Abstract
Copyright 2013 American Association for Aerosol Research
INTRODUCTION
A particle with precisely controlled dual or multiple patches of varying compositions is known as a “patchy particle.” Patchy particles have been attracting attention in recent years because of the possibility of multiple surface functionalities and their use as building blocks in mesoscale particle assemblies (Zhang and Glotzer Citation2004; Pawar and Kretzschmar Citation2010). A Janus particle is the simplest example of a patchy particle with two hemispheres of different components (Casagrande et al. Citation1989; Paunov and Cayre 2004). Wide applications of Janus particles in photonic crystals (Cayre et al. Citation2003; Liddell et al. Citation2003), drug delivery systems (Langer and Tirrell Citation2004; Champion et al. Citation2007), and sensors (Takei and Shimizu Citation1997; Ozin et al. Citation2005) have been reported.
A variety of methods have been explored for the generation of patchy particles, including templating, vapor deposition, nanosphere lithography, and capillary fluid flow methods. (Takei and Shimizu Citation1997; Haynes and Van Duyne Citation2001; Nisisako et al. Citation2004; Hong et al. Citation2006) Advantages and disadvantages of these methods and other technologies are summarized in a recent review by Pawar and Kretzschmar (Citation2010). Unfortunately, there is no simple scalable method that yields precise control of particle patchiness.
In this article, we tested ultrasonic spray pyrolysis as a potential patchy particle generation method. Spray pyrolysis is a simple one-step synthesis process and is widely studied for the generation of various metal and metal oxide particles (Nagashima et al. Citation1987; Gurav et al. Citation1993; Choa et al. Citation2003; Zhong et al. 2012; Jian et al. Citation2007; Guo et al. Citation2012; Yao et al. Citation2012). Advantages of ultrasonic spray pyrolysis include the capability of scalable production and adjustable particle size from submicron to several microns. A cosolvent was added to the precursor solution to create the reductive atmosphere and improve the safety of the technology (Kim et al. Citation2003). AgNi, CuNi, and AgCu bimetallic particles were generated by this process in our lab. The results indicated that the structure of particles was governed by thermodynamics of the metal mixtures and thus mainly depended on the particle composition and could be controlled by the operating conditions.
METHODS
The bench scale spray pyrolysis system used in our experiment includes an ultrasonic droplet generator, a reactor consisting of a quartz tube heated by a reactor tube furnace and a filtration system. For the generation of bimetal particles, metal nitrates mixture/water/ethylene glycol (EG) were used as the precursor solution. EG was used as the cosolvent, because it has a relatively high flash point, and is therefore safer for industrial applications (Zhong et al. 2003). Metal nitrates, such as AgNO3 (99.5% purity) Cu(NO3)26H2O (99.5% purity), and Ni(NO3)2·6H2O (99.99% purity), were ordered from Strem Company (Boston, MA, USA) and used without further treatment. The concentration of each metal nitrate was selected according to the desired particle composition, and the total concentration of nitrate in the solution was 1.2 M. 40 vol% EG (99%, Sigma Aldrich) was added into the solution as the cosolvent.
The precursor solution was atomized by a custom built ultrasonic generator operated at a frequency of 1.7 MHz. The precursor droplet volume mean diameter was 5 ± 2 μm, measured based on the light scattering behavior of the droplets by the Malvern Ensemble Particle Condensation and Size (EPCS) System. (Kim et al. Citation2003) The tube reactor, of total length 81 cm, was placed in the furnace and operated at temperatures ranging from 750 to 1000°C. A 2.5 L/min industrial nitrogen (99.5%, Airgas) was used to carry the droplets from generator to furnace. The residence time was defined as the time for the droplet to pass through the heated zone, from the beginning to the end of the furnace. It varied from 1.5 to 2 s depending on the furnace operating temperature. Downstream of the furnace, industrial grade 10 L/min nitrogen (95%, Airgas) was used as a quench gas to cool down the generated particles. The final product was collected by filtration.
Scanning electron microscopy (SEM, Hitachi SU-70) was used to image the particles. In order to observe the internal patchy structure of AgCu particles, Cu was removed by immersing AgCu particles into 2 M FeCl3 solution. After 2 h of ultrasonic treatment, the Cu phase was completely dissolved in the FeCl3. Pretreated particles were separated by centrifuge, washed by distilled (DI) water and dried at room temperature. All the particles were examined with an X-ray diffractometer (XRD, Bruker Smart 1000) with the diffraction angle ranging from 20o to 90o. More detailed information about crystal structure and phase abundance was obtained from Rietveld refinement.
RESULT AND DISCUSSION
AgNi bimetallic particles were generated by the cosolvent assisted spray pyrolysis process from a 60 at% AgNO3 and 40 at% Ni(NO3)2 water solution. XRD () and Rietveld refinement indicated that particles were composed of 62 at% Ag and 38 at% Ni, close to the ratio in the precursor. The small deviation was possibly due to the calculation uncertainty of the refinement. Spherical particles with diameters of 0.6 ± 0.3 μm were observed from backscatter SEM images as shown in . The particle surfaces were mostly light with a small fraction appearing dark. EDS mapping () indicated that the light regions contained a high concentration of silver, represented by red dots, and the dark areas were enriched in Ni, represented by green in the mapping image. Quantitative analysis of energy dispersive spectroscopy (EDS) suggested a composition of 75 ± 3 at% Ag and 24 ± 3 at% Ni. Compared with the XRD result, a higher concentration of Ag was obtained from EDS, which is more sensitive, suggesting that the particle surface is Ag rich, with most of the Ni concentrated in the center of the particle.
FIG. 1 (a) XRD of AgNi particles, composed of 60 at% Ag and 40 at% Ni and generated at 1000°C; (b) backscatter SEM images of AgNi particles; (c) EDS mapping of AgNi particles; (d) XRD of CuNi particles, which were composed of 60 at% Cu and 40 at% Ni and generated at 1000°C; (e) SEM images of CuNi particles; (f) EDS mapping of CuNi particles; (g) XRD of AgCu particles, composed of 60 at% Ag and 40 at% Cu and generated at 1000°C; (h) backscatter SEM images of AgCu particles with inserted SEM images of particles with Cu solid solution removed; and (i) EDS mapping of AgCu particles. (Color figure available online.)
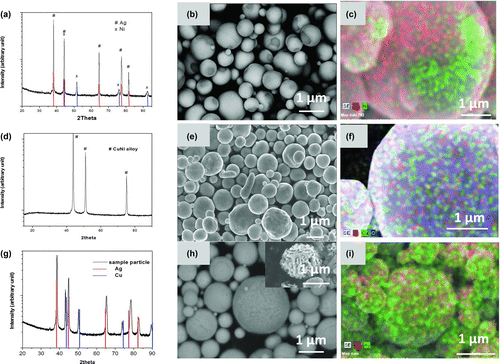
The formation of the Ag layer on the surface could be attributed to two reasons. One is the immiscibility of Ag and Ni, and the other is the low-wetting angle between Ag and Ni; in helium silver was reported to form a 9o contact angle on nickel (Nagesh and Pask 1983). During the particle formation process, one possible reaction process, based upon our observations of single component AgNO3 decomposition (Zhong et al. 2003) is that AgNO3 and Ni(NO3)2 hydrolyzed and decomposed to oxide, and then the oxides were reduced to metallic Ag and Ni. According to the Ag–Ni phase diagram (ASM 1992; see the online supplemental information), Ag and Ni have melting points at 961°C and 1455°C, and Ag and Ni are nearly immiscible below these melting points. Thus, after particles reach 1000°C, solid Ni and liquid Ag may coexist, and the two phases should separate. The small wetting angle between Ag and Ni will allow the liquid silver to spread, nearly covering the Ni surface. At the end of the furnace, 10 L/min nitrogen was used as quench gas to cool down the particles. As particles cooled, liquid Ag solidified to a nearly continuous layer on the surface of the particle.
The 60/40 at% CuNi bimetallic particles were generated by spray pyrolysis using a Cu(NO3)2/Ni(NO3)2 solution with 40 volume % of EG as the precursor. XRD, SEM, and EDS were showed in . XRD results () indicated that the particles were composed of a single alloy phase. In the SEM images (), spherical particles were observed. EDS mapping () showed a uniform distribution of Cu and Ni on the surface, represented by red and green dots respectively. Quantitative analysis of the EDS gave a surface composition of 63 ± 3 at% Cu and 37 ± 3 at% Ni, close to the initial ratio of Cu and Ni in the precursor. This also indicated that there were no differences in the composition between the surface and the center of the particles. According to the phase diagram (ASM 1992; see the online supplemental information), CuNi alloy is the thermodynamically favored state at temperatures between 354°C and 1064°C, which is the melting point of pure copper. At the operating temperature of 1000°C, Cu and Ni were mixed at the atomic level, and a single alloy phase was generated to achieve a lower free energy of the system.
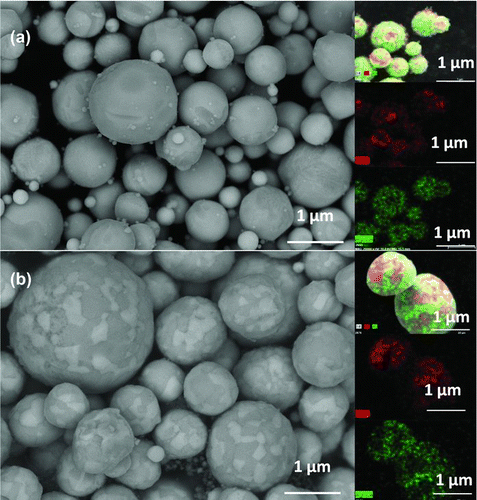
FIG. 2(a) SEM images of AgCu particles, which were composed of 60 at% Ag and 40 at% Cu and generated at 750°C. A 2.5 L/min N2 was used as carrier gas. (b) Backscatter SEM images of AgCu. Particles were composed of 40 at% Ag and 60 at% cu and generated at 750°C. 2.5 L/min N2 was used as carrier gas. (Color figure available online.)
More complicated particle structures were observed when spray pyrolysis was applied to the generation of 60/40 at% AgCu particles from AgNO3/Cu(NO3)2 mixture precursor. XRD results shown in indicated that particles were composed of two phases. One is an Ag rich solid solution. A right shift of the diffraction peaks of Ag solid solution was observed, compared with the diffraction peaks of pure Ag (ICDD PDF No. 01-071-3761) labeled by the green line. Rietveld refinement indicated that the Ag solid solution was composed of 91 at% Ag and 9 at% Cu. Cu rich solid solution was also detected with a composition of 91 at% Cu and 9 at% Ag. Diffraction peaks attributed to the Cu solid solution were shifted toward smaller angles compared with the diffraction peaks from pure Cu (ICDD PDF No. 01-071-3762), which are labeled by the red line. The composition of the particles is 62 at% Ag and 38 at% Cu, quite close to the initial ratio of 60 at% Ag and 40 at% Cu.
Backscatter SEM images are shown in . Because of composition differences, a contrast was observed in the image of the particle surface. Ag is a stronger electron scatterer than Cu, so the light area represented silver rich solid solution, and dark area is copper rich solid solution. These two solid solutions are intermingled at the nanoscale. EDS mapping () showed a mixture of green dots and red dots representing Ag and Cu respectively. There is no nanostructure detected from EDS mapping, because of the spatial resolution limit of the equipment. Quantitative analysis of EDS suggested a composition of 59 ± 2 at% Ag and 41 ± 5 at% Cu in agreement with the salt concentration in the precursor.
In order to further investigate the structure of the AgCu particles, the Cu rich solid solution phase in the particles was removed by etching using a FeCl3 solution. A SEM image of an Ag rich solid solution skeleton with a spherical outline is displayed in . Stripes were mostly in a direction normal to the surface with a distance between adjacent strip layers of around 50 nm. The lamellar structure of particles indicates a possibility of eutectic decomposition. As shown in the phase diagram (ASM 1992; see the online supplemental information), Ag–Cu alloy has an eutectic temperature of 779°C, which is lower than our furnace operating temperature of 1000°C, so in the furnace, the temperature is high enough for melting and complete mixing of Ag and Cu. During the quench process, the cooling of the particles led to a supercooled state possibly resulting in spinodal decomposition forming the bimetallic layer as we observed in .
A formation process for AgCu bimetallic particles is proposed. First, AgNO3 and Cu(NO3)2 precipitate and decompose after water and EG evaporate. The separation of Ag and Cu nitrate probably happened during the evaporation because of their different solubility in the water/EG solvent: 71.5 g AgNO3/100 g H2O saturated solution and 56.0 g Cu(NO3)2·6H2O/100 g H2O saturated solution at room temperature (Speight Citation2004). At higher temperatures, pure Ag and Cu form from the oxide. At temperatures higher than 779°C, Ag and Cu melt and mix together. In the final step, the particles composed of Ag rich and Cu rich solutions form, likely by spinodal decomposition during the quenching process, resulting in a nanoscale lamellar structure. During the AgNi particle formation process, Ag and Ni may separate as AgNO3 and Ni(NO3)2 precipitate separately because of the different solubilities (77.0 g Ni(NO3)2·6H2O/100 g H2O saturated solution at room temperature) (Speight Citation2004). Because Ag and Ni has melting temperatures of 962°C and 1455°C, at a generation temperature of 1000°C, only Ag melts and surrounds the Ni which is still in the solid state. After the particles cool down, the particle surface is nearly covered by Ag as observed in . A more detailed discussion, including estimates of relevant timescales, is given in the online supplemental information.
According to the results, the phase separation behavior appears to be mainly determined by the thermodynamic properties of the different metal components. However, further experiments indicated that the shape and size of the patchiness could be controlled by changing the operating conditions. In the generation of AgCu particles, reducing the temperature to below the eutectic point can lead to a more segregated mixture of the two components. shows a SEM image of AgCu particles generated from 60% AgNO3 and 40% Cu(NO3)2 at a temperature of 750°C, and a carrier gas flow rate of 2.5 L/min. Silver and copper rich phases coexisted in the particles, indicated by green and red in EDS mapping separately. Separation of Ag and Cu probably began during the evaporation process due to different solubilities of silver nitrate and copper nitrate, and, because the generation temperature is lower than the eutectic point, a segregated structure was preserved through to the final product particles rather than the lamellar structure observed at 1000°C.
Different ratios of patchiness can be obtained by changing the composition of precursor. shows particles composed of 40 at% Ag and 60 at% Cu. The particles were generated at a temperature of 750°C with a carrier gas flow rate of 2.5 L/min. A smaller portion of the surface was covered by Ag patches compared to particles composed of 60 at% Ag. More details of the bimetallic particle generation and properties are under investigation.
CONCLUSIONS
In conclusion, cosolvent spray pyrolysis is an effective way to make bimetallic patchy particles. AgNi and AgCu patchy particles were generated with different morphologies and structures because of the thermodynamic properties of the components. Different morphologies can also be obtained by changing the operating temperature and residence time indicating the possibility of generating particles with more functional structures. More research results on the detailed reaction steps and resulting particle properties will be reported in the future.
Supplemental File.zip
Download Zip (552 KB)Acknowledgments
[Supplementary materials are available for this article. Go to the publisher's online edition of Aerosol Science and Technology to view the free supplementary files.]
REFERENCES
- ASM . 1992 . ASM Handbook: Alloy Phase Diagrams (Asm Handbook) , 10th ed. , Vol. 3 , OH , , USA : ASM International, Materials Park .
- Casagrande , C. , Fabre , P. , Raphael , E. and Veyssie , M. 1989 . Janus Beads-Realization and Behavior at Water Oil Interfaces . Europhys. Lett., , 9 ( 3 ) : 251 – 255 .
- Cayre , O. , Paunov , V. N. and Velev , O. D. 2003 . Fabrication of Asymmetrically Coated Colloid Particles by Microcontact Printing Techniques . J. Mater. Chem. , 13 ( 10 ) : 2445–2450
- Champion , J. A. , Katare , Y. K. and Mitragotri , S. 2007 . Making Polymeric Micro-and Nanoparticles of Complex Shapes . Proc. Nat. Acad. Sci., USA. , 104 ( 29 ) : 11901–11904
- Choa , Y. , Yang , J. , Kim , B. H. , Jeong , Y. K. , Lee , J. S. and Nakayama , T. 2003 . Preparation and Characterization of Metal/Ceramic Nanoporous Nanocomposite Powders . J. Magn. Magn. Mater. , 266 ( 1–2 ) : 12 – 19 .
- Guo , J. C. , Liu , Q. , Wang , C. S. and Zachariah , M. R. 2012 . Interdispersed Amorphous MnO x –Carbon Nanocomposites with Superior Electrochemical Performance as Lithium- Storage Material . Adv. Funct. Mater. , 22 ( 4 ) : 803 – 811 .
- Gurav , A. , Kodas , T. , Pluym , T. and Xiong , Y. 1993 . Aerosol Processing of Materials . Aerosol Sc. & Tech. , 19 ( 4 ) : 411 – 452 .
- Haynes , C. L. and Van Duyne , R. P. 2001 . Nanosphere Lithography: A Versatile Nanofabrication Tool for Studies of Size-Dependent Nanoparticle Optics . J. Phys. Chem. B. , 105 ( 24 ) : 5599 – 5611 .
- Hong , L. , Jiang , S. and Granick , S. 2006 . Simple Method to Produce Janus Colloidal Particles in Large Quantity . Langmuir. , 22 ( 23 ) : 9495 – 9499 .
- Jian , N. , Li , Z. , Fan , Y. and Zhao , M. 2007 . Synthesis of Carbon Encapsulated Magnetic Nanoparticles with Giant Coercivity by a Spray Pyrolysis Approach . J. Phys. Chem. B. , 111 ( 8 ) : 2119 – 2124 .
- Kim , J. H. , Babushok , V. I. , Germer , T. A. , Mulholland , G. W. and Ehrman , S. H. 2003 . Cosolvent-Assisted Spray Pyrolysis for the Generation of Metal Particles . J. Mater. Res. , 18 ( 7 ) : 1614 – 1622 .
- Langer , R. and Tirrell , D. 2004 . Designing Materials for Biology and Medicine . Nature , 428 ( 6982 ) : 487 – 492 .
- Liddell , C. M. , Summers , C. J. and Gokhale , A. M. 2003 . Stereological Estimation of the Morphology Distribution of ZnS Clusters for Photonic Crystal Applications . Mater. Charact. , 50 ( 1 ) : 69 – 79 .
- Nagashima , K. , Morimitsu , Y. and Kato , A. 1987 . Preparation of Fine Metal Particles from Aqueous Solution s of Metal Nitrate by Chemical Flame Method . Nippon Kagaku Kaishi , : 2293 – 2300 . (12)
- Nagesh , V. K. and Pask , J. A. 1983 . Wetting of Nickel by Silver . J. Mater. Sci. , 18 ( 9 ) : 2665 – 2670 .
- Nisisako , T. , Torii , T. and Higuchi , T. 2004 . Novel Microreactors for Functional Polymer Beads.” . Chem. Eng. J , 101 ( 1–3 ) : 23 – 29 .
- Ozin , G. A. , Manners , I. , Fournier-Bidoz , S. and Arsenault , A. 2005 . Dream Nanomachines . Adv. Mater. , 17 ( 24 ) : 3011 – 3018 .
- Paunov , V. N. and Cayre , O. J. 2004 . Supraparticles and ‘Janus’ Particles Fabricated by Replication of Particle Monolayers at Liquid Surfaces Using a Gel Trapping Technique . Adv. Mater. , 16 ( 9-10 ) : 788 – 791 .
- Pawar , A. B. and Kretzschmar , I. 2010 . Fabrication, Assembly, and Application of Patchy Particles . Macromol. Rapid Commun. , 31 ( 2 ) : 150 – 168 .
- Speight , J. 2004 . Lange's Handbook of Chemistry, 70th Anniversary Edition. , 16th ed. , McGraw-Hill Professional, New York, USA .
- Takei , H. and Shimizu , N. 1997 . Gradient Sensitive Microscopic Probes Prepared by Gold Evaporation and Chemisorption on Latex Spheres . Langmuir. , 13 ( 7 ) : 1865 – 1868 .
- Yao , S. D. , Song , C. J. , Nan , F. H. , Botton , G. A. , Chen , J. W. and Fairbridge , C. 2012 . Synthesis of Hierarchical Structured Porous MoS2/SiO2 Microspheres by Ultrasonic Spray Pyrolysis . Can. J. Chem. Eng. , 90 ( 2 ) : 330 – 335 .
- Zhang , Z. L. and Glotzer , S. C. 2004 . Self-Assembly of Patchy Particles . Nano Lett. , 4 ( 8 ) : 1407 – 1413 .
- Zhong , K. , Peabody , Q. , Langrock , A. , Glicksman , H. and Ehrman , S. H. 2012 . Particle Generation by Co-Solvent Spray Pyrolysis Process: Effects of Ethanol and Ethylene Glycol . J. Mater. Res. , (accepted 29 June 2012)