Abstract
Most aerosol chemical characterization studies to date involve bulk particle analysis. The surface chemical and physical properties of aerosol particles have rarely been analyzed, despite the particles’ potential health impacts and interactions with gas in the atmosphere. Aerosol particles ranging from 0.056 to 10 μm in size collected using a 10-stage impactor sampler from a busy walkway in a downtown area of Hong Kong were analyzed using X-ray photoelectron spectroscopy (XPS), a technique providing both elemental and chemical state information about the particle surfaces. Six key elements were detected: nitrogen (N), sulfur (S), calcium (Ca), silicon (Si), oxygen (O), and carbon (C). Carbon was the dominant species on the surfaces of all particles regardless of their sizes. A higher carbon concentration was found on the surfaces of the 0.056–0.32 μm particles. The N, Si, Ca, and O concentrations were higher on the surface of the 3.2–10 μm particles than in the smaller size fractions. Sulfur was mainly found on the surface of the 0.32–1.8 μm particles. High-resolution scans of C, N, and S were obtained to provide chemical state information about these elements. Aromatic C-H and aliphatic C-H were found to be the major carbon chemical states. Fullerenic carbon was detected on the surfaces of the finest (0.056–0.32 μm) particles. Oxygen- and nitrogen-containing organics were found on the surfaces of the 0.32–1.8 μm particles. Sulfur was present in the form of sulfates as expected. Ammonium salts, amide, and nitrate were found to form especially on the surfaces of aerosol particles in the nucleation, accumulation, and coarse modes, respectively. Silicates and carbonates were only discovered on the surfaces of coarse-mode particles (3.2–10 μm). The results suggest that both the chemical elements and their chemical states were significantly dependent on the size of the aerosol particles.
Copyright 2013 American Association for Aerosol Research
INTRODUCTION
Aerosol is defined as a system of solid or liquid particles suspended in air or a gaseous environment (Seinfeld and Pandis Citation2006). Atmospheric aerosol particles have multiple impacts on the environment, with health impacts causing the most concern in urban settings (Sorensen et al. Citation2003; Pope et al. Citation2009; Klejnowski et al. Citation2012). Numerous epidemiological studies have demonstrated a positive association between exposure to aerosol particles and adverse respiratory consequences (Rogula-Kozlowska et al. Citation2008; Klejnowski et al. Citation2012). Evidence shows that the toxicological and carcinogenic effects of aerosol particles depend on their sizes and chemical compositions (Diociaiuti et al. Citation2001; Cho et al. Citation2009). Aerosol particles of a specific size range have distinct sources, formation mechanisms, and chemical compositions (Hering and Friedlander Citation1982; Huang et al. Citation2006). Particles with aerodynamic diameters less than 10 μm (PM10), in particular, can pass through the nose and mouth (Klejnowski et al. Citation2012). Particles with aerodynamic diameters less than 2.5 μm (PM2.5), which can enter the respiratory tract and reach the deeper parts of the lung, have been routinely measured to determine their toxic and carcinogenic potential (Spurny Citation2000; Kappos et al. Citation2004). Particles between 1 and 0.1 μm in diameter are believed to be the most toxic components of atmospheric aerosols (Kappos et al. Citation2004; Spindler et al. Citation2010; Klejnowski et al. Citation2012). Many studies have been performed to determine the bulk chemical composition of aerosol particles. However, surface analysis techniques have not been widely used in the study of aerosols possibly because the analyses of a large number of samples are not practical. Since the surface chemical composition of airborne particles critically determines their impact on human health due to their high specific surface areas, not to mention the fact that the surface comes into direct contact with biological fluids after inhalation (Kendall et al. Citation2001), it is important to establish the relationship between surface chemical properties and particle size.
X-ray photoelectron spectroscopy (XPS) is a popular surface analysis technique. A number of studies have used XPS to analyze particulate matters collected with filters (Hutton and Williams Citation2000; Zhu et al. Citation2001; Paoletti et al. Citation2003; Song and Peng Citation2009; Vander Wal et al. Citation2011). XPS analysis provides important information about elemental concentrations and chemical states (Faude and Goschnick Citation1997; Lei et al. Citation2003; Lau et al. Citation2009; Ng et al. Citation2011). Samples can be directly examined by XPS without any sample preparation (Gilham et al. Citation2008). The concentration of elements can be determined with an accuracy of up to 0.1 atm%. Chemical states of elements can also be determined by peak synthesis (Briggs and Seah Citation1990; Monteil-Rivera et al. Citation2000; Olivella et al. Citation2002; Paoletti et al. Citation2003).
In this study, we used XPS to analyze aerosol particles with sizes ranging from 0.056 to 10 μm collected with a MOUDI (MSP Corp, Model 100). The size-fractionated aerosol samples were collected from a walkway in Wanchai, a downtown area of Hong Kong. Hong Kong, located on China's south coast, is enclosed by the Pearl River Delta (PRD) and the South China Sea. With a land area of 1,104 km2 and a population of over seven million, Hong Kong is one of the most densely populated cities in the world. The continued increase in automobile exhaust emissions has caused severe air pollution problems (Chan and Yao Citation2008; Xue et al. Citation2011; He and Lu Citation2012). Particulates originating from mobile sources are thought to be responsible for various adverse health effects, ranging from asthma to lung cancer and cardiopulmonary disease (Rogula-Kozlowska et al. Citation2008; Cho et al. Citation2009).
The surface chemical composition of aerosol particles as a function of particle size was determined with XPS. Chemical states of carbon, nitrogen, and sulfur and their atomic concentrations were determined. This study clearly reveals the relationship between the size of aerosol particles and their chemical composition.
EXPERIMENTAL
Sample Collection
Aerosol samples were collected along Lockhart Road, Wanchai, a downtown district (22°16′40″N, 114°10′50″E) of Hong Kong. Lockhart Road was chosen because it is a heavy traffic road and is lined with tall buildings. A 10-stage micro-orifice uniform deposit impactor (MOUDI, MSP Corp., Shoreview, MN, USA) was used for the sample collection. The sampling inlet was 1.5 m above the ground. The flow rate was 30 L/min, and the parameters of each stage are shown in . As a particle entered the collector, it was classified and deposited on a specific stage according to its size. Two sets of samples were collected during daytime on 7 and 8 August 2012. The total collection time was 8 h and the roadside conditions were assumed stable. Vehicles were the dominant source of emissions. One set of samples was collected with Teflon filters on 7 August 2012 and the other with aluminum foil substrates on 8 August 2012. Particles with diameters below 0.056 μm or above 10 μm were not collected. The filters with the particles were wrapped in aluminum foil and stored in a freezer (−40°C) until analysis.
TABLE 1 Physical properties of the aerosols deposited on each stage of the impactor
XPS Analysis
The particles were analyzed using XPS to determine their surface elemental compositions and chemical states. The spectrometer (Axis Ultra DLD system, Kratos Analytical) was equipped with a 150-watt monochromatic Al Kα X-ray radiation source (1486.6 eV). A take-off angle of 90°, which approximately corresponds to a sampling depth of 10 nm, was used. In order to obtain the average chemical surface compositions of the aerosol samples, the XPS beam was focused on a larger area of about 1 cm2 that covered many particles. The samples collected with Teflon filters on 7 August were subjected to elemental composition analysis and chemical-state identification of sulfur and nitrogen. Because the Teflon could contribute erroneous signals to the carbon peak, which could affect the determination of the pi–pi shake-up satellite peak, we used the samples collected on 8 August with aluminum foil substrates for the characterization of the chemical states of carbon. The samples collected on 7 August should be very similar to those collected on 8 August due to the fact that the samples were collected at the same location and the weather conditions of these two days were very similar. However, there might not be a direct link between these two results. The pass energy of 20, 40, and 100 eV was used for high-resolution, narrow and survey scans, respectively. Elements on the surfaces of the aerosols were identified in a survey scan and the relative amounts of the detected elements were determined using narrow scans. The high-resolution scans were analyzed using a peak-synthesis software provided by Kratos. A linear background and a standard line-shape analysis with a Lorentzian-Gaussian fitting function were used in the curve-fitting analysis.
RESULTS AND DISCUSSION
Surface Elemental Composition
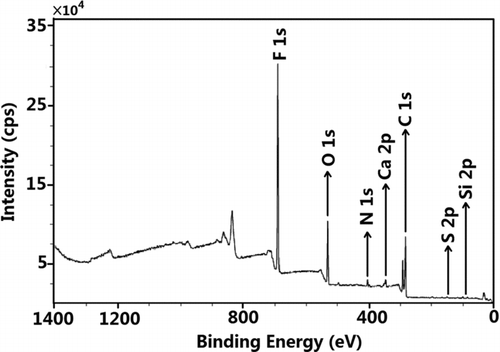
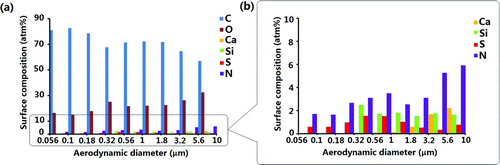
An XPS survey scan can be used to identify any elements on the surface of a sample because the survey scan spans a broad range of chemical binding energies (0 to 1400 eV). In order to determine the surface chemical composition of the aerosol particles as a function of particle size, nine groups of size-fractionated aerosol particles deposited on stage 2 to stage 10 of MOUDI were studied using XPS. The XPS spectra of the aerosols deposited on each stage show different surface chemical compositions. is the XPS survey scan of the aerosols deposited on stage 2. Nine elements, including F, C, O, N, S, Si, Ca, Na, and Mg, were detected. The contributions of the Teflon substrate to the XPS spectra were subtracted from all analyses. Na and Mg were also excluded from the analyses because their concentrations were quite low and could only be observed on the 5.6–10 μm particles. shows the atomic concentrations of different elements as a function of the particle size. The results show that carbon, followed by oxygen, was the dominant species on the surfaces of the aerosols regardless of their sizes, while the concentration of N, S, Si, and Ca were all below 10 atm%.
shows that the carbon atomic concentration reached above 80 atm% in the nucleation-mode 0.056–0.18 μm particles. For the larger accumulation-mode 0.32–1.8 μm particles, more sulfur- and nitrogen-containing compounds were discovered on their surfaces and there was a dramatic increase in the oxygen contribution. Silicon and calcium were present only on the surfaces of the particles larger than 0.18 and 1 μm in diameter, respectively. This might be due to the fact that the coarse-mode particles were mainly formed by mechanical attrition processes and hence soil dust and many industrial dusts are particles within this mode (Spurny Citation2000; Kim et al. Citation2003).
The results in clearly show that the surface chemical composition depends on the particle size. Carbon was the most abundant in the 0.056–0.1 μm particles. More elements were detected on the surfaces of the 0.1–1.8 μm particles and their surface compositions were relatively complex. Coarse-mode particle fractions, which were composed of inorganic materials, were found to have relatively low concentrations of carbon on their surfaces.
In order to obtain more in-depth information about the surfaces, the chemical states of carbon, sulfur, and nitrogen were determined.
Carbon Chemical States
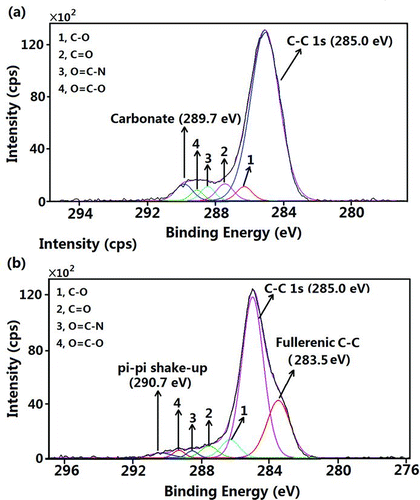
The carbon chemical states, which reveal important information about the surface chemical composition of the aerosols with various particle sizes, were distinguished using XPS high-resolution spectra obtained from the samples collected with aluminum foil substrates. and b show the C1s spectra of the aerosols deposited on stages 2 and 10, respectively. The carbonate carbon peak at 289.7 eV (Moulder et al. Citation1992) is clearly visible in . The lower binding energy of the C1s peak at 283.5 eV shown in indicates the possibility of the presence of fullerenic carbon (Chiang et al. Citation1994; Dresselhaus et al. Citation1996). The carbon pi–pi shake-up satellite peak at 290.7 eV (Muller et al. Citation2007) further confirms the presence of aromatic carbon. The fullerenes were first discovered by Kroto, Smalley, and Curl in 1985 (Kroto et al. Citation1985; Dementjev et al. Citation2000; Leiro et al. Citation2003). The fullerenic carbon has a remarkable cage-like polyhedral geometry that is the consequence of arranging sp2-bonded carbon atoms in pentagonal and hexagonal rings to form a closed shell (Grieco et al. Citation2000).
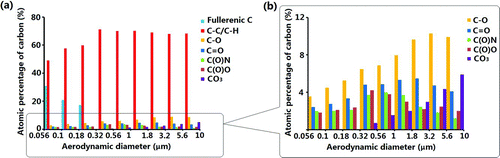
The Cls peak was resolved into five chemical states (five peaks): (1) the hydrocarbon group (C–C/ C–H), (2) the ether or alcohol group (C–O), (3) the carbonyl group (C˭O), (4) the amide group (O˭C–N), and (5) the carboxylic acid group (O˭C–O) as labeled in (Song and Peng Citation2009). The curve-fitting procedure first involved the assignment of these peaks to their corresponding binding energies of their suspected chemical states given in the literature (Beamson and Briggs Citation1992). The full width at half maximum of each chemical state was allowed to vary within physically realistic values (Liu et al. Citation2004; Chen et al. Citation2006). The fitting proceeded until the best fit was obtained between the experimental and computed spectra. The plot showing the atomic concentrations of the seven types of carbon as a function of particle size is shown in . It is obvious that the hydrocarbon group was the dominant carbon species on the surfaces of the particles. Fullerenic carbon existed only on the surfaces of the 0.056–0.32 μm particles. The concentrations of the amide and carboxylic groups were the highest on the surfaces of 0.32–1.8 μm particles. The alcohol and ketone groups were slightly more abundant in the larger particles. The O/C ratios for the fine particles (<2 μm) were calculated using the peaks areas of the oxygen-containing carbon chemical states to the total C1s peak (excluding the contribution from the inorganic carbon) and are shown in (Song and Peng Citation2009; Song et al. Citation2012). The results reveal that the O/C ratio increased as the particle size increased from 0.1 to 0.56 μm, suggesting that the accumulation-mode particles were more oxidized than the smaller fresher particles. This result was also confirmed with our ToF-SIMS spectra analysis. (Cheng et al. under review).
TABLE 2 The calculated organic O/C ratios for fine particles (0.056–1.8 μm)
Soot particles, which are produced during the combustion of fuels, are known to consist mainly of elemental carbon (EC) particles (Spurny Citation2000; Huang et al. Citation2006; Cheng et al. Citation2009). A high concentration of fullerenic carbon detected on the surfaces of the finest particles (soot particles) confirms that the nucleation-mode particles were mainly produced from the combustion of fossil fuels. The presence of this type of carbon can be explained by the deposition of gas-phase species, like polycyclic aromatic hydrocarbons (PAHs), on EC particles followed by internal rearrangement of the solid phase carbon (Grieco et al. Citation2000; Zhu et al. Citation2004). C–H was the main carbon state on the surfaces of the aerosol particles regardless of particle size due possibly to the condensation of aliphatic/aromatic organic materials on the surfaces of existing particles (Faude and Goschnick Citation1997; Song and Peng Citation2009). The surface organic composition of the accumulation-mode particles is more complex because of their longer atmospheric lifetime. More oxygen- and nitrogen-containing functional groups were found on their surfaces than on the surfaces of particles of other sizes. The carbonate distribution is similar to the calcium distribution shown in , indicating the presence of calcium carbonate particles in the coarse mode.
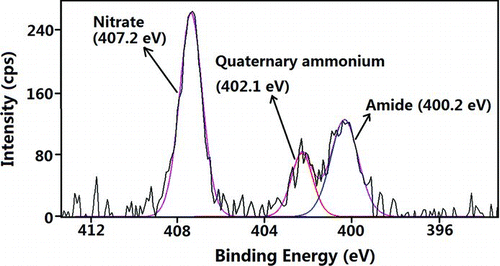
FIG. 5 PS high-resolution N1s spectrum of particles deposited on stage 4. (Color figure available online.)
From the XPS results, the soot particulates formed in the nucleation were coated with condensable materials (e.g., PAHs). As they grew to the accumulation mode, more organics from the gas phase might have condensed on the surfaces and some might have oxidized further to form oxygen-containing surface groups (Muller et al. Citation2007; Vander Wal et al. Citation2011). Thus, these particles had organic surface layers with multiple functional groups. High sulfur and nitrogen concentrations on the surfaces of the accumulation-mode particles also suggest that the reactive uptake of NOx and SO2 was quite feasible. Chemical reactions could further proceed among these adsorbed species (Prince et al. Citation2002; Vander Wal et al. Citation2011).
Sulfur and Nitrogen Chemical States
High-resolution S2p spectra (160–175 eV) reveal that there was only one peak at 168.0 eV for all samples, suggesting the presence of sulfate. shows that sulfate was abundant in the particles ranging from 0.32 to 1.8 μm in size/diameter. Sulfate is likely formed from photochemical oxidation of SO2 (Lazaridis and Skouloudis Citation1999).
High-resolution N1s spectra of the particles show three types of functional groups: the amide group (400.2 eV), the quaternary ammonium group (402.1 eV), and the nitrate group (407.2 eV) (Song and Peng Citation2009). A typical N1s spectrum is shown in . A plot showing the atomic concentration of nitrogen in these different functional groups as a function of particle size is shown in . The results suggest that nitrogen of the quaternary ammonium group (up to 90 atm%) was the major nitrogen component on the surfaces of the ultra-fine particles. Nitrate was found on the surfaces of the coarse-mode particles. The amide concentration was more abundant in the particles ranging from 0.32 to 3.2 μm in size/diameter, agreeing with the amide distribution determined by the synthesis of the C1s peaks shown in .
FIG. 6 Atomic concentration of nitrogen in different chemical states as a functional of particle size. (Color figure available online.)
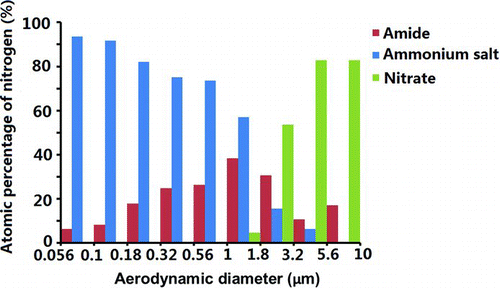
A higher concentration of ammonium salts on the surfaces of the fine particles suggests the formation of ammonium sulfate particles possibly due to the reaction between sulfuric acid and ammonia (Spurny Citation2000). The presence of amide on the surfaces of the accumulation-mode particles might be due to the reactions between condensed ammonia and the acid groups on the surfaces. The presence of nitrate on the surfaces of the coarse-mode particles was likely a result of the replacement of Cl− by NO3 − (Zhuang et al. Citation1999a). The size distributions of ammonium, sulfate, and nitrate are consistent with bulk particle analysis of MOUDI samples in Hong Kong (Zhuang et al. Citation1999b).
CONCLUSION
Traffic-related aerosol particle samples collected from a busy walkway in a downtown district of Hong Kong were examined with XPS. The surface compositions of the aerosol particles were found to be size-dependent. Carbon was the major component on the surfaces of all particles. Carbon chemical state determination showed that C–H, which included aliphatic carbon and aromatic carbon, was the dominant carbon state. Fullerenic carbon was found on the surfaces of nucleation-mode soot particles, which might have been produced from surface reactions among the condensed PAHs. As the particles grew in size, more organics from the gas phase were adsorbed on their surfaces. Some might have been oxidized to form oxygen-containing surface groups such as the carboxyl group, as indicated by the increase in the O/C ratio with particle size. The presence of carboxylic acids on the surfaces of the accumulation-mode particles also favored the deposition of ammonia gases to form amide. Therefore, the accumulation-mode particles were coated with organic layers with various functionalities. The coarse-mode particles were mainly inorganic particles with fewer organic coatings. The size distributions of inorganic species such as sulfate, ammonium salts, and nitrate in general followed previous findings in Hong Kong based on bulk chemical analysis. Most importantly, the surfaces of accumulation-mode particles had a diverse chemical composition. Since this size range accounts for a substantial percentage of the aerosol mass, the surface properties of particles falling within this size range warrants further investigation.
Acknowledgments
This work was fully supported by the University Grants Committee (Infrastructure Grant # SBI11IPO01) and the Research Grants Council (General Research Fund 610909).
REFERENCES
- Beamson , G. and Briggs , D. 1992 . High Resolution XPS of Organic Polymers, The Scienta ESCA 300 , Chichester : Wiley . Database
- Briggs , D. and Seah , M. P. 1990 . Practical Surface Analysis: Auger and X-Ray Photoelectron Spectroscopy , New York : Wiley .
- Chan , C. K. and Yao , X. 2008 . Air Pollution in Mega Cities in China . Atmos. Environ., , 42 : 1 – 42 .
- Chen , Z. J. , Lu , X. L. , Chan , C. M. and Mi , Y. L. 2006 . Manipulating the Surface Properties of Polyacrylamide with Nitrogen Plasma . Eur. Polym. J. , 42 : 2914 – 2920 .
- Cheng , Y. , Lee , S. C. , Cao , J. J. , Ho , K. F. , Chow , J. C. and Watson , J. G. 2009 . Elemental Composition of Airborne Aerosols at a Traffic Site and a Suburban Site in Hong Kong . Int. J. Environ. Pollut. , 36 : 166 – 179 .
- Cheng , W. , Weng , L. T. , Li , Y. , Lau , A. , Chan , C. K. and Chan , C. M. Aerosol Sci. Tech. , (under review)
- Chiang , L. Y. , Wang , L. Y. , Swirczewski , J. W. , Soled , S. and Cameron , S. 1994 . Efficient Synthesis of Polyhydroxylated Fullerene Derivatives via Hydrolysis of Polycyclosulfated Precursors . J. Org. Chem. , 59 : 3960 – 3968 .
- Cho , S. H. , Tong , H. Y. , McGee , J. K. , Baldauf , R. W. , Krantz , Q. T. and Gilmour , M. I. 2009 . Comparative Toxicity of Size-Fractionated Airborne Particulate Matter Collected at Different Distances from an Urban Highway . Environ. Health. Persp. , 117 : 1682 – 1689 .
- Dementjev , A. , Eletskii , A. , Bezmelnitsyn , V. and Maslakov , K. 2000 . Characterization of Carbon Atoms Chemical States in Nanotubes Containing Soot Materials and Fullerene by XPS, XAES Proceedings of AIP conference on Electronic Properties on Novel Materials, Austria, March 2000
- Diociaiuti , M. , Balduzzi , M. , De Berardis , B. , Cattani , G. Stacchini , G. 2001 . The Two PM2.5 (Fine) and PM2.5-10 (Coarse) Fractions: Evidence of Different Biological Activity . Environ. Res. , 86 : 254 – 262 .
- Dresselhaus , M. S. , Dresselhaus , G. and Eklund , P. C. 1996 . Science of Fullerenes and Carbon Nanotubes: Their Properties and Applications , 697 – 697 . San Diego : Academic Press .
- Faude , F. and Goschnick , J. 1997 . XPS, SIMS and SNMS Applied to a Combined Analysis of Aerosol Particles from a Region of Considerable Air Pollution in the Upper Rhine Valley . Fresen. J. Anal. Chem. , 358 : 67 – 72 .
- Gilham , R. J. J. , Spencer , S. J. , Butterfield , D. , Seah , M. P. and Quincey , P. G. 2008 . On the Applicability of XPS for Quantitative Total Organic and Elemental Carbon Analysis of Airborne Particulate Matter . Atmos. Environ. , 42 : 3888 – 3891 .
- Grieco , W. J. , Howard , J. B. , Rainey , L. C. and VanderSande , J. B. 2000 . Fullerenic Carbon in Combustion-Generated Soot . Carbon , 38 : 597 – 614 .
- He , H. D. and Lu , W. Z. 2012 . Urban Aerosol Particulates on Hong Kong Roadsides: Size Distribution and Concentration Levels with Time . Stoch. Env. Res. Risk A. , 26 : 177 – 187 .
- Hering , S. V. and Friedlander , S. K. 1982 . Origins of Aerosol Sulfur Size Distributions in the Los-angele Basin . Atmos. Environ. , 16 : 2647 – 2656 .
- Huang , X. F. , Yu , J. Z. , He , L. Y. and Yuan , Z. B. 2006 . Water-Soluble Organic Carbon and Oxalate in Aerosols at a Coastal Urban Site in China: Size Distribution Characteristics, Sources, and Formation Mechanisms . J. Geophys. Res-Atoms. , 111 : D22212
- Hutton , B. M. and Williams , D. E. 2000 . Assessment of X-Ray Photoelectron Spectroscopy for Analysis of Particulate Pollutants in Urban Air . Analyst , 125 : 1703 – 1706 .
- Kappos , A. D. , Bruckmann , P. , Eikmann , T. , Englert , N. , Heinrich , U. and Höppe , P. 2004 . Health Effects of Particles in Ambient Air . Int. J. Hyg. Envir. Heal. , 207 : 399 – 407 .
- Kendall , M. , Hutton , B. M. , Tetley , T. D. , Nieuwenhuijsen , M. J. , Wigzell , E. and Jones , F. H. 2001 . Investigation of Fine Atmospheric Particle Surfaces and Lung Lining Fluid Interactions Using XPS . Appl. Surf. Sci. , 178 : 27 – 36 .
- Kim , K. H. , Choi , G. H. , Kang , C. H. , Lee , J. H. , Kim , J. Y. and Youn , Y. H. 2003 . The Chemical Composition of Fine and Coarse Particles in Relation with the Asian Dust Events . Atmos. Environ. , 37 : 753 – 765 .
- Klejnowski , K. , Pastuszka , J. S. , Rogula-Kozlowska , W. , Talik , E. and Krasa , A. 2012 . Mass Size Distribution and Chemical Composition of the Surface Layer of Summer and Winter Airborne Particles in Zabrze, Poland . B. Environ. Contam. Tox. , 88 : 255 – 259 .
- Kroto , H. W. , Heath , J. R. , Oberien , S. C. , Curl , R. F. and Smalley , R. E. 1985 . C-60-Buckminsterfullerene . Nature , 318 : 162 – 163 .
- Lau , Y. T. R. , Schultz , J. M. , Weng , L. T. , Ng , K. M. and Chan , C. M. 2009 . Control of the Fold Surface Conformation of the Lamellae of an Oligomer . Langmuir , 25 : 8263 – 8267 .
- Lazaridis , M. and Skouloudis , A. 1999 . Computer Simulation of the Transport, Formation and Dynamics of Atmospheric Sulfate Particles . Water Air Soil Poll. , 112 : 171 – 185 .
- Lei , Y. G. , Ng , K. M. , Weng , L. T. , Chan , C. M. and Li , L. 2003 . XPS C 1s Binding Energies for Fluorocarbon-Hydrocarbon Microblock Copolymers . Surf. Interface Anal. , 35 : 852 – 855 .
- Leiro , J. A. , Heinonen , M. H. , Laiho , T. and Batirev , I. G. 2003 . Core-level XPS Spectra of Fullerene, Highly Oriented Pyrolitic Graphite, and Glassy Carbon . J. Electron Spectrosc. , 128 : 205 – 213 .
- Liu , S. Y. , Chan , C. M. , Weng , L. T. and Jiang , M. 2004 . Surface Quantitative Characterization of Poly (Styrene-co-4-vinyl Phenol)/Poly(Styrene-co-4-Vinyl Pyridine) Blends with Controlled Hydrogen Bonding Interactions . Polymer , 45 : 4945 – 4951 .
- Monteil-Rivera , F. , Brouwer , E. B. , Masset , S. , Deslandes , Y. and Dumonceau , J. 2000 . Combination of X-Ray Photoelectron and Solid-State C-13 Nuclear Magnetic Resonance Spectroscopy in the Structural Characterisation of Humic Acids . Anal. Chim. Acta. , 424 : 243 – 255 .
- Moulder , J. F. , Stickle , W. F. and Sobol , P. E. 1992 . Handbook of X-Ray Photoelectron Spectroscopy , Chichester : Perkin-Elmer Corporation .
- Muller , J. O. , Su , D. S. , Wild , U. and Schlogl , R. 2007 . Bulk and Surface Structural Investigations of Diesel Engine Soot and Carbon Black . Phys. Chem. Ch. Ph. , 9 : 4018 – 4025 .
- Ng , K. M. , Lau , Y. T. R. , Chan , C. M. , Weng , L. T. and Wu , J. S. 2011 . Surface Studies of Halloysite Nanotubes by XPS and ToF-SIMS . Surf. Interface Anal. , 43 : 795 – 802 .
- Olivella , M. A. , Palacios , J. M. , Vairavamurthy , A. , DelRio , J. C. and DeLasHeras , F. X. C. 2002 . A Study of Sulfur Functionalities in Fossil Fuels Using Destructive-(ASTM and Py-GC-MS) and Non-Destructive-(SEM-EDX, XANES and XPS) Techniques . Fuel , 81 : 405 – 411 .
- Paoletti , L. , DeBerardis , B. , Arrizza , L. , Passacantando , M. , Inglessis , M. and Mosca , M. 2003 . Seasonal Effects on the Physico-Chemical Characteristics of PM2.1 in Rome: A Study by SEM and XPS . Atmos. Environ. , 37 : 4869 – 4879 .
- Pope , C. A. , Ezzati , M. and Dockery , D. W. 2009 . Fine-Particulate Air Pollution and Life Expectancy in the United States . New. Engl. J. Med. , 360 : 376 – 386 .
- Prince , A. P. , Wade , J. L. , Grassian , V. H. , Kleiber , P. D. and Young , M. A. 2002 . Heterogeneous Reactions of Soot Aerosols with Nitrogen Dioxide and Nitric Acid: Atmospheric Chamber and Knudsen Cell Studies . Atmos. Environ. , 36 : 5729 – 5740 .
- Rogula-Kozlowska , W. , Pastuszka , J. S. and Talik , E. 2008 . Influence of Vehicular Traffic on Concentration and Particle Surface Composition of PM10 and PM2.5 in Zabrze, Poland . Pol. J. Environ. Stud. , 17 : 539 – 548 .
- Seinfeld , J. H. and Pandis , S. N. 2006 . Atmospheric Chemistry and Physics , Hoboken, NJ. : Wiley-Interscience .
- Song , J. Z. , He , L. L. , Peng , P. A. , Zhao , J. P. and Ma , S. X. 2012 . Chemical and Isotopic Composition of Humic-Like Substances (HULIS) in Ambient Aerosols in Guangzhou, South China . Aerosol Sci. Tech. , 46 : 533 – 546 .
- Song , J. Z. and Peng , P. A. 2009 . Surface Characterization of Aerosol Particles in Guangzhou, China: A Study by XPS . Aerosol Sci. Tech. , 43 : 1230 – 1242 .
- Sorensen , M. , Autrup , H. , Moller , P. , Hertel , O. , Jensen , S. S. and Vinzents , P. 2003 . Linking Exposure to Environmental Pollutants with Biological Effects . Mutat. Res-Rev. Mutat. , 544 : 255 – 271 .
- Spindler , G. , Bruggemann , E. , Gnauk , T. , Gruner , A. , Muller , K. and Herrmann , H. 2010 . A Four-Year Size-Segregated Characterization Study of Particles PM(10), PM(2.5) and PM(1) Depending on Air Mass Origin at Melpitz . Atmos. Environ. , 44 : 164 – 173 .
- Spurny , K. R. 2000 . Aerosol Chemical Process in the Environment , New York : Lewis .
- Vander Wal , R. L. , Bryg , V. M. and Hays , M. D. 2011 . XPS Analysis of Combustion Aerosols for Chemical Composition, Surface Chemistry, and Carbon Chemical State . Anal. Chem. , 83 : 1924 – 1930 .
- Xue , J. , Lau , A. K. H. and Yu , J. Z. 2011 . A Study of Acidity on PM(2.5) in Hong Kong Using Online Ionic Chemical Composition Measurements . Atmos. Environ. , 45 : 7081 – 7088 .
- Zhu , W. Z. , Miser , D. E. , Chan , W. G. and Hajaligol , M. R. 2004 . Characterization of Combustion Fullerene Soot, C-60, and Mixed Fullerene . Carbon , 42 : 1463 – 1471 .
- Zhu , Y. J. , Olson , N. and Beebe , T. P. 2001 . Surface Chemical Characterization of 2.5-mu m Particulates (PM2.5) from Air Pollution in Salt Lake City Using TOF-SIMS, XPS, and FTIR . Environ. Sci. Technol. , 35 : 3113 – 3121 .
- Zhuang , H. , Chan , C. K. , Fang , M. and Wexler , A. S. 1999a . Formation of Nitrate and Non-sea-salt Sulfate on Coarse Particles . Atmos. Environ. , 33 : 4223 – 4233 .
- Zhuang , H. , Chan , C. K. , Fang , M. and Wexler , A. S. 1999b . Size Distributions of Particulate Sulfate, Nitrate, and Ammonium at a Coastal Site in Hong Kong . Atmos. Environ. , 33 : 843 – 853 .