Abstract
Relative humidity (RH) affects the liquid water content of an aerosol, altering its scattering and absorption of visible light, which is important for aerosol effects on visibility and climate. Particle light extinction, light scattering, and light absorption coefficient values are reported here for laboratory-generated inorganic and organic carbon (OC) aerosols at RH values between 8% and 95%. Light scattering was measured with a nephelometer, light extinction was measured with an extinction cell, and light absorption was determined on the basis of the difference between those two values at three visible wavelengths (467, 530, and 660 nm). The instrumentation was benchmarked with nonabsorbing ammonium sulfate, absorbing polystyrene microspheres (PSMs) and nigrosin aerosol under controlled RH conditions. Agreement between dry measured scattering and extinction coefficients for ammonium sulfate was achieved within 3%. Optical closure with modeled scattering values based on measured ammonium sulfate particle size distributions was achieved within 7%. Measured single scattering albedo for dry absorbing PSMs agreed within 0.02 with the literature value. Light absorption by nigrosin increased by a factor of 1.24 ± 0.06 at all wavelengths as RH increased from 38% to 95%. Light absorption of OC aerosol that was generated from wood pyrolysis demonstrated enhancements of 2.2 ± 0.7 and 2.7 ± 1.2 between 32% and 95% RH at the wavelengths of 467 and 530 nm, but no absorption was detected at 660 nm. A spectral dependence of light absorption by OC was observed with absorption increasing from 530 nm towards the 467 nm wavelength, consistent with previously reported ex situ measurements of filter extracts. The increase in OC light absorption with RH is currently not represented in radiative transfer models even though biomass burning produces most of the primary OC aerosol in the atmosphere.
Copyright 2012 American Association for Aerosol Research
1. INTRODUCTION
The quantification of aerosol optical properties is critical for the understanding of the role of aerosols in visibility (Watson Citation2002) and in the earth's radiative energy balance. Aerosol perturbation of the radiative balance may result in warming or cooling of the earth system, and this is dependent on the balance between absorption and scattering of light. Relative humidity (RH) plays an important role in the direct radiative forcing of aerosols (Ramaswamy and Kiehl Citation1985; Haywood et al. Citation1997) because elevated RH conditions can cause aerosol particles to uptake water, altering their size and composition and thus their interaction with light (Orr et al. Citation1958).
TABLE 1 Aerosol types, aerosol generation method, and purpose for tests
Many field studies have examined and observed an increase in light scattering of aerosols with increasing RH conditions. These include but are not limited to Covert et al. (Citation1970), Carrico et al. (Citation1998), and Zieger et al. (Citation2010). Detailed laboratory studies have also been conducted to analyze and quantify changes of scattering with RH for inorganic aerosols (Tang and Munkelwitz Citation1994) or organic/inorganic mixtures (Hansson et al. Citation1998). Although radiative transfer models parameterize the changes in scattering with RH according to these studies (Boucher and Anderson Citation1995; Ghan and Zaveri Citation2007), they frequently assume that RH has no effect on aerosol light absorption. This dependence has been less frequently investigated; a decrease in the light absorption coefficient for RH values increasing above 70% was observed in ambient measurements with a photoacoustic spectrometer (Arnott et al. Citation2003) but this could have been caused by instrument artifacts, as discussed below. A decreasing absorption coefficient upon humidification was observed for wood smoke aerosols (Lewis et al. Citation2009) and was partly attributed to a morphological change in the aerosol upon humidification. Morphological change has been found to affect optical properties of soot particles coated with sulfuric acid (Zhang et al. Citation2008), but in the range of 10%–80% RH, an overall absorption cross-section increase of 1.5 times was observed. Increased light absorption by a factor of up to 3.5 in comparison to dry conditions was observed for hydrophilic soot water agglomerates under saturated water vapor conditions (Mikhailov et al. Citation2006). An enhancement in absorption by mineral dust of up to 1.5 times the initial value at 80% RH was observed by Lack, Quinn, et al. (2009).
Another facet of atmospheric aerosols that has not been thoroughly investigated is absorption by organic aerosol (OA). There are certain organic compounds that show a strong wavelength (λ) dependent absorbance near ultraviolet and blue wavelengths. Nitrated and aromatic compounds were reported as likely absorbers by Jacobson (Citation1999). Different levels of aromatization were suggested by Bond (Citation2001) to explain the wavelength dependence. The absorption of organic carbon (OC) in biomass smoke was quantified with measurements by Kirchstetter et al. (Citation2004). Absorbing OA at visible wavelengths was also identified as highly oxygenated, having high molecular weight and containing one or more nitrogen atoms (Sun et al. Citation2007).
Instrumental challenges have hampered the investigation of light absorption under elevated RH conditions. Current filter-based light absorption measurement techniques have artifacts such as responses to nonabsorbing aerosol and show oscillatory behavior above 80% RH (Arnott et al. Citation2003). Photoacoustic ambient aerosol light absorption measurements have shown a decrease in the light absorption coefficient at RH values greater than 70%. Theory indicates that some of the laser energy is consumed by mass transfer, evaporating part of the water-containing droplets and therefore lowering the apparent absorption signal by wet aerosols when compared with dry aerosols. This phenomenon could explain the observed decrease in the photoacoustic signal (Arnott et al. Citation2003). This interference has been theoretically characterized (Murphy Citation2009).
The main objective of this research is to provide measured optical properties for light-absorbing OA generated by wood pyrolysis at high RH conditions. Of particular interest is the range of RH conditions between 85% and 95%, where modeled optical properties are rarely characterized by measurements. One approach is to measure absorption by difference between light extinction and scattering (difference method). The difference method has been used in field campaigns (Weiss Citation1992; Reid et al. Citation1998; Virkkula et al. Citation2005), for laboratory studies (Mikhailov et al. Citation2006; Zhang et al. Citation2008; Khalizov et al. Citation2009), and as a reference method for the calibration of filter-based absorption measurements (Bond et al. Citation1999; Weingartner et al. Citation2003). Measurements with an extinction cell are an attractive alternative to other measurements because of the simple construction and the capacity to accommodate high relative humidities in situ without concerns about loss of energy to mass transfer. On the other hand, single path extinction cells have a limited sensitivity and require long path lengths for atmospheric applications. Furthermore, the difference method is prone to large uncertainties in the determined light absorption, especially at high single scattering albedos. The method developed here is currently not suitable for typical ambient aerosol concentrations. This work takes advantage of the laboratory setting to study aerosol properties, where aerosols with reproducible composition and high concentrations can be generated under controlled conditions.
2. METHODOLOGY AND DATA ANALYSIS PROCEDURES
2.1. Aerosol Generation and Setup Overview
The optical properties of four different types of aerosol are reported in this article (). Because this is the first report of measurements from this newly constructed extinction cell and modified nephelometer, it is important to provide benchmarking for this instrumentation and compare results with known standards to evaluate the validity of the measurements.
The first test was conducted with ammonium sulfate as a nonabsorbing benchmark for both dry and humidified conditions. This test with a well-characterized aerosol served three purposes: first, it calibrated the path length of the extinction cell under nonabsorbing conditions by comparing the measured extinction with the measured scattering; second, the absolute accuracy of the instrumentation could be evaluated by an optical model for dry and wet conditions; and third, it tested the performance of the humidity measurement and control in the optical instrumentation. The second benchmark verified the accuracy of the dry light absorption measurements using monodisperse dyed polystyrene microspheres (PSMs, XPR 1547, Thermo Scientific Inc.) with a nominal diameter of 327 nm. The optical properties of these and other absorbing PSM were characterized by Lack et al. (2006; Lack, Cappa, et al. 2009) at the 532-nm wavelength. The third benchmark test was performed with absorbing nigrosin (N4754, Sigma Aldrich Inc.) aerosols. Nigrosin has been used previously as an absorbing benchmark under dry conditions (Bond et al. Citation1999; Lack et al. Citation2006; Sedlacek and Lee Citation2007; Lang-Yona et al. Citation2009). For this work, we investigated light absorption of nigrosin as a function of RH because nigrosin is water soluble.
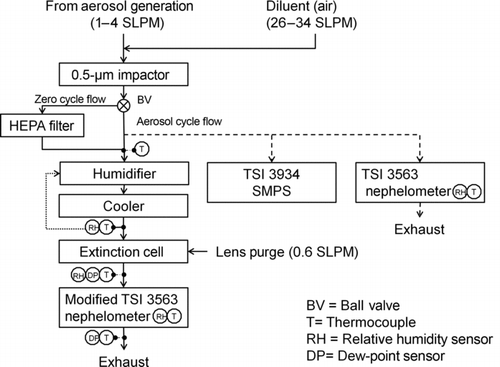
Ammonium sulfate, absorbing PSM and nigrosin aerosols were generated by atomizing their solutions with a constant output atomizer (TSI 3076). The atomizing pressure was set at 241 kPa (35 psig). Before dilution with particle-free dry air, the generated aerosol was dried with a silica gel diffusion dryer and charge neutralized with a custom made neutralizer that utilizes four ionizer plates (Staticmaster 2U500, Amstat Corp.) with 500 μCi each. The conditioned aerosol then entered a 20-L mixing chamber that smoothed concentration fluctuations. From there, the aerosol was forced into the RH-controlled instrumentation ().
A temperature-controlled wood pyrolysis reactor was operated to generate complex mixed primary OAs. Nitrogen sheath flow was used to produce anoxic conditions in the reactor, as would occur in the middle of a wood piece. These conditions allow the pyrolysis that typically generates OC, while also preventing flaming and the production of elemental carbon. This reactor was used by Subramanian et al. (Citation2007) and Chen and Bond (Citation2010) with the only difference for this project being the installation of a proportional integral differential (PID) temperature controller that allowed control within 10°C. Chen and Bond (Citation2010) showed that pyrolysis temperature is more important for absorptive properties than wood type. Pyrolysis at 340°C–360°C produced a greater mass fraction of OA that is less water soluble and more absorptive than at 210°C. In this work, OC aerosol from red oak (Quercus rubra) pyrolyzed at 425°C was investigated. The higher temperature in comparison with Chen and Bond (Citation2010) was chosen because it was difficult to detect absorption at 360°C with these in situ measurements in comparison to the instrumentation used by Chen and Bond (Citation2010) who measured extracts from integrated filter measurements. Individual 7 × 2 × 2 cm (length × width × height) wood blocks were pyrolyzed for 7 min. The generated aerosol was sampled from the reactor at 4 SLPM and diluted with particle-free dry air to a 7:1 dilution ratio. The diluted aerosol was drawn into a 208-L stainless steel storage vessel that was initially purged with nitrogen. After the pyrolysis event, the vessel was disconnected from the vacuum source and reconnected in a forced draft configuration to the RH-controlled optical instrumentation (see ). The flow rate through the storage chamber into the optical instruments ranged from 1 to 4 SLPM.
Aerosol particles with an aerodynamic diameter greater than 500 nm were removed with a two-stage impactor before entering the optical instruments (Berner et al. Citation1979). The switchable high efficiency particulate air (HEPA) filter bypass allowed clean air cycles for the determination of optical background signals without particles.
2.2. Optical Instrumentation
2.2.1. Light Extinction
The Short Path Extinction Cell (SPEC) is a modular optical extinction cell based on the design of Virkkula et al. (Citation2005). The major change from the Virkkula design is the shorter physical path length of 1.25 m instead of 6.57 m that resulted in a sensing volume of 1.75 L instead of 22.4 L and a more rigid vibration insensitive design (). The smaller volume reduced the residence time of the sample in the cell, allowing better control of high RH conditions.
FIG. 2 Short Path Extinction Cell (SPEC) overview: M is a silver plated mirror, L1 and L2 are achromatic lenses, BS is a beam splitter, PD are photo detectors, HD1 and HD2 are holographic diffusers, and LS is the light source.
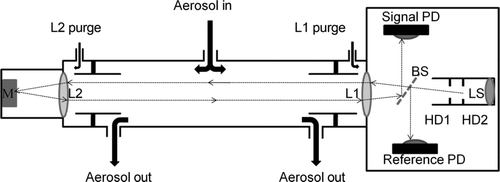
The optoelectronics assembly used the electronics hardware of a 3-λ Radiance Research Particle Soot Absorption Photometer (PSAP) that is able to detect 1 part in 106 changes in light intensity by using integrated photon counts. The PSAP's firmware programming was not modified for use in the SPEC, but the positioning of the signal photo-detector (PD) and reference PD was altered. The setup utilizes the same PDs as the original PSAP electronics (S2387-66R, Hamamatsu Inc.). However, in the extinction cell, the PDs receive light from a beam splitter (BS, NT 45-324, Edmund Optics Inc.). The PSAP light emitting diode (LED) assembly that emits pulsed light at three wavelengths blue (467 nm), green (530 nm), and red (660 nm) was used as the light source (LS). The uniformity of light across the beam path was enhanced by two holographic diffusers (HD1, HD2, NT54-498, NT53-873, Edmund Optics Inc.) and an aperture. Dry HEPA-filtered purge air at a flow rate of 0.3 SLPM was used to keep the two achromatic lenses (L1, L2, 01-LAO-238, MellesGriot Inc.) at each end of the aerosol sensing volume clean from contamination. The purge air resulted in a reduction of the physical path length by 1.1 cm (0.8%) to an actual optical path length of 123.9 cm. The actual optical path length L of the extinction cell was determined with nonabsorbing ammonium sulfate aerosol for which it was assumed that the scattering values measured with the nephelometer match the corresponding extinction values (see section 3.2.1.). In addition, the lens purge air caused dilution of the downstream aerosol flow which was accounted for as explained later. The extinction coefficient σ ep at a specified wavelength λ was calculated from the actual optical path length and the ratio between the signal-detector photon count for clean (zero) air before and after the sample period and for sample air when aerosol is present (EquationEquation (1)). Photon counts for both zero air (Sig. Cnt.)Zero and sample air (Sig. Cnt.)Sample are the respective ratios to the measurements at the reference detector (Ref. Cnt.) to account for signal drift:
The extinction sensitivity and detection limit were determined by recording the signal and reference PD counts under steady conditions for dry clean air, respectively. The uncertainties of the zero and sample period were added in quadrature to determine the uncertainty of the extinction. The extinction detection limit was set to be three times the standard deviation of the obtained extinction noise.
2.2.2. Light Scattering
A wavelength modified TSI 3563 nephelometer was used to measure light scattering and backscattering at three wavelengths (470, 530, and 660 nm). Under normal sampling conditions, heating within the nephelometer can increase the temperature as much as 4.5°C, thereby altering the RH in the nephelometer's sample volume. Several modifications were employed to reduce heating and optimize the instrument's ability to measure at high RH:
| 1. | Exchanged halogen lamp to a version with reduced wattage and thinner filament (Q20MR16C/CG40°, General Electric Inc.) | ||||
| 2. | Installed a hot mirror (46388, Edmund Optics Inc.) in front of the lamp to eliminate thermal radiation into the sampling volume of the instrument. | ||||
| 3. | Separated electronics from the instrument body (Heintzenberg and Erfurt Citation2000) | ||||
| 4. | Increased lamp ventilation with external blowers and reduced power input to lamp (Carrico et al. Citation1998) | ||||
These modifications reduced the sample heating from the original 4.5°C to 0.5 ± 0.1°C, which made scattering measurements up to 95% RH possible. Observed nephelometer sample RH reductions due to the heating were 2.6% for an inlet RH of 80% and 3.2% for an inlet RH of 95%. In addition, the optical band pass filters in front of the photomultiplier tubes were exchanged with new filters with peak wavelengths of 470, 530, and 660 nm (NT-62, Edmund Optics Inc.). This modification allowed the comparison between the scattering and extinction values without wavelength corrections to reduce uncertainty in the accurate determination of light absorption by difference.
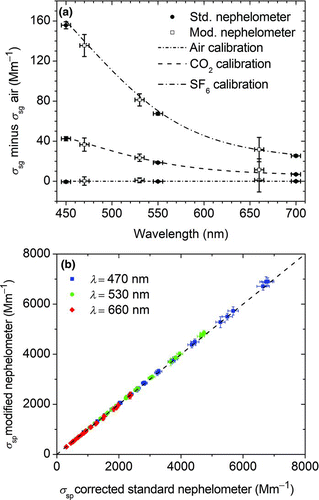
The reduced lamp power and the hot mirror resulted in decreased signal relative to noise that made the standard calibration with air and carbon dioxide (CO2) more challenging, especially at 660 nm. Therefore, longer calibration averaging times were chosen and sulfur hexafluoride (SF6, Matheson, Inc., 99.95% purity) was used as the high span gas. The calibration was verified by comparing measured CO2 and SF6 Rayleigh scattering values with values determined by Bodhaine (Citation1979) at the specific wavelengths and in comparison to an unmodified nephelometer (TSI 3563, ). The scattering sensitivity and detection limit of the modified instrument was determined with clean air similar to the method described by Anderson et al. (Citation1996). To account for scattering by water vapor and wall scattering at elevated RH values, background scattering measurements were performed at 70% and 90% RH that corrected the background scattering signal by 0.7% and 1.5%, respectively (Carrico et al. Citation2000).
FIG. 3 Evaluation of the optical modified and temperature controlled nephelometer. (a) Measured Rayleigh scattering values (σ sg) for the calibration gases air (zero), CO2 (Span1), and SF6 (Span2) for standard TSI 3563 nephelometer (circles) and modified instrument (squares). Dashed lines indicate theoretical values. (b) Comparison of scattering values from the modified nephelometer and the wavelength interpolated unmodified nephelometer determined with ammonium sulfate aerosol at different aerosol concentrations. The dashed line indicates ideal correlation. For all three wavelengths, the instruments differ less than 1.5%. (Color figure available online.)
An additional nephelometer (TSI 3563) was operated in parallel to the sample flow (see dashed line in ). This measurement was used to account for decreasing aerosol concentrations due to dilution of the storage chamber by the forced draft flow in the wood smoke experiments.
2.3. Relative Humidity Measurement and Control
The RH in the system was controlled by maintaining a constant dry-bulb temperature and varying the dew-point temperature. The dew point was set by an annular humidifier that controls water vapor diffusion through a tubular GORE TEX membrane (Carrico et al. Citation1998). The RH instrumentation included two capacitance-based RH sensors (HMP 230, Vaisala Inc.) and two chilled mirror-based dew-point sensors (Hygro M1, General Eastern Inc.). Four thermocouples (Omega, Inc.) for dry-bulb temperature measurements were distributed in the system in addition to the existing dry-bulb temperature measurement within the nephelometer (T sample). A heat exchanger was installed after the humidifier to reduce the difference in dry-bulb temperatures between the initially humidified sample flow and within the optical instrumentation.
One RH and one dew-point sensor were sent to their manufacturers for accredited calibrations. These sensors served as a standard for the remaining two sensors that were calibrated in-house over five temperature-controlled saturated salt solutions (K2CO3, NaCl, KCl, KNO3, and K2SO4) over a range of 43.2%–97.4% RH (at 20°C). The temperature calibrations of the thermocouples were verified against a National Institute of Science and Technology (NIST) reference thermometer with ice and boiling water. However, no adjustments were made since the recorded values were within 0.15 K of the reference. The overall performance of the humidity sensors was checked by placing them at their actual sampling location and varying the set point of the humidifier (see section 3.1.3.).
2.4. Particle Sizing
Aerosol number concentrations and size distributions were measured upstream of the humidifier with a Scanning Mobility Particle Sizer (SMPS, 3934, TSI Instruments). The SMPS aerosol flow was set to 0.5 LPM and the sheath flow was 5 LPM. The multiple particle charge correction inversion algorithm from the TSI aerosol instrument manager software was used. The sizing accuracy of the SMPS system was evaluated by measuring 200 nm monodisperse polystyrene latex spheres (3200A, Thermo Scientific Inc.) and comparing the resulting measured diameter reported by the SMPS. The electrostatic classifier used in the SMPS system was operated with HEPA-filtered recirculating sheath airflow.
2.5. Instrument Corrections and Data Analysis Procedures
2.5.1. Instrument Corrections
The measured extinction and scattering coefficients were corrected to standard laboratory temperature (298.15 K) and pressure (1013.15 mbar). Additionally the scattering coefficient was corrected for the dilution of the aerosol flow, Q Aerosol, caused by the lens purge flow of the extinction cell, Q Purge, shown in EquationEquation (2):
The nephelometer's angular truncation was corrected for submicrometer diameter ammonium sulfate aerosol particles (Anderson and Ogren Citation1998). For absorbing aerosol with single scattering albedo ω < 0.9, the truncation correction approach of Bond et al. (Citation2009) was followed; the correction was calculated with an assumed refractive index and Mie theory based on the measured particle size distributions. A constant nephelometer truncation correction was assumed under varying RH conditions.
2.5.2. Determination of Actual RH in Scattering Volume
Because of the slightly elevated temperature in the nephelometer, scattering is measured at a different RH than the rest of the system. The actual RH in the sensing volume of the nephelometer was calculated from the average of the two dew-point temperature values measured upstream and downstream of the nephelometer (T DP, Ave) with the T sample values provided by the nephelometer [EquationEquation (3)]:
In EquationEquation (3), β = 17.62 and λw = 243.12 K [WMO (World Meteorological Organization) 2008]. This procedure was observed to be the most accurate to determine the RH within the nephelometer's sensing volume due to sample heating within the nephelometer (Carrico et al. Citation1998; Koloutsou-Vakakis et al. Citation2001; Kus et al. Citation2004).
2.5.3. Determination of Light Absorption and Single Scattering Albedo
The extinction measurements covered the entire range of RH values measured by the nephelometer but did not occur at exactly the same RH value due to the nephelometer heating. Therefore, the extinction coefficient that would have been measured at the RH within the nephelometer was inferred by using a cubic spline interpolation of RH values above and below the RH for the extinction cell (σ ep,int). The light absorption coefficient σ ap and ω at the RH of the nephelometer were calculated from the interpolated extinction coefficient (EquationEquations (4) and (Equation5)):
The absolute uncertainties for light absorption were calculated by adding the uncertainties of the extinction and scattering measurements in quadrature.
2.6. Optical Closure Evaluation with Ammonium Sulfate
Modeling optical properties from the measured size distributions and known chemical properties of the particles allows an independent verification of directly measured optical results. A computer program based on the Mie–Lorentz light scattering (BHMIE) code of Bohren and Huffman (Citation1983) was used to calculate aerosol optical properties during both dry and humidified ammonium sulfate aerosol experiments. Particles were assumed to be homogeneous spheres of uniform density for dry and hydrated conditions. The effect of the dry particle shape at RHs below deliquescence was neglected because its correction is small (< 2%) (Mikhailov et al. Citation2009). For the humidified experiment, aerosol properties including the water content, refractive index, and density as a function of RH were taken from Tang (Citation1996) and Tang and Munkelwitz (Citation1994). Diameter growth factors for each particle size bin across each size distribution were determined from the midpoint diameter for each SMPS channel. Scattering coefficients as a function of RH were calculated by assuming that all of the particles in each size bin grow equally and the dry aerosol particle number concentration is conserved under humidified conditions (no coagulation or particle loss). Optical model uncertainties were computed from the measurement uncertainty in the size distribution (counting and sizing errors), not from the uncertainty in the thermodynamic model.
3. RESULTS AND DISCUSSION
The results are presented in five sections: instrumentation and quality control, ammonium sulfate benchmarking, dry absorbing microspheres, humidified nigrosin, and humidified OA from wood pyrolysis. While the primary purpose of this work is to examine absorption by organic matter at high RH, the other benchmarks are necessary to provide confidence in the new instrumentation.
3.1. Instrumentation Performance and Quality Control
3.1.1. Optical Instrumentation Sensitivities and Detection Limits
The root mean square (rms) noise of the measured extinction and scattering coefficients of particle-free air as a function of sample averaging time in seconds are shown in .
FIG. 4 Root mean square (rms) noise values for particle-free air extinction and scattering as a function of wavelength and sample averaging time for the constructed extinction cell and the modified nephelometer, respectively. (Color figure available online.)
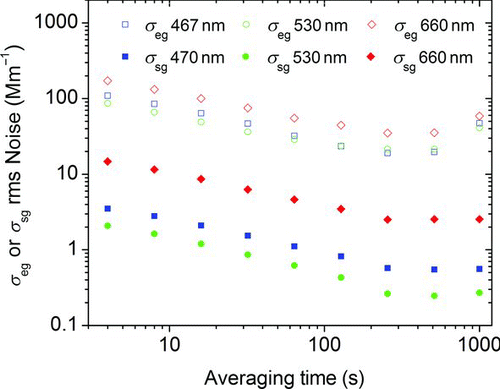
Between the averaging time of 4 and 120 s, the rms noise of both instruments follows a typical white noise behavior and decreases linearly with the square root of integration time. The lowest noise for the extinction cell is reached after an averaging time of 120–300 s, after which the noise begins to increase due to low frequency drift. The main cause of the drift is temperature fluctuations that affect the stability of the signal and reference PDs. The lowest noise level for the extinction coefficient was observed to be 19.1 Mm−1 (467 nm), 21.5 Mm−1 (530 nm), and 35.1 Mm−1 (660 nm) at an averaging time of 256 s. At the same averaging time, these values correspond to an extinction detection limit of 57.3 Mm−1 (467 nm), 64.5 Mm−1 (530 nm), and 105 Mm−1 (660 nm) if the lowest detectable signal to noise ratio is assumed to be 3. The noise and approximate detection limits of the modified TSI nephelometer do not show a drift with time after 300 s and its noise levels stabilize at 0.58 Mm−1 (470 nm), 0.27 Mm−1 (530 nm), and 2.5 Mm−1 (660 nm) at an averaging time of 128 s.
3.1.2. Modified Nephelometer Calibration and Performance
The nephelometer calibration was checked by measuring the Rayleigh scattering of the calibration gases air, CO2, and SF6 and comparing the measured values with the theory. In addition, the performance of the modified instrument was evaluated by comparing the measured particle light scattering coefficient for ammonium sulfate aerosol with wavelength-adjusted values from an unmodified instrument. The results are shown in . shows the measured Rayleigh scattering values for air (used as zero), CO2, and SF6 (used as span). The errors in the wavelengths are the uncertainties of the band pass filters reported by the vendor, and the error bars for the scattering values are the standard deviations from a 120 s sample period. Both the standard and modified instruments agree well with the theoretical values obtained by Bodhaine (Citation1979) which are indicated by dashed lines for each gas.
shows the agreement of the modified nephelometer with the wavelength-corrected unmodified instrument for different ammonium sulfate aerosol concentrations. The scattering coefficients measured at 450, 550, and 700 nm were interpolated to the wavelengths of the modified instrument (470, 530, and 660 nm) assuming a constant Ångström exponent within the wavelength intervals. The error bars in the vertical direction indicate the measured standard deviations for a 120 s sample averaging time. The error bars in the horizontal direction include interpolation uncertainty values of 1.8% (470 nm), 1.5% (530 nm), and 1.1% (660 nm) from Virkkula et al. (Citation2005) in addition to the measured standard deviation of the sample. The instruments show an excellent linear agreement with a maximum deviation of 1.41% at 530 nm, which is within the uncertainty of the wavelength interpolation. We conclude that the modifications to the nephelometer do not reduce the accuracy of the scattering measurement.
TABLE 2 Linear regression coefficients obtained for a regression between the measured scattering and measured extinction for dry ammonium sulfate to obtain correction factors for nonidealities in the extinction cell. All R 2 values were greater than 0.99 and the regression equation was σ ep, corrected = σ sp = Intercept + Slope × σ ep, measured
TABLE 3 Linear regression coefficients from comparison between the modeled and measured scattering (σ sp, model = Intercept + Slope × σ sp, measured) of dry ammonium sulfate. All R 2 values were greater than 0.99
3.1.3. Humidity Sensor Performance
The accurate determination of RH at multiple points is critical to ensure that different optical measurement techniques measure the aerosol under the same RH conditions. shows the agreement among the RH sensors at different controller set points, which are indicated with a dashed line. The whiskers show the minimum and maximum values, the box indicates the lower and upper quartiles, and the mean value is given as a small solid circle.
FIG. 5 RH sensor agreement at RH set point values of (a) 34% RH, (b) 50% RH, (c) 90% RH, and (d) 97.5% RH. V1 and V2 are the capacitive-based RH values. DP1 and DP2 are the dew-point-based RH values calculated with colocated dry-bulb temperature measurements.
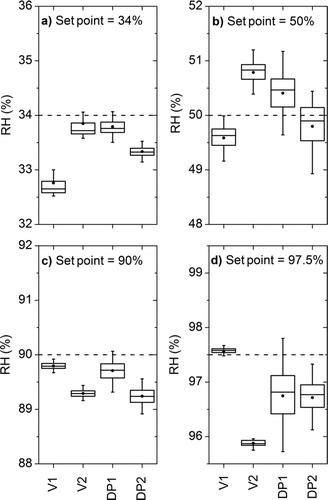
The RH values for the dew-point sensors (DP1 and DP2) were determined by using the colocated dry-bulb temperature measurements. The data are from sampling periods of 180 s after equilibration after each humidity change. The sampling system reaches stable RH equilibrium in less than 45 s for RH values below 85% and up to 180 s for RH values of 90% and 97.5%. All sensors show an agreement within their manufacturer-specified range of ±2% RH for all measurements and the obtained mean values all lie within 1.7% RH. The largest discrepancy was observed for the highest RH set point that can be attributed to several reasons, including small temperature gradients between sensor locations and issues with sensor technology at high RH values. The polymers used in capacity based measurements become water saturated (Chen and Lu Citation2005), and the uncertainty in the calculated RH values from the dew-point sensors becomes larger (Gates Citation1994).
3.2. Ammonium Sulfate Benchmark Tests
Dry ammonium sulfate was used as a nonabsorbing benchmark to verify the agreement between light scattering and extinction. Additionally this benchmark allowed an accurate evaluation of optical closure.
3.2.1. Dry Scattering and Extinction Agreement
Ideally, under nonabsorbing conditions, the measured extinction values equal the measured scattering values. In this work, nonidealities were observed and the measured extinction values were corrected to the measured scattering values by correction factors determined by linear regressions. The regression coefficients to determine the corrected extinction values (σ ep,corrected) based on measured scattering values (σ sp,measured) for 21 aerosol concentrations ranging from 400 to 3000 Mm−1 at three wavelengths are provided in .
The regression coefficients for matching the measured extinction with the measured scattering show slight wavelength dependence and offset, but the intercept values are within the noise of the instrument. The deviation from the ideal slope of unity at different wavelengths can be attributed to uncertainties in wavelength filters and LSs, and alignment of LSs. (LEDs at each wavelength do not have identical locations and therefore are not at the same focal point.) All measured extinction results are corrected with these regression coefficients, which implies that we relate the accuracy of our results to the accuracy of the nephelometer.
3.2.2. Dry Scattering Closure with Mie Model
The measured scattering values were compared with values modeled using measured size distributions to verify the accuracy of the scattering measurement (optical closure). A linear regression between the measured and modeled scattering values for 21 aerosol concentrations ranging from 400 to 3000 Mm−1 is shown in .
Results from the Mie model and the scattering measurements match with a maximum deviation (over prediction of true value) of 6% at 660 nm. This agreement is comparable to other closure studies for inorganic aerosols (Anderson et al. Citation1996).
3.2.3. Optical Properties as a Function of RH
shows humidograms of scattering and extinction coefficients for ammonium sulfate for all three wavelengths. The modeled values with their uncertainties are shown as solid and dashed lines, respectively. The abrupt increase in scattering and extinction is attributable to deliquescence of the aerosol. This appears to occur at lower RH values for scattering in comparison to extinction and theoretical values. Such reduction in the apparent deliquescence values is caused by heating of the nephelometer. For a narrow range of RH, particles may deliquesce upstream of the nephelometer, drying below the deliquescence point as RH is reduced in the instrument, but remaining in a metastable state.
FIG. 6 Measured scattering (σ sp) and extinction (σ ep) coefficients for ammonium sulfate aerosol as a function of RH. The modeled values and their uncertainties are indicated as solid and dashed lines, respectively. (Color figure available online.)
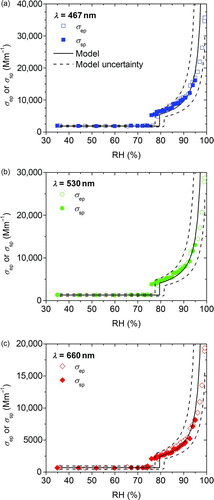
The instrumentation is able to measure light extinction at RH up to 98.5%, and there is good agreement with the modeled scattering values. The maximum RH of the scattering measurement (95%) is lower than that of the extinction due to the heating in the nephelometer. Particle losses and uncertainties in the RH measurement may explain the mismatch between measurement and modeled results at high RH. However, the extinction and scattering measurements agree well, so particles are not lost between the extinction cell and the nephelometer up to 95% RH. Therefore, particle losses may affect the model–measurement comparison and the apparent growth factor but not the ability to measure single-scattering albedo.
3.3. Dry Absorbing Microspheres Benchmark Test
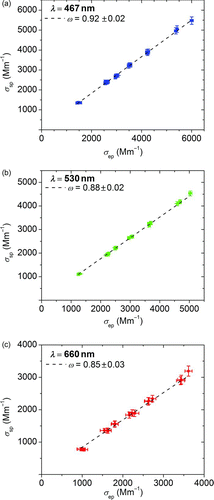
Because optical properties of the absorbing PSM have been previously determined at 532 nm wavelength (Lack et al. Citation2006; Lack, Cappa, et al. Citation2009), they were used as a benchmark to demonstrate that our instrumentation could accurately measure light absorption. shows the relationship between the measured scattering and extinction values to retrieve ω values by means of a linear regression at each wavelength. The linear fit is indicated as a dashed line. The error bars correspond to measured standard deviations for an averaging time of 120 s.
The measurements at 530 nm show good agreement with the values reported by Lack, Cappa, et al. (2009). Lack reports an ω value of 0.86 ± 0.02 at the 532 nm wavelength. This result verifies the accuracy of the optical method under dry conditions.
3.4. Humidified Nigrosin Results
Nigrosin is water soluble and therefore provides an opportunity to examine the dependence of absorption on RH (see ). Extinction and scattering error bars are measured standard deviations for an averaging time of 120 s. Error bars for absorption are the pooled standard deviations of the measured scattering and extinction values. Error bars for RH are the absolute measurement uncertainties of the RH measurements specified by the sensor manufacturers.
Extinction and scattering increase with increasing RH values due to water uptake, which results in larger aerosol size. Light absorption is highest at 530 nm, followed by 660 and 467 nm. A similar wavelength dependence has been reported for bulk measurements of nigrosin solutions (Sedlacek and Lee Citation2007). As RH increases, there is an increase in light absorption and in single scattering albedo. The absorption enhancement, described by [σ ap (95% RH)/σ ap (RH 38%)], is 1.24 ± 0.06 with no distinguishable wavelength dependence.
Mie theory was used in a preliminary effort to explain the observed enhancement. Model inputs were the measured dry size distributions, and an inferred size growth factor [D p (95% RH)/D p (38% RH)] of 1.25 based on a one-to-one volume uptake of water by nigrosin, which would match the measured scattering. The refractive index for the resulting droplets was determined using a volume mixing of the refractive indices of pure water and nigrosin (Lang-Yona et al. Citation2009). For complete and uniform dissolution of nigrosin in water, the theory predicts an absorption enhancement of 1.15. A core-shell model with an insoluble nigrosin core predicts an enhancement of 2. The measured absorption enhancement is higher than the modeled value for complete dissolution of the solute, which would be the most appropriate assumption for this water-soluble compound. This difference could be explained by the uncertainties related to the inferred growth factor from scattering. A detailed investigation with a more accurate determination of diameter growth measurements is required to understand these results further.
3.5. Humidified Organic Aerosol from Wood Pyrolysis
Nigrosin and absorbing PSM have no apparent relevance to the earth's atmosphere but served as benchmarks to build confidence in the ability of the instrumentation to measure atmospherically relevant, biomass-related OC aerosol. The optical properties of humidified primary OC aerosol generated by oak wood pyrolysis under anoxic conditions are provided in . The figure shows extinction (), scattering (), absorption inferred by the difference of the two (), and the absorption growth factor () derived by dividing the measured humidified aerosol absorption coefficient by its dry absorption coefficient [σ ap (RH)/σ ap (RH < 50%)]. Error bars in and are the measured standard deviations of the σ ep and σ sp coefficients for an integration time of 120 s, and the error bars for the absorption coefficient () and absorption growth factor () are calculated on the basis of those values.
FIG. 8 Optical properties of nigrosin as a function of RH. Extinction (σ ep) and scattering (σ sp) for 467, 530, and 660 nm are shown in (a), (b), and (c), respectively; calculated absorption (σ ap) by difference in σ ep and σ sp is shown in (d). (Color figure available online.)
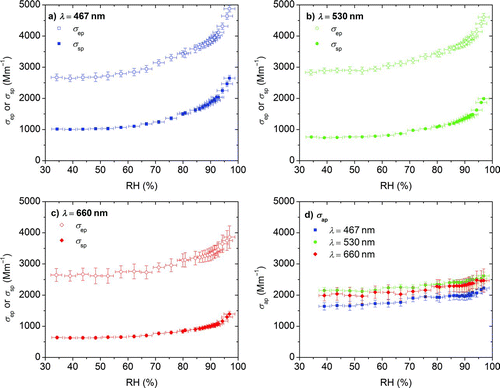
FIG. 9 Optical properties at controlled RH conditions for primary OC aerosol generated by pyrolysis of biomass. Extinction (σ ep), scattering (σ sp), and absorption (σ ap) by difference for three wavelengths are shown in (a), (b), and (c); the hygroscopic absorption growth factor is shown in (d). (Color figure available online.)
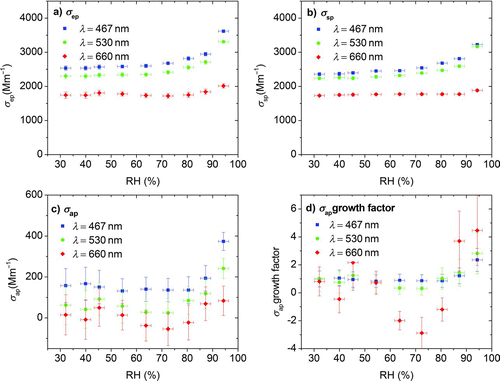
Dry and humidified absorption has a strong wavelength dependence, with the strongest absorption at the 467 nm wavelength and undetectable absorption at 660 nm. This wavelength dependence is similar to previous findings for biomass burning under dry conditions derived from filter measurements (Kirchstetter et al. Citation2004) and filter extracts measured with spectrophotometry (Chen and Bond Citation2010).
The growth of scattering and extinction is low until 75% RH where it starts to increase rapidly. For the 467 and 530 nm wavelengths, the light absorption coefficient increases with increasing RH. The determined absorption growth factors at 95% RH are 2.2 ± 0.7 and 2.7 ± 1.2 at the wavelengths of 467 and 530 nm, respectively. At 660 nm the absorption growth factor is highly variable because the dry absorption is near the detection limit of the instrumentation. Simple modeling using Mie theory indicates that only a heterogeneously mixed aerosol with insoluble absorbing compounds can explain such an absorption enhancement. If each droplet were homogeneously mixed, the increase in absorption would be much smaller, as seen in the nigrosin results.
The slight decrease in absorption upon humidification below 75% lies within the measurement uncertainty. This decrease could be real, because coated black carbon exhibits a decrease in absorption when it compacts under similar conditions (Zhang et al. Citation2008). However, to the best of our knowledge, the organic material measured here does not have a low fractal dimension and therefore cannot collapse. It is also possible that the nature of the dry OA could change during the test, either due to coagulation or loss of semivolatile material upon dilution. No significant changes in absorption were observed in tests without humidification that were otherwise identical to the experiments shown here.
Because the difference method relies on the small difference of two large numbers, the high relative uncertainties in derived absorption and absorption growth factors can be expected, especially for weakly absorbing material such as OC. However, these uncertainties do not obscure the main finding: absorption growth for OC aerosol generated by wood pyrolysis above 85% RH is significant at the 467 and 530 nm wavelengths. At the 660 nm wavelength, where absorption is at the detection limit of the instrumentation, noise propagates into the inferred absorption growth factor, but characterizing the low absorption at this wavelength is less important. Further development of optical techniques may be required to improve the quantification of light absorption as a function of RH.
4. SUMMARY
Light extinction and light scattering at high relative humidities were measured in the laboratory to determine light absorption by difference. This article reports on modifications that make these measurements possible. The nephelometer was modified to reduce heating in the sample volume from 4.5°C for the unmodified instrument to less than 0.6°C which allowed for accurate measurement of light scattering up to 95% RH. Benchmarking with humidified ammonium sulfate indicated an overall performance of the measurements within the uncertainties of the modeled values. The difference method was capable of measuring single scattering albedo of absorbing PSMs within 0.02 of previously reported results. Light absorption of nigrosin was enhanced by a factor of 1.24 ± 0.06 at 95% RH compared with 38% RH. Absorption by OC aerosols generated from wood pyrolysis increased by a factor of 2.2 ± 0.7 and 2.7 ± 1.2 between 32% and 95% RH at the wavelengths of 467 and 530 nm, respectively. Additionally, previously reported spectral dependencies of OC absorption that were determined with filter and bulk liquid measurements were confirmed in situ.
Understanding the effect of RH on light absorption is required to better describe how aerosol affects radiative transfer through the earth's atmosphere. Most global models of organic matter optical properties do not yet account for the absorption by OC emitted from biomass combustion or pyrolysis. When this absorption is represented in models, model parameterizations assume that scattering increases but absorption remains constant as RH increases. The findings presented here indicate that even parameterizations that do consider absorption measured for dry aerosol could underestimate it for RH values between 75% and 95%. Furthermore, these particles may serve as cloud condensation nuclei, and absorption within cloud droplets may also be underrepresented.
Acknowledgments
This work is supported by the research grant: “Optical Properties of Moderately-Absorbing Organic and Mixed Organic/Inorganic Particles at Very High Humidities” (Department of Energy's Atmospheric Science Program [DOE-ASP] grant no.: DE-FG02-08ER64533).
REFERENCES
- Anderson , T. L. , Covert , D. S. , Marshall , S. F. , Laucks , M. L. , Charlson , R. J. Waggoner , A. P. 1996 . Performance Characteristics of a High-Sensitivity, Three-Wavelength, Total Scatter/Backscatter Nephelometer . J. Atmos. Ocean. Technol. , 13 : 967 – 986 .
- Anderson , T. L. and Ogren , J. A. 1998 . Determining Aerosol Radiative Properties Using the TSI 3563 Integrating Nephelometer . Aerosol Sci. Technol. , 29 : 57 – 69 .
- Arnott , W. P. , Moosmuller , H. , Sheridan , P. J. , Ogren , J. A. , Raspet , R. Slaton , W. V. 2003 . Photoacoustic and Filter-Based Ambient Aerosol Light Absorption Measurements: Instrument Comparisons and the Role of Relative Humidity . J. Geophys. Res.-Atmos. , 108 : 4034 – 4045 .
- Berner , A. , Lurzer , C. , Pohl , F. , Preining , O. and Wagner , P. 1979 . Size Distribution of the Urban Aerosol in Vienna . Sci. Total Environ. , 13 : 245 – 261 .
- Bodhaine , B. A. 1979 . Measurement of the Rayleigh-Scattering Properties of Some Gases with a Nephelometer . Appl. Opt. , 18 : 121 – 125 .
- Bohren , C. E. and Huffman , D. R. 1983 . Absorption and Scattering of Light by Small Particles , Wiley and Sons, New York .
- Bond , T. C. 2001 . Spectral Dependence of Visible Light Absorption by Carbonaceous Particles Emitted from Coal Combustion . Geophys. Res. Lett. , 28 : 4075 – 4078 .
- Bond , T. C. , Anderson , T. L. and Campbell , D. 1999 . Calibration and Intercomparison of Filter-Based Measurements of Visible Light Absorption by Aerosols . Aerosol Sci. Technol. , 30 : 582 – 600 .
- Bond , T. C. , Covert , D. S. and Muller , T. 2009 . Truncation and Angular-Scattering Corrections for Absorbing Aerosol in the TSI 3563 Nephelometer . Aerosol Sci. Technol. , 43 : 866 – 871 .
- Boucher , O. and Anderson , T. L. 1995 . General Circulation Model Assessment of the Sensitivity of Direct Climate Forcing by Anthropogenic Sulfate Aerosols to Aerosol Size and Chemistry . J. Geophys. Res.-Atmos. , 100 : 26117 – 26134 .
- Carrico , C. M. , Rood , M. J. and Ogren , J. A. 1998 . Aerosol Light Scattering Properties at Cape Grim, Tasmania, During the First Aerosol Characterization Experiment (ACE 1) . J. Geophys. Res.-Atmos. , 103 : 16565 – 16574 .
- Carrico , C. M. , Rood , M. J. , Ogren , J. A. , Neususs , C. , Wiedensohler , A. and Heintzenberg , J. 2000 . Aerosol Optical Properties at Sagres, Portugal During ACE-2 . Tellus B-Chem. Phys. Meteorol. , 52 : 694 – 715 .
- Chen , Y. and Bond , T. C. 2010 . Light Absorption by Organic Carbon from Wood Combustion . Atmos. Chem. Phys. , 10 : 1773 – 1787 .
- Chen , Z. and Lu , C. 2005 . Humidity Sensors: A Review of Materials and Mechanisms . Sensor Lett. , 3 : 274 – 295 .
- Covert , D. S. , Charlson , R. J. and Ahlquist , N. C. 1970 . Study of Relationship of Chemical Composition and Humidity with Light Scattering by Aerosols . Bull. Am. Meteorol. Soc. , 51 : 968–976
- Gates , R. S. 1994 . Dew-Point Temperature Error from Measuring Dry-Bulb Temperature and Relative-Humidity . T. Asae , 37 : 687 – 688 .
- Ghan , S. J. and Zaveri , R. A. 2007 . Parameterization of Optical Properties for Hydrated Internally Mixed Aerosol . J. Geophys. Res.-Atmos. , 112 : 10
- Hansson , H. C. , Rood , M. J. , Koloutsou-Vakakis , S. , Hameri , K. , Orsini , D. and Wiedensohler , A. 1998 . NaCl Aerosol Particle Hygroscopicity Dependence on Mixing with Organic Compounds . J. Atmos. Chem. , 31 : 321 – 346 .
- Haywood , J. M. , Ramaswamy , V. and Donner , L. J. 1997 . A Limited-Area-Model Case Study of the Effects of Sub-Grid Scale Variations in Relative Humidity and Cloud upon the Direct Radiative Forcing of Sulfate Aerosol . Geophys. Res. Lett. , 24 : 143 – 146 .
- Heintzenberg , J. and Erfurt , G. 2000 . Modification of a Commercial Integrating Nephelometer for Outdoor Measurements . J. Atmos. Ocean. Technol. , 17 : 1645 – 1650 .
- Jacobson , M. Z. 1999 . Isolating Nitrated and Aromatic Aerosols and Nitrated Aromatic Gases as Sources of Ultraviolet Light Absorption . J. Geophys. Res.-Atmos. , 104 : 3527 – 3542 .
- Khalizov , A. F. , Xue , H. X. , Wang , L. , Zheng , J. and Zhang , R. Y. 2009 . Enhanced Light Absorption and Scattering by Carbon Soot Aerosol Internally Mixed with Sulfuric Acid . J. Phys. Chem. A. , 113 : 1066 – 1074 .
- Kirchstetter , T. W. , Novakov , T. and Hobbs , P. V. 2004 . Evidence that the Spectral Dependence of Light Absorption by Aerosols is Affected by Organic Carbon . J. Geophys. Res.-Atmos. , 109 : 12
- Koloutsou-Vakakis , S. , Carrico , C. M. , Kus , P. , Rood , M. J. , Li , Z. Shrestha , R. 2001 . Aerosol Properties at a Midlatitude Northern Hemisphere Continental Site . J. Geophys. Res.-Atmos. , 106 : 3019 – 3032 .
- Kus , P. , Carrico , C. M. , Rood , M. J. and Williams , A. 2004 . Measured and Modeled Light Scattering Values for Dry and Hydrated Laboratory Aerosols . J. Atmos. Ocean. Technol. , 21 : 981 – 994 .
- Lack , D. A. , Cappa , C. D. , Cross , E. S. , Massoli , P. , Ahern , A. T. Davidovits , P. 2009 . Absorption Enhancement of Coated Absorbing Aerosols: Validation of the Photo-Acoustic Technique for Measuring the Enhancement . Aerosol Sci. Technol. , 43 : 1006 – 1012 .
- Lack , D. A. , Lovejoy , E. R. , Baynard , T. , Pettersson , A. and Ravishankara , A. R. 2006 . Aerosol Absorption Measurement Using Photoacoustic Spectroscopy: Sensitivity, Calibration, and Uncertainty Developments . Aerosol Sci. Technol. , 40 : 697 – 708 .
- Lack , D. A. , Quinn , P. K. , Massoli , P. , Bates , T. S. , Coffman , D. Covert , D. S. 2009 . Relative Humidity Dependence of Light Absorption by Mineral Dust After Long-Range Atmospheric Transport from the Sahara . Geophys. Res. Lett. , 36 : 5
- Lang-Yona , M. , Rudich , Y. , Segre , E. , Dinar , E. and Abo-Riziq , A. 2009 . Complex Refractive Indices of Aerosols Retrieved by Continuous Wave-Cavity Ring Down Aerosol Spectrometer . Anal. Chem. , 81 : 1762 – 1769 .
- Lewis , K. A. , Arnott , W. P. , Moosmuller , H. , Chakrabarty , R. K. , Carrico , C. M. Kreidenweis , S. M. 2009 . Reduction in Biomass Burning Aerosol Light Absorption upon Humidification: Roles of Inorganically-Induced Hygroscopicity, Particle Collapse, and Photoacoustic Heat and Mass Transfer . Atmos. Chem. Phys. , 9 : 8949 – 8966 .
- Mikhailov , E. , Vlasenko , S. , Martin , S. T. , Koop , T. and Poschl , U. 2009 . Amorphous and Crystalline Aerosol Particles Interacting with Water Vapor: Conceptual Framework and Experimental Evidence for Restructuring, Phase Transitions and Kinetic Limitations . Atmos. Chem. Phys. , 9 : 9491 – 9522 .
- Mikhailov , E. F. , Vlasenko , S. S. , Podgorny , I. A. , Ramanathan , V. and Corrigan , C. E. 2006 . Optical Properties of Soot-Water Drop Agglomerates: An Experimental Study . J. Geophys. Res.-Atmos. , 111 : 1815–1834
- Murphy , D. M. 2009 . The Effect of Water Evaporation on Photoacoustic Signals in Transition and Molecular Flow . Aerosol Sci. Technol. , 43 : 356 – 363 .
- Orr , C. Jr. , Hurd , F. K. and Corbett , W. J. 1958 . Aerosol Size and Relative Humidity . J. Colloid Sci. , 13 : 472 – 482 .
- Ramaswamy , V. and Kiehl , J. T. 1985 . Sensitivities of the Radiative Forcing Due to Large Loadings of Smoke and Dust Aerosols . J. Geophys. Res.-Atmos. , 90 : 5597 – 5613 .
- Reid , J. S. , Hobbs , P. V. , Liousse , C. , Martins , J. V. , Weiss , R. E. and Eck , T. F. 1998 . Comparisons of Techniques for Measuring Shortwave Absorption and Black Carbon Content of Aerosols from Biomass Burning in Brazil . J. Geophys. Res.-Atmos. , 103 : 32031 – 32040 .
- Sedlacek , A. and Lee , J. 2007 . Direct Aerosol Absorption Measurements Using Photothermal Interferometry Paper presented at the US DOE ASP Science Team Meeting
- Sedlacek , A. and Lee , J. 2007 . Photothermalinterferometric Aerosol Absorption Spectrometry . Aerosol Sci. Technol. , 41 : 1089 – 1101 .
- Subramanian , R. , Roden , C. A. , Boparai , P. and Bond , T. C. 2007 . Yellow Beads and Missing Particles: Trouble Ahead for Filter-Based Absorption Measurements . Aerosol Sci. Technol. , 41 : 630 – 637 .
- Sun , H. L. , Biedermann , L. and Bond , T. C. 2007 . Color of Brown Carbon: A Model for Ultraviolet and Visible Light Absorption by Organic Carbon Aerosol . Geophys. Res. Lett. , 34 : 5
- Tang , I. N. 1996 . Chemical and Size Effects of Hygroscopic Aerosols on Light Scattering Coefficients . J. Geophys. Res.-Atmos. , 101 : 19245 – 19250 .
- Tang , I. N. and Munkelwitz , H. R. 1994 . Water Activities, Densities, and Refractive-Indexes of Aqueous Sulfates and Sodium-Nitrate Droplets of Atmospheric Importance . J. Geophys. Res.-Atmos. , 99 : 18801 – 18808 .
- Virkkula , A. , Ahlquist , N. C. , Covert , D. S. , Sheridan , P. J. , Arnott , W. P. and Ogren , J. A. 2005 . A Three-Wavelength Optical Extinction Cell for Measuring Aerosol Light Extinction and Its Application to Determining Light Absorption Coefficient . Aerosol Sci. Technol. , 39 : 52 – 67 .
- Watson , J. G. 2002 . Visibility: Science and Regulation . J. Air Waste Manage. Assoc. , 52 : 628 – 713 .
- Weingartner , E. , Saathoff , H. , Schnaiter , M. , Streit , N. , Bitnar , B. and Baltensperger , U. 2003 . Absorption of Light by Soot Particles: Determination of the Absorption Coefficient by Means of Aethalometers . J. Aerosol Sci. , 34 : 1445 – 1463 .
- Weiss , R. E. 1992 . Optical Extinction Properties of Smoke from the Kuwait Oil Fires . J. Geophys. Res.-Atmos. , 97 : 14537 – 14540 .
- World Meteorological Organization . 2008 . WMO Guide to Meteorological Instruments and Methods of Observation World Meteorological Organization (WMO), Geneva, Switzerland
- Zhang , R. Y. , Khalizov , A. F. , Pagels , J. , Zhang , D. , Xue , H. X. and McMurry , P. H. 2008 . Variability in Morphology, Hygroscopicity, and Optical Properties of Soot Aerosols During Atmospheric Processing . P. Natl. Acad. Sci. USA , 105 : 10291 – 10296 .
- Zieger , P. , Fierz-Schmidhauser , R. , Gysel , M. , Strom , J. , Henne , S. Yttri , K. E. 2010 . Effects of Relative Humidity on Aerosol Light Scattering in the Arctic . Atmos. Chem. Phys. , 10 : 3875 – 3890 .