Abstract
New particle formation has been proposed to happen via activation of nanometer-sized condensation nuclei (nano-CN), which can be large molecules or molecular clusters. Electrically neutral nano-CN have mostly been outside the measurement range until recently. To address the concentrations and plausible chemical composition of the nano-CN, we measured the neutral particle size distribution down to mobility diameter ∼1.3 nm with a pulse-height CPC in Hyytiälä, southern Finland during springs 2007–2009. We also performed laboratory experiments with variable concentrations of precursors and oxidants in order to reproduce the atmospheric observations. The atmospheric nano-CN data were compared to ion size distributions (∼0.8–7.5 nm) measured with a BSMA ion spectrometer, and the mass spectra of natural ions detected with an APi-TOF mass spectrometer. We detected elevated concentrations of nano-CN, especially in the nocturnal boundary layer. However, the night-time nano-CN did not grow to sizes larger than a few nanometers. High temperature favored these night-time events, and the nano-CN concentration correlated well with the oxidation products of monoterpenes. The night-time negative ion mass spectra were dominated by large oxidized organic molecules and also the flow tube experiments support the idea that the nano-CN consist mostly of oxidized organic molecules. Nights with elevated neutral nano-CN concentration also showed enhanced concentrations of charged sulphuric acid monomer and dimer compared to other nights. However, all of the observed nano-CN does not necessarily participate to the atmospheric new particle formation process.
1. INTRODUCTION
It was long believed that atmospheric new particle formation, which is frequently observed in many kinds of environments throughout the world (CitationKulmala et al. 2004a), mainly takes place during daytime. Nocturnal particle formation was brought to light by CitationLee et al. (2008), who suggested that also night-time nucleation can be a significant source of atmospheric aerosol particles and cloud condensation nuclei. Nocturnal particle formation events have been previously observed, for example, in the Australian eucalypt forest (CitationSuni et al. 2008), near orographic clouds (CitationWiedensohler et al. 1997) and in the northern boreal forest (CitationVehkamäki et al. 2004a). CitationJunninen et al. (2008) found that the concentration and mean size of small ions in Hyytiälä, southern Finland, often increased in the night-time, but the ions rarely grew to sizes above 2 nm. CitationLehtipalo et al. (2009) discovered elevated concentrations of neutral nano-CN during night-time in Hyytiälä. What is preventing night-time particle formation in Hyytiälä?
The molecular level processes behind new particle formation are not completely understood. The prevailing nucleation mechanism, at least in the boreal forest environment, is suggested to be activation of atmospheric clusters or molecules approximately 1–2 nm in diameter (CitationKulmala 2003; CitationKulmala et al. 2006, 2007a). These nanometer-sized condensation nuclei (nano-CN), which were previously often referred to merely as clusters, could be electrically either neutral or charged. Also kinetic type of nucleation has been proposed (CitationMcMurry 1980), and ions can participate in new particle formation via ion-induced or ion-mediated nucleation (CitationTurco et al. 1998; CitationYu and Turco 2000). Ions in the size range below 1.5 nm can be easily detected and are known to be omnipresent in the atmosphere (CitationHõrrak et al. 1998). The concentration of small ions is, however, typically not high enough to explain the observed atmospheric particle formation rates in the boundary layer (CitationLaakso et al. 2004; CitationIida et al. 2006). Although some studies report the crucial role of ions in particle formation (CitationYu and Turco 2008; CitationYu et al. 2008), the contribution of ion-induced nucleation in Hyytiälä typically seems to be less than 10% (CitationGagné et al. 2008). Due to instrumental limitations, direct observations of neutral nano-CN in the atmosphere have been scarce. CitationWeber et al. (1995) detected molecular clusters when ultrafine particles were present, and recently CitationZhao et al. (2010) reported elevated concentrations of H2SO4 clusters up to the tetramer during new particle formation. The continuous existence of a neutral particle pool below 3 nm was first detected in Hyytiälä by CitationKulmala et al. (2007a), and CitationSipilä et al. (2008).
Nano-CN are constantly formed in the atmosphere, but they are effectively scavenged by the pre-existing particle population (CitationKerminen et al. 2001). The composition and size of nano-CN are likely to vary depending on environmental conditions and atmospheric composition, possibly consisting of a mixture of clusters and molecules. Different chemical compounds might be needed for forming the nano-CN and activating them for growth. The proposed candidates for the chemical compounds involved in nucleation in the boundary layer include for example sulphuric acid or sulphates (CitationWeber et al. 1995, 1997; CitationVehkamäki et al. 2004b; CitationKulmala et al. 2004b), and organics (CitationBonn et al. 2008). Quantum chemical studies suggest that ammonia and amines can enhance nucleation from sulphuric acid-water solutions (CitationTorpo et al. 2007; CitationKurtén et al. 2008). On coastlines, like in Mace Head on the west coast of Ireland, nucleation from biogenic iodine compounds is observed during daytime low tide (CitationO’Dowd et al. 2002b), and CitationLehtipalo et al. (2010) showed, that the particle bursts in Mace Head result more likely from homogenous nucleation than activation of nano-CN.
Several field and laboratory studies show a connection between particle formation and sulphuric acid (CitationWeber et al. 1997; CitationHanson and Eisele 2002; CitationSihto et al. 2006; CitationPetäjä et al. 2009; CitationNieminen et al. 2009; CitationKuang et al. 2008; CitationSipilä et al. 2010; CitationEhn et al. 2010). The atmospheric reaction path leading to formation of sulphuric acid involves photochemical reactions, so it is not likely to initiate nocturnal nucleation. There are also studies showing that sulphuric acid and water alone cannot account for particle formation and growth (CitationWeber et al. 1996; CitationKulmala et al. 1998; CitationFiedler et al. 2005; CitationBoy et al. 2005). Recent laboratory experiments suggest that both sulphuric acid and organics are involved in the particle formation process (CitationMetzger et al. 2010).
Volatile organic compounds (VOCs) are emitted by vegetation and thus always present inside the forest canopy. VOCs are oxidized in the atmosphere producing vapors, some of which can form clusters and/or condense on particles, as the oxidation products often have lower saturation vapor pressure than their precursors (CitationAsher et al. 2002). Due to boundary layer meteorology, VOCs emitted by the vegetation tend to accumulate at night time on the surface level. For example monoterpenes have the highest concentrations in the night-time in Hyytiälä (CitationSellegri et al. 2005; CitationRinne et al. 2005; CitationRuuskanen et al. 2009). The potential of different VOCs, including monoterpenes and sesquiterpenes, to form secondary organic aerosol have been demonstrated in numerous laboratory experiments, also using real plant emission from boreal forest tree species (CitationMentel et al. 2009). CitationEerdekens et al. (2009) showed that intense nocturnal bursts of particles in Hyytiälä were connected to elevated concentrations of monoterpenes and anthropogenic pollution.
Measuring the chemical composition of atmospheric clusters and nano-particles represents a challenge due to the small mass and relatively low concentration of the objects. Getting information about the composition of newly formed particles was long confined to indirect methods, like the CPC-battery (CitationKulmala et al. 2007b; CitationRiipinen et al. 2009) or studying the volatility (CitationWehner et al. 2005; CitationEhn et al. 2007b) and hygroscopicity (CitationSakurai et al. 2005; CitationEhn et al. 2007a) of particles. Developments in mass spectrometry are currently reforming our possibilities to study nucleation at the molecular level. CitationJunninen et al. (2010) introduced an APi-ToF (Atmospheric Pressure Interface Time-of-Flight Mass Spectrometer), which is capable of detecting the composition of ambient ions up to a mass to charge ratio of 2000 Th with high resolution and accuracy, and is thus well suited for nucleation studies, as shown by CitationEhn et al. (2010).
Clearly ion measurements alone are not sufficient to resolve the nucleation mechanism, since neutral nucleation seems to dominate under most environmental conditions. Our measurements of neutral nano-particles with a pulse-height CPC (CitationSipilä et al. 2009) can shed light on the nucleation mechanism and nocturnal nano-CN. We compare the results to ion spectrometer measurements and chemical compounds identified with the APi-TOF. Also measurements of nano-CN in a flow tube are presented as support. We aim at understanding the reasons behind the elevated night-time concentrations, and thus the possible sources and composition of the nano-CN. We believe that studying nocturnal events can give us insight to the critical steps of the new particle formation process.
2. MATERIAL AND METHODS
2.1. Field Measurements
The field measurements took place at the SMEAR II station (61°51’N, 24°17’E, 181 m ASL) in Hyytiälä, southern Finland. The station is equipped with comprehensive aerosol and meteorological instrumentation. The measurement site is surrounded by coniferous Scots pine–dominated forest. For detailed site description see CitationHari and Kulmala (2005). The data was collected during EUCAARI (CitationKulmala et al. 2009) spring campaign periods on 14 March–26 June 2007, 1–31 May 2008, and 28 April–26 May 2009.
The particle size-distribution from 3 to 1000 nm was measured with a twin-DMPS system (CitationAalto et al. 2001). For measuring the ion mobility distribution a Balanced Scanning Mobility Analyzer (BSMA, CitationTammet 2006) with a mobility range 0.032–3.2 cm2V−1s−1 (∼0.8–7.5 nm in mobility diameter) was deployed. The size distribution of neutral particles ∼1.3–5 nm was measured with a pulse height CPC (PH-CPC, discussed in Section 2.3).
The composition of natural ions was measured with an APi-TOF mass spectrometer. The APi-TOF consists of an atmospheric pressure interface (APi) coupled to a time-of-flight mass spectrometer (TOF). The function of the APi is to deliver the ions reaching the instrument inlet to the TOF as efficiently as possible while pumping away the gas molecules. The TOF then measures the mass/charge (m/Q) of the ions with high mass accuracy and resolution. For additional technical details, see CitationJunninen et al. (2010). The APi-TOF was deployed in Hyytiälä in spring 2009, and identified a large part of the small ions present (CitationEhn et al. 2010).
The concentrations of selected volatile organic compounds (VOCs) in ambient air were measured with a proton-transfer-reaction mass spectrometer (PTR-MS, Ionicon GmbH, Innsbruck, Austria). We used the data of monoterpenes (C10H16), which is a group of compounds with the molecular mass of 136 amu. Monoterpenes are highly reactive, and their main oxidizing compounds in the air are the hydroxyl radical (OH) and ozone (O3). The concentration of oxidized monoterpenes (MToxy ) was estimated using equation
2.2. Flow Tube Experiments
Experiments were carried out in the Leibniz Institute for Tropospheric Research laminar flow tube (IfT-LFT) with a total length of 5.05 m and an inner diameter of 8 cm. A detailed description of the system is given by CitationBerndt et al. (2006) and CitationSipilä et al. (2010). In our experiments we used highly purified air (99.9999999%, Linde 5.0, with further purification by GateKeeper CE-500KF-O-4R, AERONEX) as carrier gas. Water vapor was introduced in the chamber by flushing a stream of carrier gas through the saturator containing de-ionized (Milli-Q) water. Alkenes used in the experiments were premixed with a gas metering unit with synthetic air. O3 was generated by an ozone generator (UVP OG-2). The PH-CPC was connected at the end of the flow tube together with an ozone analyzer and a proton-transfer reaction mass spectrometer (PTR-MS) for measuring the decay of alkenes used. The reaction of the alkenes with O3 leads to the production of OH radicals, which can subsequently oxidize background SO2 producing sulphuric acid. Therefore, in some experiments we applied a chemical ionization mass spectrometer (CIMS, CitationEisele and Tanner 1991; CitationPetäjä et al. 2009) for monitoring the sulphuric acid formation.
Experiments were performed at RH = 22%, [O3] ≈ 25ppb and total residence time in the flow tube of 95 or 426 s. The studied alkenes include limonene, α-pinene, 1-methyl-1-cyclo-hexene, and tetra-methyl-ethylene with initial concentrations ranging from ∼1010 to ∼1012 molecules cm−3. Depending on the experiment a maximum of a few percent of the initial alkene-concentration reacted with ozone.
2.3. The Pulse-Height CPC
The pulse height analysis technique (CitationSaros et al. 1996) relies on detecting the intensity of light scattered by particles after their condensational growth in the CPC. Due to the supersaturation gradient inside the condenser, particles activate for growth at different axial positions depending on their size. The smaller the particle, the later it will be activated leading to smaller final droplet sizes. Clearly there is an upper size limit, after which only total concentration, but no size information of particles can be achieved, since all larger particles are activated practically simultaneously.
The PH-CPC comprises a TSI-3025A ultrafine CPC with modified optics (CitationDick et al. 2000) and a multichannel analyzer. For increasing the detection efficiency of small particles, the supersaturation inside the condenser was increased from nominal until homogenous nucleation of butanol appeared. The pulse height analysis technique allowed us to distinguish homogenous nucleation from activation of clusters and resolve the size distribution of particles below 5 nm. The homogenous nucleation level was also measured after each atmospheric measurement by filtering clusters and small particles away with a diffusion tube. Detailed description of the method and data inversion is published by CitationSipilä et al. (2009).
It should be noted that the activation probability of nano-particles inside a CPC depends on their composition (CitationO’Dowd et al. 2002a; CitationKulmala et al. 2007b) and charge (CitationWinkler et al. 2008). Therefore for example a butanol CPC will detect butanol-soluble particles at lower cut-off size than insoluble particles, and charged particles easier than neutral ones. The PH-CPC's size response and detection efficiency has been calibrated using positive insoluble ions (silver and 241-Am charger generated ions). Furthermore, the activation probability depends on the total particle concentration activated inside the condenser due to the vapor consumption of the growing droplets. The factors affecting the size and concentration response of the PH-CPC has been further discussed by CitationSipilä et al. (2009).
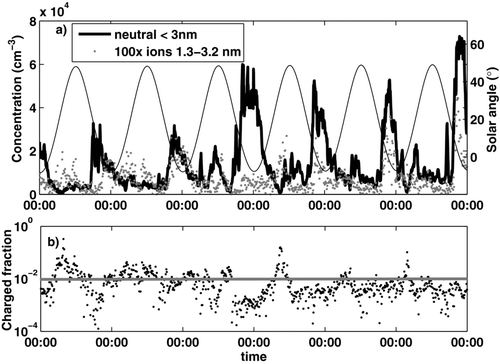
FIG. 1 Nocturnal particle formation in Hyytiälä, May 19–24, 2008. (a) Neutral nano-CN concentration (black line), intermediate (1.3–3.2 nm positive + negative) ion concentration multiplied by 100 (dots), and solar angle (thin line, right axis). (b) Charged fraction of 1–3 nm nano-CN (black dots), and its median value of the whole May 2008 as a horizontal line.
3. RESULTS
3.1. The Occurrence of Nano-CN in the Night-Time
In Hyytiälä the highest concentrations of neutral particles below 3 nm (referred to hereafter just as nano-CN) were regularly measured in the night-time during all our three measurement campaign periods in March–June 2007, May 2008, and May 2009. The overall concentration of nano-CN ranged from thousands to tens of thousands per cubic centimeter (CitationLehtipalo et al. 2009, 2010)—at least one order of magnitude higher than the concentration of small ions (CitationHirsikko et al. 2005)—but the night-time concentrations could occasionally reach more than 105 cm−3.
A distinct rise in the nano-CN concentration was observed on about two-thirds of nights within a few hours from sunset. presents a 6-day time series from May 2008 (19.–24.5.2008) where a nocturnal event took place every night starting around 18:00 local time. The elevated concentrations lasted for several hours, occasionally even for days. On most of these event nights, we also observed growth of the concentration and mean size of small ions. There were also nights with elevated neutral cluster concentrations, but little or no increase in ion clusters, or the opposite. Intensive bursts of ions were usually related to rain. Rain and waterfalls are known to produce large amounts of negative intermediate ions (CitationLaakso et al. 2007; CitationTammet et al. 2009). No clear sign preference was detected in either size or concentration response of the ions, except for the rain events when negative ions grew larger. Events related to rain were excluded from further analysis. summarizes the frequency of nocturnal events in the three measurement periods. A nocturnal maximum was observed on about 63% of nights in both neutral and ion data. CitationJunninen et al. (2008) observed small ion growth on ∼16% of nights from a 4-year ion spectrometer dataset in Hyytiälä; however, May was the peak month in the annual variation and had a much higher event frequency.
TABLE 1 Frequency of nocturnal events detected with the PH-CPC (neutral) and the BSMA (ions) in May. Events/all days, when data was available
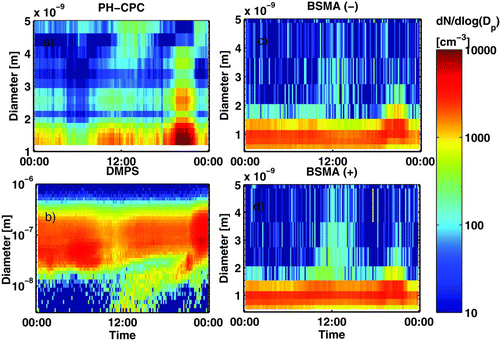
presents an example of a nocturnal nano-CN event measured with the PH-CPC, the DMPS, and the BSMA. There was a weak daytime new particle formation event starting after 8:00 in the smallest sizes, which was detected by the DMPS (cut-off size 3 nm) at 10:00. The newly formed particles grew by condensation during the day, but the nano-CN concentration stayed pretty stable until both the neutral and ion concentration started rising rapidly after 18:00. Also the DMPS detected small particles around 21:00, when the concentration of nano-CN peaked at ∼20 000 cm−3. The sunset in Hyytiälä was around 20:00. The nano-CN concentration dropped back to daytime values after 22:00, when pollution was advecting to the site (confirmed by NOx- and SO2- measurements, not shown). This can also be seen in the DMPS as higher concentration of accumulation mode particles, which scavenged smaller particles by coagulation.
FIG. 2 Particle size distributions measured with the (a) PH-CPC (1.3–5 nm, neutral), (b) DMPS (3–1000 nm, total), (c) BSMA (0.8–5 nm, negative ions), and (d) BSMA (0.8–5 nm, positive ions) in Hyytiälä, May 3, 2008.
The diurnal variation of the nano-CN concentration, mean mobility of small ions, and the charged fraction of nano-CN are presented in (median from May 2008). The median concentration of negative ions was on average somewhat smaller than positive ions, and the mean mobility higher signifying smaller size. However, the relative change in mobility and concentration during early evening was similar for negative and positive ions. The average ratio of ions to all observed nano-CN (ions + neutral) in the size range 1.3–3 nm was close to 1%, which is approximately the steady-state charge fraction of particles of this size (CitationHoppel and Frick 1986). The charged fraction was at its minimum during the nocturnal events. The maximum ion concentration was reached on average about an hour later than the maximum in neutral nano-CN concentration. The concentration of the neutral nano-CN usually dropped back to daytime values around midnight, whereas the elevated ion concentration lasted until the next sunrise and mixing of the boundary layer. The minimum in the charged fraction is an indication of a source of neutral nano-CN near ground, since if the rise in concentration would be solely due to lowering of the boundary layer, the ion cluster concentration would be expected to increase in the same proportion.
FIG. 3 Median diurnal variation of (a) neutral nano-CN (right axis) and small ions (left axis), (b) the mean mobility of the ions, and (c) charged fraction of the nano-CN in Hyytiälä in May 2008.
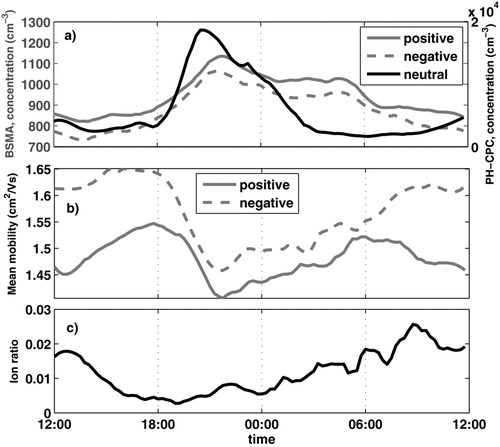
FIG. 4 Conditions favoring night-time nano-CN formation. Median diurnal variation in May 2008 separately for event (black line) and non-event nights (gray dashed line). Note the atypical time axis.
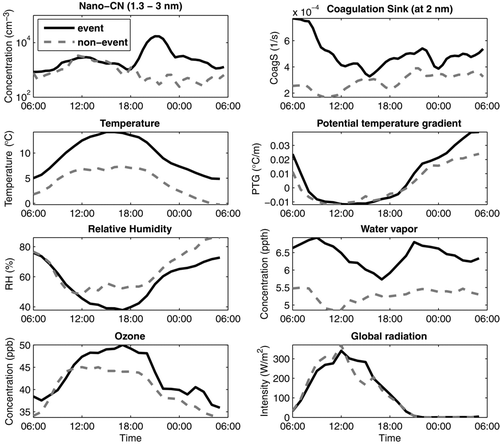
To understand what distinguishes the nights with and without a nocturnal event, the data set from May 2008 was divided into two, based on whether or not we saw a clear increase in the neutral nano-CN concentrations. The diurnal trend of several meteorological and atmospheric variables was inspected separately for the event and non-event cases (). We observed that the coagulation sink could not explain why some nights had higher nano-CN concentrations; in fact, the background aerosol population seemed to be larger on event-nights. However, the temperature was clearly higher on the event-nights, and there was slightly more ozone. Also, the water vapor concentration was higher, but the relative humidity was approximately the same or even a bit lower on the event than non-event nights. There was almost no difference in global radiation and, neither did the potential temperature gradient, as a measure of atmospheric stability, differ between the event and non-event nights. Also, no connection between daytime new particle formation events and the nocturnal events could be found in this study.
3.2. The Chemical Composition of Nano-CN
The quest for resolving the composition of the nano-CN was started by looking at the correlation of neutral nano-CN concentration with the concentration of oxidized organics and sulphuric acid.
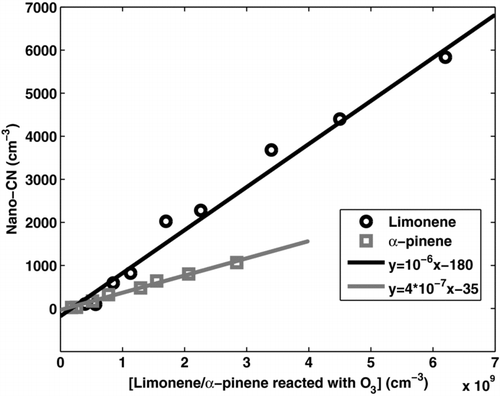
demonstrates that an almost similar nano-CN signal that is detected in field measurements can be produced in the flow tube experiments by oxidized organic vapors from limonene or α-pinene only. The initial concentration of alkenes affected only the concentration of the detected nano-CN, but the not their place on the channel axis (i.e., size). The average signal of the night-time field data is a bit broader towards higher channels, which means that there are also larger nano-CN present in the atmosphere than in the IfT-LFT experiments. The correlation between the nano-CN number concentration and the organic vapor (limonene or α-pinene) reacted with ozone is linear (), which indicates that the nano-CN in the chamber experiments are either single molecules or clusters involving only one molecule from the ozonolysis reaction instead of clusters with multiple organic molecules. However, the detection efficiency of these oxidized molecules with the PH-CPC is probably very low. Nano-CN formation was observed also in case of MCH (methyl-cyclo-hexene, C7H12) and TME (tetra-methyl-ethylene, C6H12) ozonolysis (not shown in ), but the effect was clearly weaker than observed for the terpenes.
FIG. 5 PH-CPC raw data at the flow tube experiments. Average count rate per channel number at different initial concentration of limonene. The vertical line represents the peak channel of the homogenous nucleation mode (pulse counts for [Limonene]initial = 0 cm−3 result from homogeneous nucleation). Typical night-time field data (average of 4 h, scaled to same channel axis) as thick black line. All particles larger than about 5 nm accumulate around channel 670.
![FIG. 5 PH-CPC raw data at the flow tube experiments. Average count rate per channel number at different initial concentration of limonene. The vertical line represents the peak channel of the homogenous nucleation mode (pulse counts for [Limonene]initial = 0 cm−3 result from homogeneous nucleation). Typical night-time field data (average of 4 h, scaled to same channel axis) as thick black line. All particles larger than about 5 nm accumulate around channel 670.](/cms/asset/8aa2ee42-3719-4ed6-94f6-c0b638486662/uast_a_547537_o_f0005g.gif)
FIG. 6 Nano-CN concentration detected by the PH-CPC at flow tube experiments with different concentrations of limonene and α-pinene reacted with ozone. Lines are linear fittings to the data.
In the flow tube experiments the signal from H2SO4 exceeded the CIMS detection limit of [H2SO4] = 5·104 cm−3 only in the case of highest initial alkene concentrations, and even then stayed below a few 105 cm−3. As could be expected, the sulphuric acid concentration was dependent on the initial alkene concentration, and thus, the contribution of sulphuric acid in the cluster formation together with the alkene oxidation products would yield a non-linear dependency of nano-CN concentration on the oxidized alkene concentration. As a linear dependency was observed, it can be concluded that in the IfT-LFT experiment sulphuric acid was not significantly participating in nano-CN formation.
Also the field data of nano-CN concentrations showed a good correlation with the calculated concentration of oxidized monoterpenes both in the daytime and in the night-time (R = 0.98 and 0.90, respectively, ). The best fittings to the data were obtained with slopes of 0.62 and 0.52 between the logarithms of the concentrations. However, only reactions with ozone were accounted for, and especially in daytime, the oxidation with OH radical should contribute to the oxidation products. The ozonolysis of monoterpenes is a yield of OH in the night-time, and also NO3 chemistry might play a role depending on the NO2 concentrations. However, it should be noted, that not all oxidation products contribute to the formation of nano-CN. No correlation was found between nano-CN and the sulphuric acid proxy (CitationPetäjä et al. 2009) calculated from measured SO2 and radiation.
FIG. 7 Correlation between the monoterpene oxidation products and the nano-CN concentration in Hyytiälä separately for daytime (06:00–18:00) and night-time (18:00–6:00) values. Lines are linear fittings to the data on a logarithmic scale.
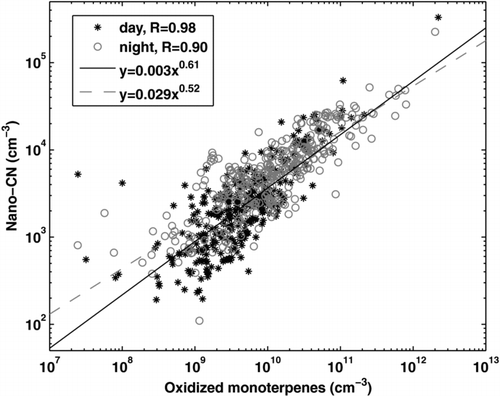
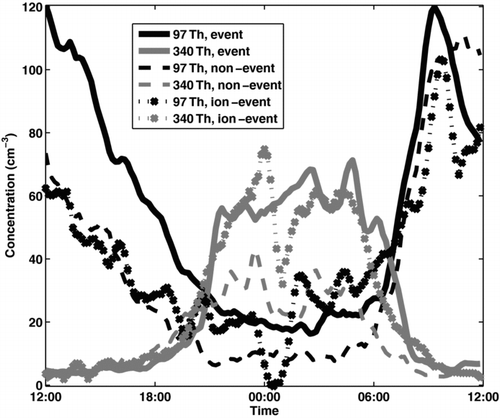
FIG. 8 Diurnal pattern of mass 97 Th (sulphuric acid monomer, HSO− 4) and mass 340 Th (possibly C10H14NO− 12, Ehn at al. 2010) separately on days with a nocturnal event (solid line), an ion event (dotted line with crosses), or no event (dashed line).
During early May 2009, when the PH-CPC and APi-TOF were measuring simultaneously in Hyytiälä, we recorded 5 days with no night-time event (“non-event”), 5 days with an event in the ion data but no clear increase in neutral nano-CN (“ion event”), and 4 days with a night-time event in both ion and neutral data (“event”). The ion spectra were compared to the nano-CN concentration measurements separately on these three cases. We focused the analysis on negative ions, as the positive did not show as large variations and the signal was spread out into a large amount of unidentified masses (CitationEhn et al. 2010).
CitationEhn et al. (2010) showed that whereas the daytime ions in Hyytiälä consisted of strong acids, like sulphuric and nitric acid, the nocturnal mass spectra of negative ions was dominated by a pattern of peaks around 280–420 Th, and similar but weaker pattern at 460–620 Th. The peaks were thought to consist of highly oxygenated organic molecules possibly containing 10 carbon and one nitrogen atom. We studied the diurnal variation of these oxidized organics, represented in by peak m/Q 340 Th (possibly C10H14NO12 −), and the sulphuric acid monomer (HSO4 −). As can be seen from , the concentration of m/Q 340 started rising after 18:00 on all cases, but remained clearly lower on the non-event nights compared to event or ion-event nights. The sulphuric acid monomer concentration, on the other hand, follows solar radiation and goes down towards the night, but the concentration seemed to be higher on the day preceding an event night compared to an ion-event or non-event night. Thus at the time when the night-time event started, there was more sulphuric acid left. This is in line with the observations of CitationJunninen et al. (2008), who noticed that the calculated median concentration of gaseous sulphuric acid was higher on nights with a nocturnal event, than on non-event nights.
We also calculated the correlation coefficient for the time series of each unit mass detected by the APi-TOF in the negative ion spectrum and the nano-CN concentration measured by PH-CPC or BSMA. The time series correlograms for neutral particles both in daytime and night-time, and for comparison also for negative ions in night-time are presented in for each unit mass. The identification of the compounds was, however, based on the exact masses of the detected peaks. The daytime neutral nano-CN correlated best with sulphur-containing ions, i.e., sulphuric acid monomer HSO− 4 (m/Q 97 Th), dimer (H2SO4)HSO− 4 (m/Q 195 Th) and trimer (H2SO4)2HSO− 4 (m/Q 293 Th), and SO− 5 (m/Q 112 Th). Also mass 259 Th stands out from the correlogram event though its absolute concentration was low. According to CitationEhn et al. (2010) it could be a cluster of malonic acid (C3H4O4) and C2H3SO− 6 or a cluster of sulphuric acid monomer and C5H6O6.
FIG. 9 Time series correlogram of the negative masses detected by the APi-ToF and the concentration of nano-CN measured by PH-CPC (neutral) or BSMA (ions) April 29–May 26, 2009. Daytime was defined as 06:00–18:00, and night-time 18:00–6:00.
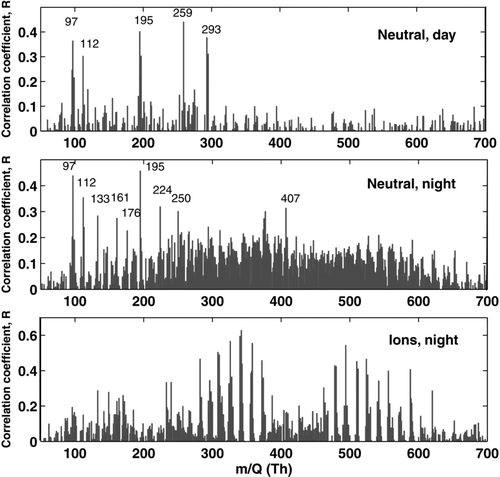
The night-time correlograms for ions and the neutral nano-CN were remarkably different. The negative ion concentration measured with the BSMA correlated, as expected, quite well with the peaks of oxidized organics that dominated the API-TOF signal in the night-time. The same peaks did not, however, stick out in the correlogram with neutral nano-CN. The neutral night-time correlogram showed the same sulphur containing ions as in daytime, plus a few other masses like m/Q 133 Th (malic acid, C4H5O5 −), m/Q161 (C5H5O6 −, possibly a tricarboxylic acid), m/Q 224 (cluster of m/Q 161 and nitric acid) and yet unidentified peaks at m/Q 176 Th, m/Q 250 Th, and m/Q 407 Th. The identified organic compounds are some of the most oxidized compounds observed with the APi-TOF.
4. DISCUSSION AND CONCLUSIONS
The development of instrumentation—both high-resolution DMAs, condensation particle counters with low cut-of-sizes and mass spectrometers—have brought aerosol measurements down to molecular sizes, where it is not always possible to distinguish between particles, clusters or large molecules. Only parallel measurements with different independent methods can lead us to understand atmospheric nucleation. At the molecular limit, the particles cannot be considered just as physical objects, but the chemical composition plays an important role. Our measurements prove that CPCs can detect molecules of oxidized VOCs, which are omnipresent inside the forest canopy. When developing CPCs and interpreting the data, this should be taken into account.
There is currently no direct way of getting information on the chemical composition of neutral nano-CN without charging them. Due to differences in their proton affinities, some compounds become charged more easily than others. This leads to a bias if interpreting the ion spectrum to represent also the ambient neutral composition.
We have shown that the nano-CN signal in the PH-CPC can be produced in a flow tube by oxidizing organic molecules (limonene or α-pinene), and that the nano-CN concentration in the field correlates well with the calculated concentration of monoterpene oxidation products. Also, the naturally charged negative ions in Hyytiälä consist mostly of organic molecules in the night-time. On about 60% of nights, we observed a sudden increase in nano-CN concentrations, a night-time event. On average, the temperature was higher on events nights than non-event nights, and there was slightly more ozone. However, the peak in nocturnal event frequency in Hyytiälä is in May (CitationJunninen et al. 2008), whereas the highest concentration of monoterpenes is observed later in summer (CitationRuuskanen et al. 2009).
During nights with high concentrations of neutral nano-CN, we observed larger concentrations of the sulphuric acid monomer and dimer in the ion spectrum than on other nights, and the neutral nano-CN correlated better with the sulphuric acid clusters than any other single compounds measured with the APi-TOF. Also CitationJunninen et al. (2008) showed that event-nights tended to have higher concentrations of sulphuric acid. This could be due to the fact, that the reaction between ozone and VOCs produces OH, which in turn reacts with SO2 producing sulphuric acid.
The results show that nano-CN are readily formed during night-time, even when no new particle formation is observed. It might be that nucleation and cluster formation happens also during night-time, but the nano-CN grow too slowly to reach sizes where they would be detected by traditional instrumentation. The reason could be that the production of condensing vapors, which are needed for particle growth, requires photochemical reactions. CitationNieminen et al. (2009) detected a threshold value for sulphuric acid concentration, 3·105 cm−3, above which most of the daytime events in Hyytiälä occurred. The start of an event is also related to boundary layer mixing and thus to sunlight (CitationNilsson et al. 2001). On the other hand, even though the nano-CN can be activated in supersaturated butanol vapor inside the PH-CPC, there is yet no evidence that they participate in the atmospheric nucleation process.
Acknowledgments
This work has been partially funded by European Commission 6th Framework programme project EUCAARI, contract no 036833-2 (EUCAARI) and Academy of Finland Center of Excellence program (project number 1118615). K.L. acknowledges Maj and Tor Nessling Foundation for financial support. K. Pielok is acknowledged for technical assistance.
REFERENCES
- Aalto , P. , Hämeri , K. , Becker , E. , Weber , R. , Salm , J. , Mäkelä , J. M. , Hoell , C. , O’Dowd , C. D. , Karlsson , H. , Hansson , H.-C. , Väkevä , M. , Koponen , I. K. , Buzorius , G. and Kulmala , M. 2001 . Physical Characterization of Aerosol Particles in Boreal Forests . Tellus. , 53B : 344 – 358 .
- Asher , W. E. , Pankow , J. F. , Erdakos , G. B. and Seinfeld , J. H. 2002 . Estimating the Vapor Pressures of Multi-Functional Oxygen-Containing Organic Compounds Using Group Contribution Methods . Atmos. Environ. , 36 : 1483 – 1498 .
- Berndt , T. , Böge , O. and Stratmann , F. 2006 . Formation of Atmospheric H2SO4/H2O Particles in the Absence of Organics: A Laboratory Study . Geophys. Res. Lett. , 33 : L15817 doi: 10.1029/2006GL026660
- Bonn , B. , Kulmala , M. , Riipinen , I. , Sihto , S.-L. and Ruuskanen , T. 2008 . How Biogenic Terpenes Govern the Correlation between Sulphuric Acid Concentrations and New Particle Formation . J. Geophys. Res. , 113 : D12209 doi: 10.1029/2007JD009327
- Boy , M. , Kulmala , M. , Ruuskanen , T. M. , Pihlatie , M. , Reissell , A. , Aalto , P. P. , Keronen , P. , Dal Maso , M. , Hellen , H. , Hakola , H. , Janson , R. , Hanke , M. and Arnold , F. 2005 . Sulphuric Acid Closure and Contribution to Nucleation Mode Particle Growth . Atmos. Chem. Phys. , 5 : 863 – 878 .
- Dick , W. D. , McMurry , P. H. , Weber , R. J. and Quant , R. 2000 . White-Light Detection for Nanoparticle Sizing with the TSI Ultrafine Condensation Particle Counter . J. Nanoparticle Res. , 2 : 85 – 90 .
- Eerdekens , G. , Yassaa , N. , Sinha , V. , Aalto , P. P. , Aufmhoff , H. , Arnold , F. , Fiedler , V. , Kulmala , M. and Williams , J. 2009 . VOC Measurements within a Boreal Forest during Spring 2005: On the Occurrence of Elevated Monoterpenes Concentrations during Night Time Intense Particle Concentration Events . Atmos. Chem. Phys. , 9 : 8331 – 8350 .
- Ehn , M. , Petaja , T. , Aufmhoff , H. , Aalto , P. , Hameri , K. , Arnold , F. , Laaksonen , A. and Kulmala , M. 2007a . Hygroscopic Properties of Ultrafine Aerosol Particles in the Boreal Forest: Diurnal Variation, Solubility and the Influence of Sulfuric Acid . Atmos. Chem. Phys. , 7 : 211 – 222 .
- Ehn , M. , Petaja , T. , Birmili , W. , Junninen , H. , Aalto , P. and Kulmala , M. 2007b . Non-Volatile Residuals of Newly Formed Atmospheric Particles in the Boreal Forest . Atmos. Chem. Phys. , 7 : 677 – 684 .
- Ehn , M. , Junninen , H. , Petäjä , T. , Kurtén , T. , Kerminen , V.-M. , Schobesberger , S. , Manninen , H. E. , Ortega , I. K. , Vehkamäki , H. , Kulmala , M. and Worsnop , D. R. 2010 . Composition and Temporal Behavior of Ambient Ions in the Boreal Forest . Atmos. Chem. Phys. Discuss , 10 : 8513 – 8530 .
- Eisele , F. L. and Tanner , D. J. 1991 . Ion-Assisted Troposheric OH Measurements . J. Geophys. Res. , 96 ( D5 ) : 9295 – 9308 . doi: 10.1029/91JD00198
- Fiedler , V. , Dal Maso , M. , Boy , M. , Aufmhoff , H. , Hoffmann , J. , Schuck , T. , Birmili , W. , Hanke , M. , Uecker , J. , Arnold , F. and Kulmala , M. 2005 . The Contribution of Sulphuric Acid to Atmospheric Particle Formation and Growth: A Comparison between Boundary Layers in Northern and Central Europe . Atmos. Chem. Phys. , 5 : 1773 – 1785 .
- Gagné , S. , Laakso , L. , Petäjä , T. , Kerminen , V.-M. and Kulmala , M. 2008 . Analysis on One Year of Ion-DMPS Data from the SMEAR II Station, Hyytiälä . Tellus. , 60B : 318 – 329 .
- Hakola , H. , Tarvainen , V. , Laurila , T. , Hiltunen , V. , Hellén , H. and Keronen , P. 2003 . Seasonal Variation of VOC Concentrations above a Boreal Coniferous Forest . Atmos. Environ. , 12 : 1623 – 1634 .
- Hanson , D. R. and Eisele , F. L. 2002 . Measurement of Prenucleation Molecular Clusters in the NH3, H2SO4, H2O System . J. Geophys. Res. , 107 ( D12 ) : 4158 doi: 10.1029/2001JD001100
- Hari , P. and Kulmala , M. 2005 . Station for Measuring Ecosystem-Atmosphere Relations (SMEAR II) . Boreal Environ. Res. , 10 : 315 – 322 .
- Hirsikko , A. , Laakso , L. , Hõrrak , U. , Aalto , P. P. , Kerminen , V.-M. and Kulmala , M. 2005 . Annual and Size Dependent Variation of Growth Rates and Ion Concentrations in Boreal Forest . Boreal Environ. Res. , 10 : 357 – 369 .
- Hoppel , W. A. and Frick , G. M. 1986 . Ion Attachment Coefficients and the Steady State Charge Distribution on Aerosols in a Bipolar Ion Environment . Aerosol Sci. Technol. , 5 : 1 – 21 .
- Hõrrak , U. , Salm , J. and Tammet , H. 1998 . Bursts of Intermediate Ions in Atmospheric Air . J. Geophys. Res. , 103 : 13909 – 13915 .
- Iida , K. , Stolzenburg , M. , McMurry , P. , Dunn , M. J. , Smith , J. N. , Eisele , F. and Keady , P. 2006 . Contribution of Ion-Induced Nucleation to New Particle Formation: Methodology and Its Application to Atmospheric Observations in Boulder, Colorado . J. Geophys. Res. , 111 : D23201 doi: 1029/2006JD007167
- Junninen , H. , Ehn , M. , Petäjä , T. , Luosujärvi , L. , Kotiaho , T. , Kostiainen , R. , Rohner , U. , Gonin , M. , Fuhrer , K. , Kulmala , M. and Worsnop , D. R. 2010 . A High-Resolution Mass Spectrometer to Measure Atmospheric Ion Composition . Atmos. Meas. Tech. Discuss. , 3 : 1039 – 1053 .
- Junninen , H. , Hulkkonen , M. , Riipinen , I. , Nieminen , T. , Hirsikko , A. , Suni , T. , Boy , M. , Lee , S.-H. , Vana , M. , Tammet , H. , Kerminen , V.-M. and Kulmala , M. 2008 . Observations on Nocturnal Growth of Atmospheric Clusters . Tellus. , 60B : 365 – 371 .
- Kerminen , V.-M. , Pirjola , L. and Kulmala , M. 2001 . How Significantly Does Coagulational Scavenging Limit Atmospheric Particle Production? . J. Geophys. Res. , 106 ( 24 ) : 119 – 126 .
- Kuang , C. , McMurry , P. H. , McCormick , A. V. and Eisele , F. L. 2008 . Dependence of Nucleation Rates on Sulfuric Acid Vapor Concentration in Diverse Atmospheric Locations . J. Geophys. Res. , 113 : D10209 doi: 10.1029/2007JD009253
- Kulmala , M. , Toivonen , A. , Mäkelä , J. and Laaksonen , A. 1998 . Analysis of the Growth of Nucleation Mode Particles Observed in Boreal Forest . Tellus. , 50B : 449 – 462 .
- Kulmala , M. , Pirjola , L. and Mäkelä , J. M. 2000 . Stable Sulphate Clusters as a Source of New Atmospheric Particles . Nature. , 404 : 66 – 69 .
- Kulmala , M. 2003 . How Particles Nucleate and Grow? . Science. , 302 : 1000 – 1001 .
- Kulmala , M. , Vehkamäki , H. , Petäjä , T. , Dal Maso , M. , Lauri , A. , Kerminen , V.-M. , Birmili , W. and McMurry , P. H. 2004a . Formation and Growth Rates of Ultrafine Atmospheric Particles: A Review of Observations . J. Aerosol Sci. , 35 : 143 – 176 .
- Kulmala , M. , Kerminen , V.-M. , Anttila , T. , Laaksonen , A. and O’Dowd , C. D. 2004b . Organic Aerosol Formation via Sulphate Cluster Activation . J. Geophys. Res. , 109 : D04205 doi: 10.1029/2003JD003961
- Kulmala , M. , Lehtinen , K. E. J. and Laaksonen , A. 2006 . Cluster Activation Theory as an Explanation of the Linear Dependence between Formation Rate of 3 nm Particles and Sulphuric Acid Concentration . Atmos. Chem. Phys , 6 : 787 – 793 .
- Kulmala , M. , Riipinen , I. , Sipilä , M. , Manninen , H. , Petäjä , T. , Junninen , H. , Dal Maso , M. , Mordas , G. , Mirme , A. , Vana , M. , Hirsikko , A. , Laakso , L. , Harrison , R. M. , Hanson , I. , Leung , C. , Lehtinen , K. E. J. and Kerminen , V.-M. 2007a . Towards Direct Measurement of Atmospheric Nucleation . Science. , 318 : 89 – 92 .
- Kulmala , M. , Mordas , G. , Petäjä , T. , Grönholm , T. , Aalto , P. P. , Vehkamäki , H. , Hienola , A. I. , Herrmann , E. , Sipilä , M. , Riipinen , I. , Manninen , H. E. , Hämeri , K. , Stratmann , F. , Bilde , M. , Winkler , P. M. , Birmili , W. and Wagner , P. E. 2007b . The Condensation Particle Counter Battery (CPCB): A New Tool to Investigate the Activation Properties of Nanoparticles . J. Aerosol Sci. , 38 : 289 – 304 .
- Kulmala , M. , Asmi , A. , Lappalainen , H. K. , Carslaw , K. S. , Pöschl , U. , Baltensperger , U. , Hov , Ø. , Benquier , J.-L. , Pandis , S. N. , Facchini , M. C. , Hansson , H.-C. , Wiedensohler , A. and O’Dowd , C. D. 2009 . Introduction: European Integrated Project on Aerosol Cloud Climate and Air Quality interactions (EUCAARI)—Integrating Aerosol Research from Nano to Global Scales . Atmos. Chem. Phys. , 9 : 2825 – 2841 .
- Kurtén , T. , Loukonen , V. , Vehkamäki , H. and Kulmala , M. 2008 . Amines are Likely to Enhance Neutral and Ion-Induced Sulfuric Acid-Water Nucleation in the Atmosphere More Effectively than Ammonia. . Atmos. Chem. Phys. , 8 : 4095 – 4103 .
- Laakso , L. , Anttila , T. , Lehtinen , K. E. J. , Aalto , P. P. , Kulmala , M. , Hõrrak , U. , Tammet , H. and Joutsensaari , J. 2004 . Kinetic Nucleation and Ions in Boreal Particle Formation Events . Atmos. Chem. Phys. , 4 : 2353 – 2366 .
- Laakso , L. , Hirsikko , A. , Grönholm , T. , Kulmala , M. , Luts , A. and Parts , T.-E. 2000 . Water Falls as Sources of Small Charged Aerosol Particles . Atmospheric Chemistry and Physics , 7 : 2271 – 2275 .
- Lee , S.-H. , Young , L.-H. , Benson , D. R. , Suni , T. , Kulmala , M. , Junninen , H. , Campos , T. L. , Rogers , D. C. and Jensen , J. 2008 . Observations of Night-Time New Particle Formation in the Troposphere . J. Geophys. Res , 113 : D10210 doi: 10.1029/2007JD009351
- Lehtipalo , K. , Sipilä , M. , Riipinen , I. , Nieminen , T. and Kulmala , M. 2009 . Analysis of Atmospheric Neutral and Charged Molecular Clusters in Boreal Forest Using Pulse-Height CPC . Atmos. Chem. Phys. , 9 : 4177 – 4184 .
- Lehtipalo , K. , Kulmala , M. , Sipilä , M. , Petäjä , T. , Vana , M. , Ceburnis , D. , Dupuy , R. and O’Dowd , C. 2010 . Nanoparticles in Boreal Forest and Coastal Environment: A Comparison of Observations and Implications of the Nucleation Mechanism . Atmos. Chem. Phys. , 10 : 7009 – 7016 . doi: 10.5194/acp-10-7009-2010
- McMurry , P. H. 1980 . Photochemical Aerosol Formation from SO2: A Theoretical-Analysis of Smog Chamber Data . J. Colloid Interface Sci. , 78 : 513 – 527 . doi: 10.1016/0021-9797(80)90589-5
- Mentel , Th. F. , Wildt , J. , Kiendler-Scharr , A. , Kleist , E. , Tillmann , R. , Dal Maso , M. , Fisseha , R. , Hohaus , Th. , Spahn , H. , Uerlings , R. , Wegener , R. , Griffiths , P. T. , Dinar , E. , Rudich , Y. and Wahner , A. 2009 . Photochemical Production of Aerosols from Real Plant Emissions . Atmos. Chem. Phys. , 9 : 4387 – 4406 .
- Metzger , A. , Verheggen , B. , Dommen , J. , Duplissy , J. , Prevot , A. S. , Weingartner , E. , Riipinen , I. , Kulmala , M. , Spracklen , D. V. , Carslaw , K. S. and Baltensperger , U. 2010 . Evidence for the Role of Organics in Aerosol Particle Formation under Atmospheric Conditions . Proc. Natl. Acad. Sci. USA. , 107 : 6646 – 6651 .
- Nieminen , T. , Manninen , H. , Sihto , S.-L. , Yli-Juuti , T. , Mauldin , R. L. , Petäjä , T. , Riipinen , I. , Kerminen , V.-M. and Kulmala , M. 2009 . Connection of Sulphuric Acid to Atmospheric Nucleation in Boreal Forest . Environ. Sci. Technol. , 43 : 4715 – 4721 .
- Nilsson , E. D. , Rannik , Ü. , Kulmala , M. , Buzorius , G. and O’Dowd , C. D. 2001 . Effects on Continental Boundary Layer Evolution, Convection, Turbulence, and Entrainment on Aerosol Formation . Tellus. , 53B : 441 – 461 .
- O’Dowd , C. D. , Aalto , P. , Hämeri , K. , Kulmala , M. and Hoffmann , T. 2002a . Atmospheric Particles from Organic Vapours . Nature. , 416 : 497 – 498 .
- O’Dowd , C. D. , Jimenez , J. L. , Bahreini , R. , Flagan , R. C. , Seinfeld , J. H. , Hämeri , K. , Pirjola , L. , Kulmala , M. , Jennings , S. G. and Hoffmann , T. 2002b . Marine Aerosol Formation from Biogenic Iodine Emissions . Nature. , 417 : 632 – 636 .
- Petäjä , T. , Mauldin , R. L. III , Kosciuch , E. , McGrath , J. , Nieminen , T. , Paasonen , P. , Boy , M. , Adamov , A. , Kotiaho , T. and Kulmala , M. 2009 . Sulfuric Acid and OH Concentrations in a Boreal Forest Site . Atmos. Chem. Phys. , 9 : 7435 – 7448 .
- Riipinen , I. , Manninen , H. , Yli-Juuti , T. , Boy , M. , Sipilä , M. , Ehn , M. , Junninen , H. , Petäjä , T. and Kulmala , M. 2009 . Applying the Condensation Particle Counter Battery (CPCB) to Study the Water-Affinity of Freshly-Formed 2–9nm Particles in Boreal Forest . Atmos. Chem. Phys. , 9 : 3317 – 3330 .
- Rinne , J. , Ruuskanen , T. M. , Reissell , A. , Taipale , R. , Hakola , H. and Kulmala , M. 2005 . On-line PTR-MS Measurements of Atmospheric Concentrations of Volatile Organic Compounds in a European Boreal Forest Ecosystem . Boreal Environ. Res. , 10 : 425 – 436 .
- Ruuskanen , T. M. , Taipale , R. , Rinne , J. , Kajos , M. K. , Hakola , H. and Kulmala , M. 2009 . Quantitative Long-Term Measurements of VOC Concentrations by PTR-MS: Annual Cycle of Ambient Concentrations at a Boreal Forest Site . Atmos. Chem. Phys. Discuss , 9 : 81 – 134 .
- Sakurai , H. , Fink , M. A. , McMurry , P. H. , Mauldin , L. , Moore , K. F. , Smith , J. N. and Eisele , F. L. 2005 . Hygroscopicity and Volatility of 4–10 nm Particles during Summertime Atmospheric Nucleation Events in Urban Atlanta . J. Geophys. Res. , 110 : D22S04 doi: 10.1029/2005JD005918
- Saros , M. , Weber , R. J. , Marti , J. and McMurry , P. H. 1996 . Ultrafine Aerosol Measurement Using a Condensation Nucleus Counter with Pulse Height Analysis . Aerosol Sci. Technol , 25 : 200 – 213 .
- Sellegri , K. , Hanke , M. , Umann , B. , Arnold , F. and Kulmala , M. 2005 . Measurements of Organic Gases during Aerosol Formation Events in the Boreal Forest Atmosphere during QUEST . Atmos. Chem. Phys , 5 : 373 – 384 .
- Sihto , S.-L. , Kulmala , M. , Kerminen , V.-M. , Dal Maso , M. , Petäjä , T. , Riipinen , I. , Korhonen , H. , Arnold , F. , Janson , R. , Boy , M. , Laaksonen , A. and Lehtinen , K. E. J. 2006 . Atmospheric Sulphuric Acid and Aerosol Formation: Implications from Atmospheric Measurements for Nucleation and Early Growth Mechanisms . Atmos. Chem. Phys. , 6 : 4079 – 4091 .
- Sipilä , M. , Lehtipalo , K. , Kulmala , M. , Petäjä , T. , Junninen , H. , Aalto , P. P. , Manninen , H. E. , Vartiainen , E. , Riipinen , I. , Kyrö , E.-M. , Curtius , J. , Kürten , A. , Borrmann , S. and O’Dowd , C. D. 2008 . Applicability of Condensation Particle Counters to Measure Atmospheric Clusters . Atmos. Chem. Phys. , 8 : 4049 – 4060 .
- Sipilä , M. , Lehtipalo , K. , Attoui , M. , Neitola , K. , Petäjä , T. , Aalto , P. P. , O’Dowd , C. D. and Kulmala , M. 2009 . Laboratory Verification of PH-CPC's Ability to Monitor Atmospheric Sub-3nm Clusters . Aerosol Sci. Technol. , 43 : 126 – 135 .
- Sipilä , M. , Berndt , T. , Petäjä , T. , Brus , D. , Vanhanen , J. , Stratmann , F. , Patokoski , J. , Mauldin , R. L. III , Hyvärinen , A.-P. , Lihavainen , H. and Kulmala , M. 2010 . The Role of Sulfuric Acid in Atmospheric Nucleation . Science. , 327 : 1243 – 1246 .
- Suni , T. , Kulmala , M. , Hirsikko , A. , Bergman , T. , Laakso , L. , Aalto , P. P. , Leuning , R. , Cleugh , H. , Zegelin , S. , Hughes , D. , van Gorsel , E. , Kitchen , M. , Vana , M. , Hõrrak , U. , Mirme , S. , Mirme , A. , Sevanto , S. , Twining , J. and Tadros , C. 2008 . Formation and Characteristics of Ions and Charged Aerosol Particles in a Native Australian Eucalypt Forest . Atmos. Chem. Phys , 8 : 129 – 139 .
- Tammet , H. , Hõrrak , U. and Kulmala , M. 2009 . Negatively Charged Nanoparticles Produced by Splashing of Water . Atmos. Chem. Phys. , 9 : 357 – 367 .
- Tammet , H. 2006 . Continuous Scanning of the Mobility and Size Distribution of Charged Clusters and Nanometer Particles in Atmospheric Air and the Balanced Scanning Mobility Analyzer BSMA . Atmos. Res. , 82 : 523 – 535 .
- Torpo , L. , Kurtén , T. , Vehkamäki , H. , Laasonen , K. , Sundberg , M. R. and Kulmala , M. 2007 . Significance of Ammonia in Atmospheric Nanoclusters . J. Phys. Chem. A. , 111 : 10671 – 10674 .
- Turco , R. P. , Zhao , J. X. and Yu , F. 1998 . A New Source of Tropospheric Aerosols: Ion-Ion Recombination . Geophys. Res. Lett. , 25 : 635 – 638 .
- Vehkamäki , H. , Dal Maso , M. , Hussein , T. , Flanagan , R. , Hyvärinen , A. , Lauros , J. , Merikanto , J. , Mänkkönen , P. , Pihlatie , M. , Salminen , K. , Sogacheva , L. , Thum , T. , Ruuskanen , T. M. , Keronen , P. , Aalto , P. P. , Hari , P. , Lehtinen , K. E. J. , Rannik , Ü. and Kulmala , M. 2004a . Atmospheric Particle Formation Events at Värriö Measurement Station in Finnish Lapland 1998–2002 . Atmos. Chem. Phys. , 4 : 2015 – 2023 .
- Vehkamäki , H. , Napari , I. , Kulmala , M. and Noppel , M. 2004b . Stable Ammonium Bisulphate Clusters in the Atmosphere . Phys. Rev. Lett. , 93 : 148501 – 148504 . doi: 10.1103/PhysRevLett.93.148501
- Weber , R. J. , Marti , J. J. and McMurry , P. H. 1996 . Measured Atmospheric New Particle Formation Rates: Implications for Nucleation Mechanisms . Chem. Eng. Comm. , 151 : 53 – 64 .
- Weber , R. J. , Marti , J. J. , McMurry , P. H. , Eisele , F. L. , Tanner , D. J. and Jefferson , A. 1997 . Measurements of New Particle Formation and Ultrafine Particle Growth Rates at a Clear Continental Site . J. Geophys. Res. , 102 : 4375 – 4385 .
- Weber , R. J. , McMurry , P. H. , Eisele , F. L. and Tanner , D. J. 1995 . Measurement of Expected Nucleation Precursor Species and 3–500-nm Diameter Particles at Mauna Loa Observatory, Hawaii . J. Atmos. Sci. , 52 : 2242 – 2257 .
- Wehner , B. , Petaja , T. , Boy , M. , Engler , C. , Birmili , W. , Tuch , T. , Wiedensohler , A. and Kulmala , M. 2005 . The Contribution of Sulfuric Acid and Non-Volatile Compounds on the Growth of Freshly Formed Atmospheric Aerosols . Geophys. Res. Lett. , 32 : L17810 doi: 10.1029/2005GL023827
- Wiedensohler , A. , Hansson , H.-C. , Orsini , D. , Wendisch , M. , Wagner , F. , Bower , K. N. , Chourlarton , T. W. , Wells , M. , Parkin , M. , Acker , K. , Wieprecht , W. , Facchini , M. C. , Lind , J. A. , Fuzzi , S. , Arends , B. G. and Kulmala , M. 1997 . Night-Time Formation and Occurrence of New Particles Associated with Orographic Clouds . Atmos. Environ. , 16 : 2545 – 2559 .
- Winkler , P. M. , Steiner , G. , Vrtala , A. , Vehkamäki , H. , Noppel , M. , Lehtinen , K. E. J. , Reischl , G. P. , Wagner , P. E. and Kulmala , M. 2008 . Heterogeneous Nucleation Experiments Bridging Scale from Molecular Ion Clusters to Nanoparticles . Science. , 319 : 1374 – 1377 .
- Yu , F. and Turco , R. P. 2000 . Ultrafine Aerosol Formation via Ion-Mediated Nucleation . Geophys. Res. Lett. , 27 : 883 – 886 .
- Yu , F. and Turco , R. P. 2008 . Case Studies of Particle Formation Events Observed in Boreal Forests: Implications for Nucleation Mechanisms . Atmos. Chem. Phys. , 8 : 6085 – 6102 .
- Yu , F. , Wang , Z. , Luo , G. and Turco , R. P. 2008 . Ion-Mediated Nucleation as an Important Source of Tropospheric Aerosols . Atmos. Chem. Phys. , 8 : 2537 – 2554 .
- Zhao , J. , Eisele , F. L. , Titcombe , M. , Kuang , C. G. and McMurry , P. H. 2010 . Chemical Ionization Mass Spectrometric Measurements of Atmospheric Neutral Clusters Using the Cluster-CIMS . J. Geophys. Res. , 115 : D08205 doi: 10.1029/2009JD012606