two AGI-30 impingers, two SKC BioSamplers, the BWWC-EC, and the BWWC-NC. The values of m l,o in Equation (Equation2) were based on measurements of the tracer in the hydrosol after running the samplers for 30 s; and the values of m l,t were based on analyses of subsequent batches of liquid at elapsed times of 15, 30, 45, and 60 min. Sample handling was conducted using procedures that minimized washing of the potential particle deposition surfaces. The WWC-NC only had sufficient fluid capacity to allow operation for about 20 min, so test samples were evaluated at elapsed times of 10 and 20 min.
Retention Efficiency of Culturable Particles
In addition to irrecoverable wall losses and re-aerosolization, for organisms there can also be a loss of culturability. The culturable particle retention efficiency, η RE,C , can be expressed as:
RESULTS
Sampling Efficiency
AGI-30 Impinger
Results from the sampling efficiency experiments are shown in , where η SE is plotted as a function of aerodynamic diameter. Between four and eight replicates tests were conducted with each unit at each particle size, and the data in provide error bars that represent ± 1 standard deviation about a mean. For a particle size of 1 μ m, the two AGI-30 impingers, , showed significantly different results, with one unit having a sampling efficiency of about 27% and the other about 6%. Peak sampling efficiency is about 67% for both units and that peak occurs at a size of 4 μ m. The largest particle size tested was 5.1 μ m, at which size one unit showed a sampling efficiency of 59% while the other was 39%, however, the error bars for the two units overlap and a statistical t-test provides a value of p > 0.1, so the difference in these mean values of sampling efficiencies of these two units is not significant. The cutpoint particle size (particle diameter for which the sampling efficiency is 50% in the portion of curve where efficiency increases with particle size) is 3–3.5 μ m AD.
FIG. 2 Sampling efficiencies of the bioaerosol collectors. (a) AGI-30 impinger. (b). SKC BioSampler impinger. (c). Batch-type wetted wall cyclone with compensation for evaporation (BWWC-EC). (d) Batch-type wetted wall cyclone with no compensation for evaporation (BWWC-NC). Error bars are ± 1 standard deviation about a mean.
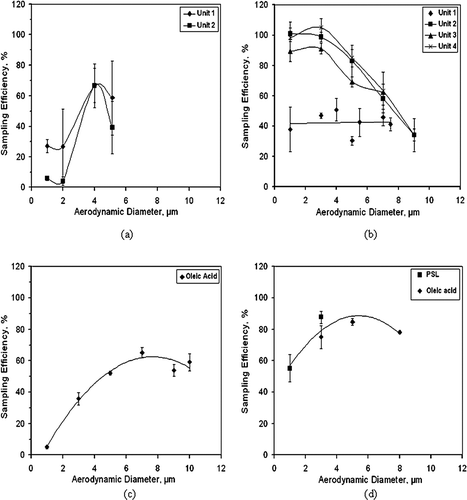
Because the efficiency values for 5.1 μ m particles in this study were 59% and 39%, which are low in comparison to the value of about 80% reported by CitationGrinshpun et al. (1994), we conducted additional tests using two new carefully selected devices. The results with those units showed 84% efficiency for 5 μ m AD oleic acid droplets. For the 5 μ m size, a statistical t-test shows the pooled mean results for first two units are statistically different than the pooled mean results for the second two units, with p = 0.0007. This implies that there can be significant performance variations between manufactured units.
SKC BioSampler
Sampling efficiency results for four SKC BioSamplers are shown in . The first SKC sampler (Unit 1) that was tested showed about 40% sampling efficiency for all sizes from 1 μ m to 7.5 μ m. This information was conveyed to the manufacturer and three additional units were delivered (Units 2–4), which provided average sampling efficiencies of 96% and 98% for 1 μ m and 3 μ m particles, respectively. For large particle sizes, the sampling efficiency drops, with the average efficiency of Units 2–4 being 34% for a particle size of 9 μ m. The cutpoint of the device is less than 1 μ m. Four replicate tests were conducted for each of the data points shown in .
The drop in efficiency values for the larger particle sizes is due to deposition of particles on the inner surfaces of the sampler, including the inside walls of the nozzles. Check tests were conducted where the air leaving the exhaust port was passed through a sampling filter. For 5 μ m liquid and solid particles, the amount of material collected by the filter was less than 2% of the amount of particulate matter that entered the sampler, indicating the losses were not due to carryover of sample-containing droplets into the exhaust stream, but rather had to be due to internal deposition.
Based on our experience, with both the AGI-30 and SKC impingers, of having measured differences in the performance of devices supplied by vendors at different times, users might consider a quality assurance plan for acceptance and use of devices. This would be especially warranted for critical application or studies where the impingers are used as reference devices.
Batch-Type Wetted Wall Cyclone with Compensation for Liquid Evaporation (BWWC-EC)
The performance of the BWWC-EC device in terms of sampling efficiency is shown in . Four replicates tests were conducted for all particle sizes except for the 9 μ m size, where triplicate tests were conducted. The efficiency is 5% for a particle size of 1 μ m, and it increases with particle size until it reaches a peak of 65% at a particle size of about 7 μ m, and then decreases for larger particle sizes, showing a value of 59% at a particle size of 10 μ m. The portion of the curve that shows decreasing efficiency with increasing particle size is due to internal losses in the device, e.g., losses of large particles in the inlet nozzle.
The BWWC-EC has a cutpoint of approximately 4.5 μ m. In general, as a goal, the design cutpoint of contemporary bioaerosol samplers should be no greater than about 1 μ m.
Batch-Type Wetted Wall Cyclone with No Compensation for Evaporation (BWWC-NC)
The sampling efficiency of the BWWC-NC is shown in , where it may be observed that the cutpoint is approximately 1 μ m, and the efficiency is approximately 80% over the size range of 3–8 μ m. Four replicate tests were conducted at each particle size.
Duplicate experiments were conducted with 3 μ m solid and liquid particles, and the results showed a mean efficiency of 88% for the solid PSL particles and a mean efficiency of 75% for the liquid oleic acid droplets. A t-test shows there is no significant difference between these mean efficiency values at the 95% confidence level.
Because of the importance of collecting the larger particles, the results showing the BWWC-NC has a sampling efficiency of about 78% for 8 μ m particles is noteworthy. Also, the cutpoint of 1 μ m should enable the device to collect reasonable quantities of aerosol particles comprised of single spores of organisms similar to BG, which have an aerodynamic diameter of about 1 μ m.
Retention Efficiency of Aerosol Samplers
Polystyrene Spheres
Retention efficiency as a function of time, for the four aerosol samplers, is shown in . Two sizes of solid polystyrene particles were used, namely, 1 and 10 μ m and three to five replicates were conducted for each test condition. Results from tests conducted with two AGI-30 units were combined, as were the results from tests with the two SKC BioSampler devices. For the AGI-30 samplers, , after 1 h of sampler operation, the retention efficiency of 10 μ m PSL particles was 86%, while that for the 1 μ m PSL particles was 26%. Results from tests with the SKC BioSamplers, , show that after 1 h the retention efficiency for 10 μ m PSL particles is 87% and that for the 1 μ m particles is 59%. Even though both the AGI-30 and the SKC BioSampler are impingers, the AGI-30 shows significantly lower retention efficiency for the 1 μ m PSL, which is likely due to the more violent bubbling action causing more re-aerosolization (CitationLin et al. 1997) and more transport of liquid to surface areas of the glass vessel away from the main pool of liquid. When some of that liquid evaporates from the wall, the 1 μ m particles may adhere to the wall and not be rinsed even when additional liquid is splashed against the wall. Assuming the bonding force of a particle to a wall in the impinger is proportional to particle diameter and the removal force is proportional to the square of the particle diameter, the larger 10-μ m particles would be more easily removed from a surface.
FIG. 3 Retention efficiency of polystyrene particles. (a) AGI-30. (b) SKC BioSampler. (c). BWWC-EC. (d) BWWC-NC. The AGI and SKC BioSampler are represented by AGI and BS in the legend. Error bars are ± 1 standard deviation about a mean.
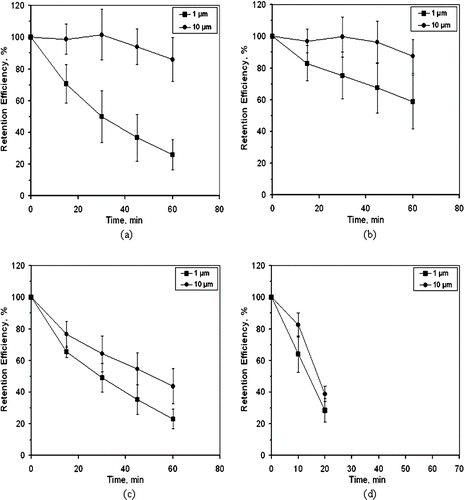
After 1 h of operation, the BWWC-EC device showed retention efficiency values of 44% for 10 μ m and 23% for 1 μ m PSL particles, . In tests with the BWWC-NC, after 20 min the amount of hydrosol remaining in the sampler was only 3.4 mL so the retention tests were terminated at that point. With reference to , results show that at 20 min, 39% of the 10 μ m and 29% of the 1 μ m particles were recovered with the collection liquid of the BWWC-NC.
For all samplers, the retention efficiencies for the 10 μ m size are higher than for the 1 μ m size at all time periods. The losses are consistent with the patterns for the mechanism called re-aerosolization by CitationLin et al. (1997), where larger losses are associated with smaller particle sizes.
Culturable Organisms
The retention efficiencies of culturable bioaerosol particles (BG and PA) were determined after 1 h of operation using new sets of two the AGI-30 impingers and two SKC BioSamplers. PSL particles, 1 μ m diameter, were added to the hydrosols to provide a non-viable reference for these tests. Six replicates were performed for each test condition.
The results for BG spores, , show that after 1 h, the average culturable retention efficiency of the AGI-30 was 67% and that of the SKC BioSampler was 79%. In comparison, for these tests, the retention efficiency of the non-viable 1 μ m PSL was 71% for the AGI-30 and 82% for the SKC BioSampler. Statistical t-tests suggest that for both sampler types, there is no difference between the retention of the PSL and BG, with p-values being > 0.4. Regarding the PA vegetative cells, after 1 h of operation the retention efficiency of the AGI-30 was 48% and that of the SKC BioSampler was 54%. In contrast, for non-viable 1 μ m diameter PSL, the retention efficiencies were 86% for AGI-30 and 91% for SKC BioSampler. Statistical t-tests on the null hypothesis (no difference in performance of the samplers with PSL or PA) show the p-values < 10–4, which suggests that there is a significant loss of vegetative cell culturability as a result of the 1 h operation in either impinger.
FIG. 4 Retention efficiencies of the AGI-30 (AGI) and SKC Biosampler (BS) for (a) Bacillus atrophaeus (BA) spores, and (b) Pantoea agglomerans (Pa) vegitative cells after 1 h. Comparative results are shown for non-viable 1 μ m polystyrene spheres (PSL), which were added to each hydrosol suspension to provide a reference. Error bars are ± 1 standard deviation about a mean value.
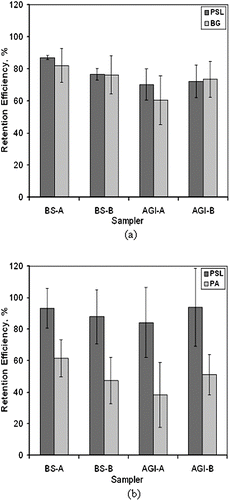
The retention efficiency results for 1 μ m PSL shown in (> 70% for the AGI-30 and > 80% for the SKC BioSampler) are higher than those shown in , where at the end of 1 h, the retention efficiency of the AGI-30 was 26% and that of the SKC BioSampler was 59%. Because new AGI-30 and SKC impingers were used for the tests with PSL and viable organisms, the PSL results from this data set cannot be directly compared with those shown in .
Change in Volume of Collection Fluid During Sampling
Measurements were made of the change in the volume fluid with time for the four systems and the results are shown in . The dry bulb temperature and relative humidity in the laboratory during these tests were about 25°C and 47%, respectively. Initial volumes of 20 mL were placed into two AGI-30 impingers, and they were operated at a flow rate of 12.5 L/min for test periods of 15, 30, 45, and 60 min. At the end of the 1-h tests, , an average of 10.6 mL remained in AGI-30 impingers, so the average rate of evaporation was 0.16 mL/min. Similar results were obtained with two SKC BioSamplers, , where 20 mL was placed into each of three units, and the units operated for the same time periods as the AGI-30. An average of 8.5 mL remained after the 1-h operational period, which gives an average evaporation rate of 0.19 mL/min. Because the evaporation rates and air sampling rates are approximately the same for the AGI and SKC impingers, the liquid loss is likely due to evaporation rather than entrainment or aerosolization of the collection liquid. Were liquid entrainment to be significant, the AGI-30 would show a larger rate of liquid loss, as the bubbling action is more severe in that unit.
FIG. 5 Change in liquid volume with time. Collectors operated in laboratory environment. Losses are primarily due to evaporation. (a) AGI-30 impinger. (b) SKC BioSampler. (c) BWWC-EC. (d) BWWC-NC. Error bars are ± 1 standard deviation about a mean.
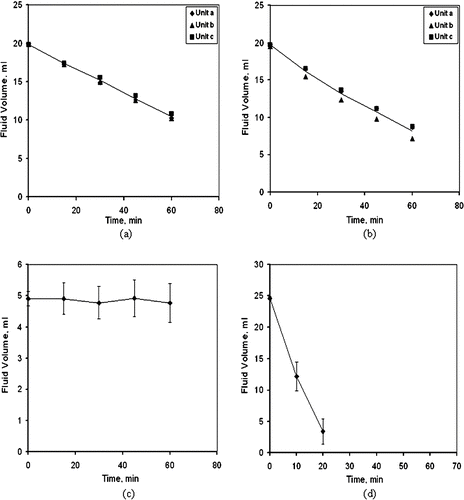
For tests with the BWWC-EC, , which has an actual air sampling flow rate of 307 L/min, an initial liquid volume of about 5 mL was maintained within about ±10% over the four sampling time periods, which shows the evaporation control system works well. The BWWC-NC unit, , which samples at a flow rate of 306 L/min, was supplied with an initial liquid volume of 25 mL; however, the unit could not be operated more than about 20 min because the liquid volume was reduced to 3.4 mL at that point. During the 20-min operational period, the average evaporation rate was 0.83 mL/min.
CONCLUSIONS AND DISCUSSION
The sampling and retention efficiency of four commercially available batch-type bioaerosol samplers were characterized in this study. Two of the samplers are impingers (AGI-30 and SKC BioSampler), and two are cyclone-type collectors (BWWC-EC and BWWC-NC), which collect particles on a liquid film setup by the swirling action of the air flow. In the context of detection of biowarfare aerosols, the current size range of interest is 1–10 μ m AD, so it is suggested that the cutpoints (particle size for which the collection efficiency is 50%) should be ≤ 1 μ m AD, and the collection efficiency should ≥ 80% for 10 μ m of the samplers. Because the impingers have been used as reference devices in bioaerosol experiments and the cyclones can be used in conjunction with field monitoring of bioaerosols, it is important to understand the performance of the devices regarding their sampling and retention efficiency.
AGI-30 Impingers
Two AGI-30 impingers, which have a nominal airflow rate of 12.5 L/min flow rate and an initial liquid volume of 20 mL, were tested for sampling efficiency with non-biological aerosols (PSL and oleic acid), and the results showed a cutpoint particle size of about 3–3.5 μ m AD and a peak sampling efficiency of 67% at a particle size of 4 μ m AD. Differences in sampling efficiency were observed with the two units, with one unit showing a sampling efficiency of 27% and the other a value of 6% for 1 μ m AD particles. Also, tests with 5.1 μ m AD particles showed sampling efficiency values of 59% and 39% for the two AGI-30 impingers. Two subsequently-procured units were tested, and the results showed the sampling efficiency for 5 μ m particles was 84% for both units. The low efficiency of the AGI-30 impingers for 1 μ m particles and the peak efficiency of 67% or 84%, depending upon the manufacturer's batch, which occurs at 4–5 μ m AD, suggests that the AGI-30 is less than an ideal reference sampler for experiments dealing with the particle size range of 1–10 μ m AD.
Retention efficiencies for PSL particles, after 1 h of operation, were 86% for 10 μ m AD and 26% for 1 μ m AD particles. The retention tests, which were conducted with a different set of AGI-30 impingers than the sampling efficiency tests, employed a suspension containing both organisms and 1 μ m PSL particles. Results showed that after 1 h of operation, the retention efficiency for Bacillus atrophaeus spores was 67% and that for Pantoea agglomerans vegetative cells was 48%; and, the associated retention efficiencies for the 1 μ m PSL tracers were 71% and 86%, respectively. Statistically, for these tests with BG and PA, there was no significant difference between the retention of the BG and 1 μ m PSL, but there was a highly significant difference between retention of the vegetative PA cells and the 1 μ m PSL. These results, which showed about 1/3 of BG spores and 1/2 of PA vegetative cells were lost, or lost culturability, after 1 h of operation further reduce the potential utility of the AGI-30 as a reference sampler. However, the AGI-30 is simple in design and easy to operate so it is a useful tool in some bioaerosol sampling applications.
Measurements were made of the evaporation losses of liquid when air at 25°C and 47% relative humidity was sampled. The results showed the rate was 0.16 mL/min or about half of an initial 20 mL batch of liquid was lost during a 1-h operational period.
SKC BioSampler
The first 12.5 L/min SKC BioSampler that was tested showed sampling efficiency values that were approximately constant at 40% for particle sizes from 1 to 7.5 μ m AD. After discussions with the vendor, three additional SKC BioSamplers were purchased, which performed much better. The cutpoint particle size of the functional units was not determined, but the sampling efficiency was measured as 96% at a particle size of 1 μ m AD, so the cutpoint is well below the 1 μ m AD value. The peak sampling efficiency was about 98% at a particle size of 3 μ m AD; however, at a particle size of 9 μ m AD, the sampling efficiency was 34%, which is not compatible with a goal of effective sampling of particles with sizes as large as 10 μ m AD. This low efficiency may be due to particle losses in the acceleration nozzles of the impinger.
The retention efficiencies for 1 and 10 μ m diameter PSL particles were 59% and 87%, respectively. Culturable retention efficiencies for BG spores and PA vegetative cells, measured with a third set of SKC units, were 79% and 54%, respectively. One-micrometer PSL particles added to the cell suspensions in this latter set of tests showed retention efficiencies after 1 h of 82% for the BG tests and 91% for PA tests. Statistically, there is not a significant difference between the retention, after 1 h, of BG and 1 μ m PSL (p > 0.4); however, there is a highly significant difference between the retention efficiency of the culturable PA cells and 1 μm PSL (p = 4 × 10–6). This again shows that there can be a significant loss of vegetative cell culturability when the organisms are retained the sampling liquid of an operating impinger over an extended period of time.
The evaporation of liquid at typical room conditions, 25°C and 47% relative humidity was measured. Results showed an average loss of 0.19 mL/min.
Batch-Type Wetted Wall Cyclone with Evaporation Compensation
The BWWC-EC, which was tested, has a design sampling flow rate of 265 L/min and an actual sampling flow rate of 307 L/min. Cutpoint was approximately 4.5 μ m and the peak sampling efficiency was approximately 65% at particle size of 7 μ m. The efficiency is about 59% at a particle size of 10 μ m, but only 5% at a particle size of 1 μ m. This implies that the system would perform well with clusters of bioparticles, but would not be very effective for single spore aerosols with sizes on the order of 1 μ m AD. However, even with a reasonably good collection efficiency of 59% for the 10 μ m AD particles, there is still missed opportunity for collection of large-sized particulate matter that potentially could substantially contribute to a bioaerosol identification process.
Retention efficiency tests with 1 and 10 μ m PSL spheres showed efficiency values of 23% and 44%, respectively, after 1 h of operation. The liquid control system, which provides makeup liquid to compensate for evaporation, performed well with the amount of collection fluid varying by only about ± 10% over a 1-h period.
Batch-type Wetted Wall Cyclone with No Compensation for Evaporation
The (BWWC-NC) has a design air sampling flow rate of 300 L/min and an initial liquid volume of 25 mL. Sampling efficiency tests showed the device has a cutpoint of about 1 μ m AD and that the efficiency is about 80% for particles in the size range of 3 to 8 μ m AD. The BWWC-NC would perform well in sampling both single spores with sizes on the order of 1 micrometer and larger clusters of particles. However, due to evaporation, the system could not sample laboratory air for more than 20 min, and so application of the version of the device tested herein would be restricted to short-term collection intervals, which would be considerably less than 20 min if hot, dry outdoor air were being sampled. Retention efficiency values after a 20-min operational period showed 29% of the 1 μ m PSL particles and 39% of the 10 μ m PSL particles were recovered from the collection fluid.
When sampling room air at 25°C and 47% relative humidity, the average evaporation rates are about 0.2 mL/min for the AGI and SKC impingers, and about 0.8 mL/min for the BWWC-NC. If the evaporation rates are normalized to the airflow rate, evaporation in the impingers is about 14 mL of liquid per cubic meter of sampled air. In contrast, for the BWWC-NC, the evaporation is about 3 mL/m3. The substantially greater relative evaporation in the impingers is a consequence of the increased surface area of the liquid associated with bubbling, as compared with the area of the relatively flat surface of liquid in the wetted wall cyclone. As a consequence, the air leaving an impinger is approximately saturated with water vapor whereas in the cyclone only the air near the wall is saturated.
General Comments
Tests of the AGI-30 and SKC BioSampler impingers showed significant differences in performance of some of the units that were delivered at different times by the vendors. The BWWC-EC has a quoted air sampling flow rate of 265 L/min, but the measured value was 307 L/min. Users who plan to employ bioaerosol samplers in critical studies, where the actual aerosol concentration needs to be known, e.g., use of an impinger as a reference sampler, should consider implementation of a quality assurance plan for acceptance and use of devices.
Two of the samplers, the SKC and the BWWC-NC, showed cutpoints that are 1 μ m or less, and only one sampler, the BWWC-NC, showed about 80% efficiency for 10 μ m AD particles. For collection of aerosol particles in the nominal size range of 1–10 μ m AD, it would be desirable if all samplers were to provide a cutpoint that is ≤ 1 μ m AD and collection of ≥ 80% for 10 μ m AD particles.
Acknowledgments
The assistance of Aubrey Hottell, Lee Cash, Brad King, Aaron Arumugarajah, Jessica Seifert, Daniel Sanford, and Dr. Maria King is gratefully acknowledged.
Disclaimer: The samplers included in this study were tested in the “as-received” condition. Neither the selection of type of samplers nor the results of this study imply any judgmental considerations on the part of the Government.
REFERENCES
- Birenzvige , A. , Akiyemi , A. N. and Zaromb , S. A. 1998 . Portable High-throughput Liquid-Absorption Air Sampler for Respirable Aerosol Particles . Aerosol Sci. Technol. , 29 : 133 – 140 .
- Buchanan , L. M. , Harstad , J. B. , Phillips , J. C. , Lafferty , E. , Dahlgren , C. M. and Decker , H. M. 1972 . Simple Liquid Scrubber for Large-Volume Air Sampling . Appl. Microbiology , 23 : 1140 – 1144 . (1972)
- Cox , C. S. 1976 . Inactivation Kinetics of Some Microorganisms Subjected to a Variety of Stresses . Appl. Environ. Microbiol. , 31 : 836 – 846 .
- Erlich , R. , Miller , S. and Idoine , L. S. 1966 . Evaluation of Slit Sampler in Quantitative Studies of Bacterial Aerosols . Appl. Microbiology , 14 : 328 – 330 .
- Errington , F. P. and Powell , E. O. 1969 . A Cyclone Separator for Aerosol Sampling in the Field . J. Hyg. , 67 : 387 – 399 .
- Grinshpun , S. A. , Chang , C. W. , Nevalainen , A. and Willeke , K. 1994 . Inlet Characteristics of Bioaerosol Samplers . J. Aerosol Sci. , 25 : 1503 – 1508 .
- Grinshpun , S. A. , Willeke , K. , Ulevicius , V. , Juozaitis , A. , Terzieva , S. , Donnelly , J. , Stelma , G. N. and Brenner , K. P. 1997 . Effect of Impaction, Bounce and Reaerosolization on the Collection Efficiency of Impingers . Aerosol Sci. Technol. , 26 : 326 – 342 .
- Hogan , C. J. , Kettleson , E. M. , Lee , M. H. , Ramaswami , B. , Angenent , L. T. and Biswas , P. 2005 . Sampling Methodologies and Dosage Assessment Techniques for Submicrometre and Ultrafine Virus Aerosol Particles . J. Appl. Microbiol , 99 : 1422 – 1434 .
- Hu , S. and McFarland , A. R. 2007 . Numerical Performance Simulation of a Wetted Wall Bioaerosol Sampling Cyclone. (2007) . Aerosol Sci. Technol. , 41 : 160 – 168 .
- Kesavan , J. , Bottiger , J. R. and McFarland , A. R. 2008 . Bioaerosol Concentrator Performance: Comparative Tests with Viable and with Solid and Liquid Nonviable Particles . J. Appl. Microbiology , 104 : 285 – 295 .
- Kesavan , J. and Doherty , R. W. 1999 . Test Procedure for Removing Polystyrene Latex Microspheres from Membrane Filters , Aberdeen Proving Ground, MD 21010 : Edgewood Research Development and Engineering Center, US Army Soldier and Biological Chemical Command . ECBC-TN-001
- Kesavan , J. , Doherty , R. W. , Wise , D. G. and McFarland , A. R. 2001 . Factors that Affect Fluorescein Analysis , Edgewood, MD : U. S. Army Edgewood Chemical Biological Center, Aberdeen Proving Ground . Report ECBC-TR-208, AD-A 397 677
- Lin , S. , Reponen , T. , Willeke , K. , Wang , Z. , Ginshpun , S. A. and Trunov , M. 2000 . Survival of Airborne Microorganisms During Swirling Aerosol Collection . Aerosol Sci. Technol. , 32 : 184 – 196 .
- Lin , X. , Willeke , K. , Ulevicius , V. and Grinshpun , S. A. 1997 . Effect of Sampling Time on the Collection of All-Glass Impingers . Am. Ind. Hyg. Assoc. J. , 58 : 480 – 488 .
- May , K. R. and Harper , G. J. 1957 . The Efficiency of Various Liquid Impinger Samplers in Bacterial Aerosols . Brit. J. Ind. Med. , 14 : 287 – 297 .
- McFarland , A. R. , Haglund , J. S. , King , M. D. , Hu , S. , Phull , M. S. , Moncla , B. W. and Seo , Y. 2010 . Aerosol Sci. Technol. , 44 : 241 – 252 .
- Olan-Figueroa , E. , McFarland , A. R. and Ortiz , C. A. 1982 . Flattening Coefficients for DOP and Oleic Acid Droplets Deposited on Treated Glass Slides . Am. Ind. Hyg. Assoc. J. , 43 : 395 – 399 .
- Powitz , R. W. and Balsamo , J. J. Jr. 2002 . Impingers: AGI–30 (All Glass Impinger) . J. Environ. Health , 64 : 33
- Robinson , E. , MacLeod , J. A. and Lapple , C. E. 1959 . A Meteorological Tracer Technique Using Uranine Dye . J. Meteorology , 16 : 63 – 67 .
- Saaski , E. W. , Jug , C. C. and McCrae , D. A. High Efficiency Wetted Surface Cyclonic Air Sampler . U.S. Patent 6,532,835 . 2003 . March 18, 2003
- Stewart , S. L. , Grinshpun , S. A. , Willeke , K. , Terzieva , S. , Ulevicious , V. and Donnelly , J. 1995 . Effect of Impact Stress on Microbial Recovery on an Agar Surface . Appl. Environ. Microbiol. , 56 : 3468 – 3472 .
- U. S. National Research Council . 2005 . Sensor Systems for Biological Agent Attacks: Protecting Buildings and Military Bases. , Washington, DC : National Academies Press . Committee on Materials and Manufacturing Processes for Advanced Sensors
- White , L. A. , Hadley , D. J. , Davids , D. E. and Naylor , R. 1975 . Improved Large-Volume Sampler for the Collection of Bacterial Cells from Aerosol . Appl. Microbiology , 29 : 335 – 339 .
- Willeke , K. , Grinshpun , S. A. , Ulevicius , V. , Terzieva , S. , Donnelly , J. , Stewart , S. and Juozaitis , A. 1995 . Microbial Stress Bounce and Re-Aerosolization in Bioaerosol Samplers . J. Aerosol Sci. , 26 ( Suppl. 1 ) : S883 – S884 .
- Willeke , K. , Lin , X. and Grinshpun , S. A. 1998 . Improved Aerosol Collection by Combined Impaction and Centrifugal Motion . Aerosol Sci. Technol. , 28 : 439 – 456 .
- Zaromb , S. , Justus , A. , Munyon , W. , Reilly , D. and Chen , B. 1997 . A Novel Portable Grab Sampler for Tritiated Water Vapor . Health Physics , 72 : 481 – 485 .