Abstract
We conducted a detailed evaluation of a method for measuring the mass concentrations and size distributions of black carbon (BC) particles in rainwater and snow. The method uses an ultrasonic nebulizer (USN) and a single particle soot photometer (SP2). The USN disperses sample water into micron-size droplets at a constant rate and then dries them to release BC particles into the air. The masses of individual BC particles are measured by the SP2, using the laser-induced incandescence technique. The loss of BC particles during the extraction from liquid water to air depends on their sizes. We determined the size-dependent extraction efficiency using polystyrene latex (PSL) spheres with 12 different diameters between 107 and 1025 nm. The PSL concentrations in water were measured by the light extinction at 532 nm. The extraction efficiency of the USN showed a broad maximum of about 10% in the diameter range 200–500 nm and decreased substantially at larger sizes. The accuracy and reproducibility of the measured mass concentration of BC in sample water after long-term storage were about ±25% and ±35%, respectively. We tested the method by analyzing rainwater and surface snow samples collected in Okinawa and Sapporo, respectively. The measured number size distributions of BC in these samples showed negligible contributions of BC particles larger than 300 nm to the total number of BC particles. A dominant fraction of BC mass in these samples was observed in the diameter range 100–500 nm.
Copyright 2013 American Association for Aerosol Research
1. INTRODUCTION
Black carbon (BC) aerosols are emitted by incomplete combustion of fossil fuels and biomass. They strongly absorb short-wave solar radiation and contribute significantly to global warming (Intergovernmental Panel on Climate Change [IPCC] Citation2007; Bond et al. Citation2013). BC particles deposited in or on snow can reduce snow albedo and may accelerate snow melting (Warren and Wiscombe Citation1980; Clarke and Noone Citation1985). The size distribution of BC in surface snow, in addition to its concentration, can be important for determining the extent of these effects (Schwarz et al. Citation2013). The temporal and spatial distributions of BC are controlled by its emission, transport, and removal during transport. It is necessary to understand these processes quantitatively to estimate the impact of BC on climate. The concentration and size distribution of BC in rainwater and snow are useful parameters for detailed understanding of the wet deposition of BC.
Three principal methods are currently used for quantifying the mass concentration of BC material in liquid water samples. The first method utilizes the thermo-optical technique (Ogren et al. Citation1983; Hadley et al. Citation2008; Wang et al. Citation2011). In this method, the liquid water sample is filtered, and the BC particles retained on the filter are thermally converted into CO2. The total mass of BC is derived by quantifying the CO2 concentration while monitoring the transmittance or reflectance of the heated filter. This method can also provide the mass concentration of organic carbon (OC) suspended in water samples. The major uncertainty of the method derives from the collection efficiency of the filter and separation of the BC/OC contributions in the analysis. Different protocols of the analytical procedures may introduce additional uncertainties. Wang et al. (Citation2011) also reported the effect of dust on the quantification of BC. The second method, which also uses a filter to collect particulate matter suspended in liquid samples, utilizes the integrating sphere/integrating sandwich spectrometer (ISSW) (Doherty et al. Citation2010; Granfell et al. Citation2011). The ISSW uses a multiple-wavelength light emitting diode and measures the attenuation of the light passing through the filter as a function of wavelength. Absorption by BC and non-BC material is differentiated by assuming the absorption angstrom exponent of the non-BC material. The mass concentration of BC is quantified from the BC absorption, via assumption of a mass absorption coefficient of BC. The major uncertainties in this method derive from the collection efficiency of the filter used and the assumed optical properties. The third method, which we evaluate in this study, consists of an ultrasonic nebulizer (USN) and a single particle soot photometer (SP2) (McConnell et al. Citation2007; Kaspari et al. Citation2011; Ohata et al. Citation2011; Schwarz et al. Citation2012). The USN disperses a liquid sample into micron-size droplets and then dries them to release BC particles into the air. The extracted particles are transferred to the SP2, which measures the masses of the individual BC particles. Therefore, this method can generally be used to measure the number and mass size distributions of BC in liquid water. Another advantage of this method is that it typically requires less than 5 mL of sample water for analysis, substantially less than that required for the filter-based techniques. In addition, the SP2 is little affected by dust. The main uncertainty of this method derives from determining the efficiency of the USN in releasing BC particles in liquid water to air. In most of the aforementioned studies, the efficiency is considered to be a constant independent of the properties of the particles in the liquid samples. However, Schwarz et al. (Citation2012) showed that the efficiency depends strongly on particle size, and the size dependence was different for the USN and the collison-type nebulizer (CTN) used in the study.
Despite the increasing use of the USN–SP2 method for measuring BC particles in rainwater, snow, and ice samples, in-depth evaluation of the method has not been completed. The purpose of this study is to make a systematic evaluation of this method. This includes determination of the size-dependent extraction efficiency of the USN, assessments of the accuracy and reproducibility of the measured mass concentration of BC in sample water, and comparison of the data obtained with the USN and the CTN.
In Section 2, we describe the experimental setup and laboratory water samples used in this study: suspensions of commercially available BC samples and polystyrene latex (PSL) spheres. In Section 3, we evaluate the size-dependent efficiency of the USN using PSL spheres and assess the accuracy of the measured mass concentration of BC in water using three kinds of laboratory BC samples. Then, we discuss the reproducibility of the measured mass concentration and size distribution of BC in rainwater collected in Tokyo (Section 4). We also examine the effect of agitation of BC samples by an ultrasonic bath prior to analysis on the measurement. In Section 5, we test the method by analyzing rainwater and snow samples collected at different locations in Japan and report their average BC size distributions. In Section 6, the size distributions of water measured by the present method are assessed by using the CTN. A summary and our conclusions are presented in Section 7.
2. METHODS
2.1. Experimental Setup
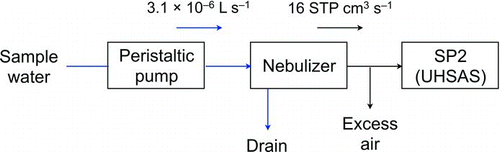
The experimental setup () is the same as that used in our previous study (Ohata et al. Citation2011). In addition, we used an ultra-high sensitivity aerosol spectrometer (UHSAS) for measurement of the PSL spheres (). A detailed description of the measurement system, which consists of a peristaltic pump (REGRO Analog; ISMATEC SA., Feldeggstrasse, Glattbrugg, Switzerland), a USN (U-5000AT; Cetac Technologies Inc., Omaha, NE, USA), and an SP2, is provided in Ohata et al. (Citation2011). In brief, the pump feeds sample water to the USN at a constant flow rate of 3.1 × 10−6 L s−1, and the USN converts a fraction of the sample water into micron-size (∼10 μm) droplets. The droplets evaporate while passing through a tube heated to 140°C, and the nonvolatile particles in the droplets are released into the air. A cooling stage at 3°C then removes the water vapor from the air. The extracted particles are transferred to the SP2 at a constant gas flow rate of 16 cm3 s−1 at standard temperature and pressure (STP). The masses of individual BC particles with mass-equivalent diameters of between 70 and 850 nm are measured without interference from nonabsorbing particles, using the laser-induced incandescence technique (Stephens et al. Citation2003; Schwarz et al. Citation2006; Moteki and Kondo Citation2007). The SP2 also has two avalanche photodiodes (APDs) for detecting scattering signals, which allow quantification of scattering particles with sizes between around 170 and 850 nm.
The UHSAS is an optical particle counter covering a wide range of scattering particles with sizes between about 60 and 1500 nm. Characterization of the UHSAS can be seen in Cai et al. (Citation2008). The UHSAS utilizes an intracavity Nd3+:YLF laser with a wavelength of 1054 nm and detects scattering signals by two pairs of Mangin mirrors and APDs. In the present study, we used the SP2 and the UHSAS to measure PSL spheres with sizes of 202–771 nm and 107–1025 nm, respectively, to determine the size-dependent extraction efficiency of the USN. PSL spheres with diameters of 202, 309, and 402 nm were used to check instrumental performance, and PSL concentrations in air measured by the SP2 and UHSAS were in agreement within 10%.
The extraction efficiency (ϵ) of the USN was determined as a function of the PSL diameter (D PSL) by the following equation:
Here, N SP2/UHSAS is the number concentration of PSL spheres in air measured by the SP2 or UHSAS (cm−3), F neb is the nebulizer gas flow rate (cm3 s−1), n samp (D PSL) is the size-resolved number concentration of PSLs in water (L−1), and V pump is the liquid flow rate of the pump (L s−1). n samp was determined by an extinction measurement, as detailed in Section 2.2. The values of the constants F neb and V pump are 16 STP cm3 s−1 and 3.1 × 10−6 L s−1, respectively. We assumed
2.2. PSL Number Concentration in Water
The diameters of the PSL spheres used in this study were 107, 152, 202, 254, 309, 402, and 814 nm (JSR Inc., Ibaraki, Japan) and 220, 356, 505, 771, and 1025 nm (Polysciences Inc., Warrington, PA, USA). n samp (D PSL) was determined for each PSL suspension by placing the PSL suspension in a rectangular acrylic cell and measuring the attenuation of a 532-nm laser beam (GSHG-3020F; KTG Co. Ltd., Kochi, Japan). n samp (D PSL) was determined by using the Lambert–Beer law (Hinds Citation1999):
The attenuation of the laser beam passing through the cell was maintained at >0.2 by using deionized water to control the concentrations of the PSL suspensions (the concentration of the original PSL suspension was ∼1012 L−1). Multiple scattering has little effect on the attenuation under this condition (Hinds Citation1999). The measured n samp (D PSL) was in the range of 109–1011 L−1, which was generated by diluting the original PSL suspensions with concentrations of ∼1012 L−1.
The measured n samp (D PSL) for the PSL spheres with diameters of 220, 356, 505, 771, and 1025 nm (Polysciences Inc., Warrington, PA, USA) agreed to within ±8% of the n samp (D PSL) calculated from the solid fraction weight of the PSL samples provided by the manufacturer. The number concentrations of the PSL suspensions (JSR Inc., Ibaraki, Japan) were generally not provided by the manufacturer, so we determined the number concentration of the 202-nm PSL suspensions (JSR Inc., Ibaraki, Japan) by drying it and weighing the residual solid PSL. The value obtained agreed with the laser-determined n samp to within ±8%.
2.3. Laboratory BC Samples
Three kinds of laboratory BC samples were tested: two fullerene soot samples (Alpha Aeser Inc., Wardhill, MA, USA, Stock No. 40971, Lots F12S011 and G25N20), which are dry powders; and AquaBlack 162 (Tokai Carbon Co. Ltd., Tokyo, Japan), a carbon black liquid ink. The incandescence–BC mass relationships for the SP2 were similar for the Lot F12S011 fullerene soot and ambient BC in Tokyo; therefore, this batch of fullerene soot is a suitable laboratory BC sample for calibrating the SP2 (Moteki and Kondo Citation2010; Baumgardner et al. Citation2012). In the present study, we also used fullerene soot from another batch (Lot G25N20). Ladorde et al. (Citation2012) have reported that the incandescence–BC mass relationship for the SP2 would be different between batches of fullerene soot. The procedure for generating known concentrations of fullerene soot in water is described in Schwarz et al. (Citation2012). The mixtures of fullerene soot and pure water were stored in a glass beaker for several days to allow large BC particles to settle out of solution. For the fullerene soot samples, a few weight percentage of methanol was added to fully disperse the BC particles in water. The upper portions of the mixtures were then slowly transferred to five glass bottles by using a peristaltic pump. The mass concentrations of BC in water in three of the bottles were determined by the gravimetric method, in which water is evaporated and the mass of the residual solid BC is measured with an electronic balance. The standard deviation of the measured mass concentrations of BC in the three bottles was less than 10%. The remaining bottles were used as master bottles, for which suitable mass concentrations of BC in water were generated by diluting the samples with a known amount of deionized water.
The BC particles in AquaBlack 162 were stably dispersed in water without sedimentation or coagulation, due to the manufacture's treatment of the surface of individual BC particles by carboxyl groups. The mass fraction of solid BC particles was 19.2% of the total (liquid and solid) mass of AquaBlack 162, according to the manufacturer, which agreed to within 5% with our measurement made by drying and weighing the sample. We controlled the mass concentration of BC in AquaBlack 162 samples by diluting them with deionized water.
FIG. 2 Normalized mass size distributions of laboratory BC samples: fullerene soot (FS, Lots F12S011 and G25N20) and AquaBlack 162. Bars indicate 1σ values derived from repeated measurements. The vertical axis is normalized by the integrated area of each size distribution between 70 and 850 nm to be 1. The diameter is equivalent assuming a void-free density of 2.0 g cm−3.
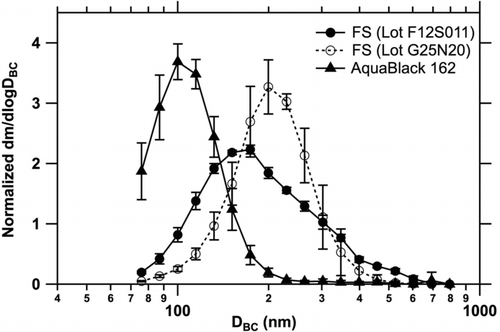
shows the measured mass size distributions of BC in the laboratory-generated BC samples. The size distributions were determined by taking the size-dependent extraction efficiency of the USN into consideration (discussed in Section 3.1). The detection range of the SP2 (70–850 nm) covers the whole mass size distribution of BC in the gravimetrically settled fullerene soot samples, and the mass of BC particles with diameters smaller than 70 nm and larger than 850 nm is negligible. For AquaBlack 162, the mass fraction of BC particles smaller than 70 nm was estimated to be (7 ± 3)%, by fitting a lognormal function to the BC mass size distribution.
3. SIZE-DEPENDENT NEBULIZER EFFICIENCY
A large fraction of the BC particles in the water samples is lost during nebulization and transport in the USN tubing. One reason for the loss is that some of the sample water is not converted into droplets on the vibrating surface of the USN and is removed through the drain together with the BC particles. The collision of some of the generated droplets with the inner walls of the tubing before evaporation also results in the loss of BC particles. These loss mechanisms immediately remove about 20%–30% of the BC mass. Another possible reason for the loss is that some aerosolized BC particles attach to the wall due to inertial impaction or thermophoresis effects. After repeated use of the nebulizer for a few years, we observed many black spots on the glass wall just downstream of the 140°C heater zone. This suggests that the thermal gradient between the heated airflow and the walls is relatively large in the area neighboring the heater zone, and therefore some BC particles deposit onto the relatively cold walls by thermophoresis. The size dependence of the extraction efficiency of the USN is possibly due to these loss mechanisms.
FIG. 3 Size-dependent extraction efficiency of the USN. Bars show 1σ values derived from repeated measurements over 6 months. For PSL spheres with diameters of 107, 152, 814, and 1025 nm, two PSL suspensions of known concentration were measured for each diameter within 1 day, and therefore their 1σ values were much smaller than those of the other data. The solid curve indicates Gaussian fitting to the data. Relative extraction efficiency of the USN reported in Schwarz et al. (Citation2012) is also shown. The efficiencies for PSL spheres with diameters of 220 and 350 nm were similar and therefore set to be 1, and the efficiencies for the other size are relative to them.
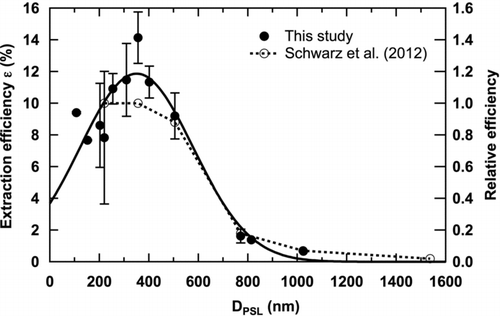
TABLE 1 Uncertainty and bias of the mass concentration of BC in liquid samples measured by the present method a
Using EquationEquation (1) and the size-resolved PSL suspensions detailed in Section 2.2, we determined the size-dependent extraction efficiency of the USN (). The number concentration of PSL spheres in air detected by the SP2 during each measurement was stable to within 10%. For these measurements, we counted each PSL sphere only within the appropriate size range. The fraction of doublets, which consist of two coagulated PSL spheres, was <9% from the SP2 and UHSAS data and was excluded from our analysis. The efficiency showed a broad maximum of about 10% in the diameter range 200–500 nm and decreased substantially at larger sizes. The size-dependent efficiency, in percent, was fitted with a Gaussian function:
The lower efficiency (<2%) for particles with diameters larger than about 800 nm as compared with the efficiency (∼10%) for particles with diameters of 200–500 nm is qualitatively consistent with that reported by Schwarz et al. (Citation2012), as shown in . Only a relative change versus diameters of the efficiencies of the two USNs is compared here, since their absolute values depend on various parameters, including V pump (Ohata et al. Citation2011). It is shown that the size dependence of the efficiency of the two USNs is very similar for the range of 200–1000 nm. The slight decrease in efficiency for smaller particles (<200 nm; ) may be caused by the thermophoretic effect because the thermophoretic velocity is larger for smaller particles (Hinds Citation1999).
The relative standard deviation of the discrepancies between the measured ϵ (D PSL) and the fitted curve (EquationEquation (5)) is 18% for the PSL spheres with diameters between 107 and 505 nm. Typical errors for dM SP2/dlogD BC, F neb, and V pump, in EquationEquation (3) were estimated to be 15%, 5%, and 5%, based on the repeated measurements. The overall uncertainty of the measured BC concentration in water (m samp) was calculated to be ±25% for this size range of BC and under the assumption that EquationEquation (2) is valid. The uncertainties in the mass concentration of BC in the liquid samples, together with the uncertainties derived in the following sections, are summarized in .
FIG. 4 BC mass concentrations in laboratory BC samples measured by the gravimetric method versus those measured by the present method. The solid line is the line fitted to all data and the dashed line is 1:1.
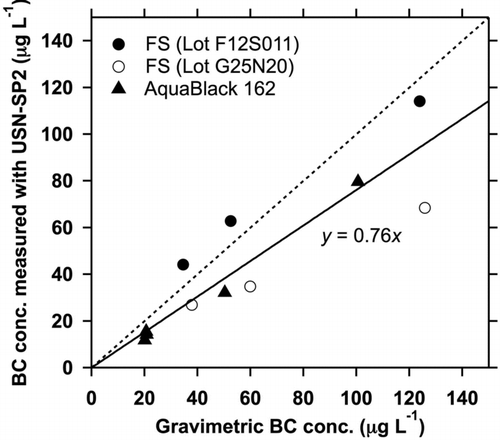
TABLE 2 BC mass concentrations in laboratory BC samples measured by the gravimetric method (m grav) versus those measured by the present method (m USN–SP2). Average number and mass size distributions of BC in the samples are also shown
To check the accuracy of the method, we measured the mass concentrations of the laboratory BC samples by using EquationEquations (3) and Equation(5) with the assumption of EquationEquation (2) and compared the concentrations with those determined by the gravimetric method described in Section 2.3 (). The agreement and BC size distribution for each sample are summarized in . and indicate that for samples containing BC particles with a mass median diameter of about 100–200 nm, the accuracy of the mass concentration of BC in water measured by the present method with the assumption of EquationEquation (2) is ±24%, which is within the uncertainty calculated above. This result supports the applicability of EquationEquation (2) to the measurement of BC in liquid samples by the present method, although this does not fully address the applicability of EquationEquation (2) to unknown samples containing BC. The mass size distributions of BC in water samples presented in this study were similar to those of the Lot F12S011 fullerene soot samples (Section 5). Therefore, the accuracy for measurement of these samples is estimated to be ±25%.
When the size distribution of BC in sample water is very different from that of the laboratory-generated BC, the uncertainty of the measured BC mass concentration in water can be large, because the relative uncertainty of ϵ for particles larger than 500 nm or smaller than 100 nm is larger than that for particles between 100 and 500 nm (). Schwarz et al. (Citation2012) found that the mass size distributions of BC in snow samples can be much larger than those in ambient air, and the measurement uncertainty under those conditions was estimated to be ±60% including the uncertainties of the extended SP2 calibration range and the efficiency of the CTN.
Extracted BC particles can be internally mixed with water-soluble species or coagulate with other insoluble species during the nebulization process. However, our previous studies showed that addition of ammonium sulfate (0.1–100 mg L−1) to a rainwater sample has negligible effects on the nebulizer efficiency (Ohata et al. Citation2011) and measured size distributions of BC (unpublished data). This concentration range of ammonium sulfate covers the typical values in rainwater observed in Japan (Okuda et al. Citation2005). This experimental result indicates that the effects of shift in particle size due to internal mixing of BC with non-BC compounds during the nebulization process on the measurement of BC should be very small.
4. REPRODUCIBILITY
4.1. Change of Size Distribution and Mass Concentration of BC in Water During Storage
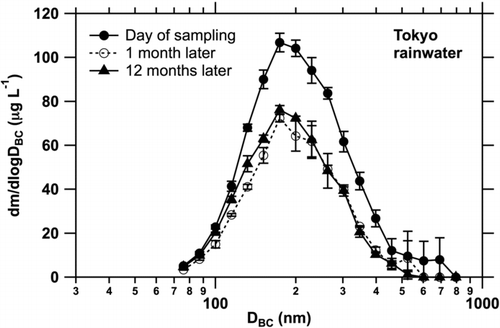
Rainwater was collected in glass beakers on 11 November 2011 in Tokyo to test the reproducibility of the measured BC size distributions and BC mass concentrations in samples after long-term storage. Immediately (<2 h) after sampling, the sample water was transferred to a glass bottle and analyzed. The mass concentration of BC in this sample was 48 μg L−1, which is typical for rainwater in mid-latitude urban sites (Ogren et al. Citation1984; Dasch and Cadle Citation1989). After analysis, the sample was kept in a refrigerator and reanalyzed about 1 month later and again 12 months later. The rainwater sample was agitated by an ultrasonic bath (UB) for 15 min just before analysis to disperse BC particles and detach them from the wall of the container. shows the measured mass size distribution of BC in this sample. The change in measured mass size distribution during storage was negligibly small, indicating that the effect of coagulation of BC particles in water during storage was not be significant for this sample. The change in measured total mass concentration of BC during storage was within 35% ().
FIG. 5 Mass size distributions of BC in rainwater repeatedly measured after long-term storage. Bars indicate 1σ values.
Reproducibility of the measured size distribution and mass concentration after long-term storage was also determined for another rainwater sample collected on 16 April 2011 at Cape Hedo (26.9°N, 128.3°E) in Okinawa over the East China Sea (remote site). Although this sample was not analyzed on the day of sampling, the shape of the BC size distribution measured after 2 and 9 months showed no significant difference, and the BC mass concentrations measured after 2 and 9 months were higher by 33% and lower by 10% than that of the first measurement, respectively. Therefore, reproducibility of the measured mass concentration for this sample was also within 35%.
Repeated measurements of a liquid sample within 1 day were reproducible to within ±10%. However, for samples stored for a longer time, the uncertainty of the overall measurement system, including both the stability of the liquid sample and the nebulizer efficiency, can cause a relatively low reproducibility (in our study, ±35% after 12 months of storage). A better reproducibility of the BC mass concentrations in Tokyo rainwater after the storage within 1 month has been reported by Ohata et al. (Citation2011).
4.2. Attachment of BC Particles to the Walls of Glass Container During Storage
To assess the loss of BC particles due to their attachment to the walls of the glass container during storage, five rainwater samples collected in Tokyo were analyzed after storage for 9 months in the refrigerator, with and without agitation by a UB for 15 min just before analysis. We found that when the samples were simply shaken by hand (i.e., no UB agitation), the mass concentrations of BC in all five samples were lower by (18 ± 13)% on average than the concentrations measured after UB agitation. This experiment was performed within 1 day, and therefore the underestimation is statistically significant, considering the good reproducibility that is obtained when samples are analyzed within a day.
The systematic underestimation described above indicates that the UB agitation just before analysis and transfer of sample water to other bottles is required for an improved analytical accuracy. The degree of underestimation reported here may depend on other factors, such as the period of sample storage and the ratio of water volume to area exposed to the glass surface. The size distribution of BC was little changed by UB agitation, indicating that UB agitation did not significantly break BC particles with diameters between 100 and 500 nm into smaller particles.
As shown in Figure 8 of Ohata et al. (Citation2011), the loss of BC particles to the glass walls during about 2 h at room temperature was negligible for a rainwater sample. Therefore, UB agitation during measurement was not necessary, because the time required for the analysis of a water sample was typically less than 10 min.
5. BC SIZE DISTRIBUTIONS IN RAINWATER AND SNOW SAMPLES
5.1. Field Samples
We analyzed rainwater samples collected in Tokyo and at Cape Hedo. In Tokyo, rainwater was collected in glass beakers and stored in glass bottles in a refrigerator. These samples were used to determine the reproducibility of the measurements (Section 4). At Cape Hedo, rainwater was collected daily in polyethylene bottles between April and July 2010. After storage for a few weeks in a refrigerator, the samples in the polyethylene bottles were transferred to glass bottles and stored in a refrigerator until analysis. The results of a long-term (>2 years) study of BC particles in rainwater from Cape Hedo and their climatologic implications will be reported in a separate article. In the present study, we determined the average size distribution of BC in rainwater samples collected during the period mentioned above to evaluate the USN–SP2 method.
We also applied the USN–SP2 method to snow samples collected in Sapporo, a semiurban area in northern Japan. Samples of the snow layer between the surface and 10-cm depth were collected in polyethylene bags twice per week from December 2011 to February 2012, and the samples were stored in a freezer for several months. Just before analysis, a fraction of each sample was put in a glass bottle and melted in a UB for 15 min.
5.2. BC Size Distributions in Water Samples
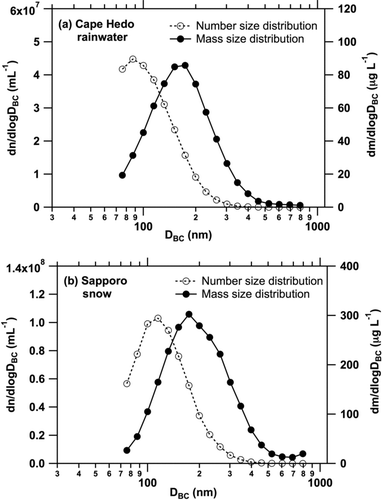
shows the average mass and number size distributions of BC in Cape Hedo rainwater (25 samples) and in Sapporo snow (10 samples). The average mass concentrations of BC in Cape Hedo rainwater and in Sapporo snow were 38 ± 52 and 142 ± 89 μg L−1, respectively. The average mass size distributions in for both the Cape Hedo rainwater and Sapporo snow show that the contributions of BC particles smaller than 70 nm and larger than 850 nm to the total mass of BC are estimated to be less than 5%, by fitting lognormal functions. The slight increase in the mass size distribution for size ranges larger than 700 nm () is due to a small number of large BC particles detected in some samples. For these rainwater and snow samples, which contain a dominant fraction of BC mass in the diameter range 100–500 nm, the USN–SP2 method enables measurement of the total BC mass concentration with an uncertainty of about ±25% (Section 3). Contributions of BC particles larger than 300 nm to the total number of BC particles were negligible for both the Cape Hedo and Sapporo samples.
If we assume, for example, a constant nebulizer efficiency of 10%, the measured mass concentrations of BC particles between 70 and 850 nm in the rainwater and snow samples are (15 ± 7)% lower than those determined using the size-dependent nebulizer efficiency, on average. Therefore, for liquid samples, which are known to contain a dominant fraction of BC in the diameter range 100–500 nm, it is possible to use a constant value for the nebulizer efficiency in deriving BC mass concentrations with a reasonable (∼30%) accuracy.
6. ULTRASONIC NEBULIZER COMPARED WITH COLLISON-TYPE NEBULIZER
To assess the contributions of BC particles with diameters larger than 500 nm to the total BC mass concentrations, we compared BC size distributions measured with a CTN with those measured with the USN. The CTN utilizes compressed air to extract BC particles from liquid water to air. A detailed description of a CTN of the same design as used in this study is given in Schwarz et al. (Citation2012). Because the CTN was used only to assess the effect of large BC particles, a detailed evaluation of the accuracy and reproducibility of data obtained with the CTN was not made in this study.
FIG. 7 (a) Normalized number and mass size distributions of BC in a Cape Hedo rainwater sample measured with the USN and the CTN. Bars indicate 1σ values of repeated measurements. (b) Normalized number and mass size distributions of BC in three Sapporo snow samples measured with the USN and the CTN (averaged). Bars indicate 1σ values. (Color figure available online.)
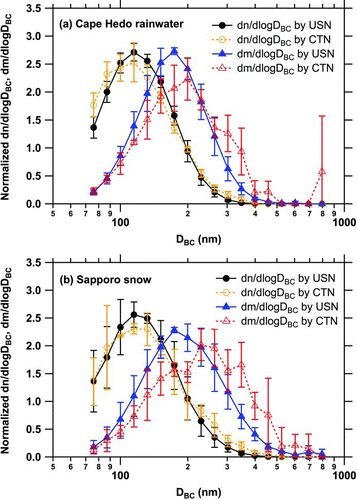
The extraction efficiency of the CTN was determined using PSL suspensions (Section 2.2). The efficiency depended little on PSL sizes (similar results were obtained for the 220-, 356-, 505-, and 771-nm particles), and the efficiency was (0.10 ± 0.010)%. shows normalized size distributions of BC for the Cape Hedo rainwater sample and those averaged for three Sapporo snow samples measured with the USN and CTN. This comparison confirms a negligible contribution of BC particles larger than 500 nm to the total BC amount in these samples.
To assess the effects of the ultrasonic vibrating surface of the USN on the measured BC size distribution, we collected USN-generated droplets of sample water from the first drain of the USN and then measured the BC size distribution in the droplets by renebulizing them with the CTN. To collect the droplets efficiently from the drain, the carrier gas was not injected into the USN during collection. The collected water included both the droplets and the fraction of water not converted into droplets. For the Cape Hedo rainwater sample, the measured BC size distributions in the USN-generated droplets were similar to those in the original rainwater sample measured with the CTN.
The USN has an advantage over the CTN in the much higher efficiency of extracting particles in water with particle size of about 100–500 nm. Due to the high extraction efficiency, the volume of rainwater and snow samples required for BC measurement with the USN was typically less than 5 mL, and sampling time to determine BC mass concentration is shorter. It is much less practical to apply the CTN to the quantification of BC concentration in the clean water samples (e.g., snow or ice samples in the polar region) because of insufficient counting statistics of BC particles under these conditions. For higher BC concentrations, application of the CTN is more easily achieved. For the determination of size distribution of unknown samples, the CTN nebulization efficiency is less dependent on particle size and thus can provide a less uncertain measurement of BC size distribution.
The BC size distributions in snow samples collected in semiurban Sapporo area () were smaller than those in fresh snow samples collected in rural and semirural Colorado (Schwarz et al. Citation2012, Citation2013). The negligible mass fraction of BC particles with diameters of 500–850 nm to the total BC mass in three Sapporo snow samples measured with both the USN and the CTN () indicates variability in actual BC size distribution in snow consistent with Schwarz et al. (Citation2013) and highlights the continued need for better constraints on the limits of BC size in snow.
7. SUMMARY AND CONCLUSION
In this study, we evaluated a method for quantifying the mass of BC particles suspended in rainwater and snow samples. The accuracy, reproducibility, and bias of the mass concentration of BC in liquid samples are summarized in .
The method uses a USN and an SP2. The USN extracts BC particles from liquid water to air with efficiency ϵ. Extracted particles are detected by the SP2. The extraction efficiency ϵ depends on particle size. We determined the size-dependent efficiency ϵ(D) using PSL suspensions; PSL number concentrations in water were measured by the extinction of a laser beam, based on Mie theory. The efficiency ϵ showed a broad maximum of about 10% in the PSL diameter range 200–500 nm and decreased significantly for diameters larger than 800 nm. The accuracy of the measured BC mass concentration in sample water was estimated to be ±25% for samples containing a dominant fraction of BC mass in the diameter range 100–500 nm. The accuracy was assessed with laboratory BC samples containing BC particles with mass median diameters of about 100–200 nm. Hence, the present method is appropriate for use with even extremely clean samples with size distributions that are consistent with typical atmospheric BC size distributions. The reproducibility of the measured mass concentration in rainwater after refrigerated storage in glass containers for 12 months was ±35%. Agitation of the sample water by a UB reduced the effect of attachment of BC particles to the wall of the container.
We applied this method to rainwater samples collected at Cape Hedo in Okinawa over the East China Sea and surface snow samples collected in Sapporo. A dominant fraction of the BC number concentration in these samples was observed for particle diameters smaller than 300 nm, and these samples did not likely contain a significant mass of BC particles with diameters larger than 500 nm. The lack of substantial BC mass in BC particles with diameters larger than 500 nm in a rainwater, and three snow samples was confirmed by SP2 measurements using a CTN, whose efficiency is less dependent on particle size than that of the USN.
Acknowledgments
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), the Strategic International Cooperative Program of the Japan Science and Technology Agency (JST), the Global Environment Research Fund of the Japanese Ministry of the Environment (A-0803 and A-1101), the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 246736, and the GRENE Arctic Climate Change Research Project. The authors would like to thank T. Mori for laboratory support. They would also like to thank N. Tomoyose, S. Kadena, and A. Iwasaki for rainwater sampling at Cape Hedo, and T. Aoki, K. Kuchiki, and K. Kawamura for snow sampling in Sapporo.
REFERENCES
- Baumgardner , D. , Popovicheva , O. , Allan , J. , Bernardoni , V. , Cao , J. Cavalli , F. 2012 . Soot Reference Materials for Instrument Calibration and Intercomparisons: A Workshop Summary with Recommendations . Atmos. Meas. Tech. , 5 : 1869 – 1887 .
- Bohren , C. F. and Huffman , D. R. 1983 . Absorption and Scattering of Light by Small Particles , New York : John Wiley & Sons .
- Bond , T. C. , Doherty , S. J. , Fahey , D. W. , Forster , P. M. , Berntsen , T. DeAngelo , B. J. 2013 . Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment . J. Geophys. Res. , 118 : 1 – 173 . doi: 10.1002/jgrd.50171
- Cai , Y. , Montague , D. C. , Mooiweer-Bryan , W. and Deshler , T. 2008 . Performance Characteristics of the Ultra High Sensitivity Aerosol Spectrometer for Particles Between 55 and 800 nm: Laboratory and Field Studies . J. Aerosol Sci. , 39 : 759 – 769 .
- Clarke , A. D. and Noone , K. J. 1985 . Soot in the Arctic Snowpack: A Cause for Perturbations in Radiative Transfer . Atmos. Environ. , 19 : 2045 – 2053 .
- Dasch , J. M. and Cadle , S. H. 1989 . Atmospheric Carbon Particles in the Detroit Urban Area: Wintertime Sources and Sinks . Aerosol Sci. Technol. , 10 ( 2 ) : 236 – 248 .
- Doherty , S. J. , Warren , S. J. , Granfell , T. C. , Clarke , A. D. and Brandt , R. E. 2010 . Light-Absorbing Impurities in Arctic Snow . Atmos. Chem. Phys. , 10 : 11647 – 11680 .
- Granfell , T. C. , Doherty , S. J. , Clarke , A. D. and Warren , S. G. 2011 . Light Absorption from Particulate Impurities in Snow and Ice Determined by Spectrophotometric Analysis of Filters . Appl. Opt. , 50 : 2037 – 2048 .
- Hadley , O. L. , Corrigan , C. E. and Kirchstetter , T. W. 2008 . Modified Thermal-Optical Analysis using Spectral Absorption Selectivity to Distinguish Black Carbon from Pyrolized Organic Carbon . Environ. Sci. Technol. , 42 : 8459 – 8464 .
- Hinds , W. C. 1999 . Aerosol Technology , New York : John Wiley & Sons .
- IPCC . 2007 . Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the 4th Assessment Report of the IPCC , Cambridge, UK : Cambridge University Press .
- Kaspari , S. D. , Schwikowsk , M. , Gysel , M. , Flanner , M. G. , Kang , S. Hou , S. 2011 . Recent Increase in Black Carbon Concentrations from a Mt. Everest Ice Core Spanning 1860–2000 AD . Geophys. Res. Lett. , 38 : L04703 doi: 10.1029/2010GL046096
- Ladorde , M. , Mertes , P. , Zieger , P. , Dommen , J. , Baltensperger , U. and Gysel , M. 2012 . Sensitivity of the Single Particle Soot Photometer to Different Black Carbon Types . Atmos. Meas. Tech. , 5 : 1031 – 1043 .
- McConnell , J. R. , Edwards , R. , Kok , G. L. , Flanner , M. G. , Zender , C. S. Saltzman , E. S. 2007 . 20th-Century Industrial Black Carbon Emissions Altered Arctic Climate Forcing . Science , 317 : 1381 – 1384 .
- Moteki , N. and Kondo , Y. 2007 . Effects of Mixing State of Black Carbon Measurement by Laser-Induced Incandescence . Aerosol Sci. Technol. , 41 : 398 – 417 .
- Moteki , N. and Kondo , Y. 2010 . Dependence of Laser-Induced Incandescence on Physical Properties of Black Carbon Aerosols: Measurements and Theoretical Interpretation . Aerosol Sci. Technol. , 44 : 663 – 675 .
- Ogren , J. A. , Charlson , R. J. and Groblicki , P. J. 1983 . Determination of Elemental Carbon in Rainwater . Anal. Chem. , 55 : 1569 – 1572 .
- Ogren , J. A. , Groblicki , P. J. and Charlson , R. J. 1984 . Measurement of the Removal Rate of Elemental Carbon from the Atmosphere . Sci. Tot. Environ. , 36 : 329 – 338 .
- Ohata , S. , Moteki , N. and Kondo , Y. 2011 . Evaluation of a Method for Measurement of the Concentration and Size Distribution of Black Carbon Particles Suspended in Rainwater . Aerosol Sci. Technol. , 45 : 1326 – 1336 .
- Okuda , T. , Iwase , T. , Ueda , H. , Suda , Y. , Tanaka , S. Dokiya , Y. 2005 . Long-Term Trend of Chemical Constituents in Precipitation in Tokyo Metropolitan Area, Japan, from 1990 to 2002 . Sci. Tot. Environ. , 339 : 127 – 141 .
- Schwarz , J. P. , Doherty , S. J. , Li , F. , Ruggiero , S. T. , Tanner , C. E. Perring , A. E. 2012 . Assessing Single Particle Soot Photometer and Integrating Sphere/Integrating Sandwich Spectrophotometer Measurement Techniques for Quantifying Black Carbon Concentration in Snow . Atmos. Meas. Tech. , 5 : 2581 – 2592 .
- Schwarz , J. P. , Gao , R. S. , Fahey , D. W. , Thomson , D. S. , Watts , L. A. Wilson , J. C. 2006 . Single-Particle Measurement of Mid Latitude Black Carbon and Light-Scattering Aerosols from the Boundary Layer to the Lower Stratosphere . J. Geophys. Res. , 111 : D16207 doi: 10.1029/2006JD007076
- Schwarz , J. P. , Gao , R. S. , Perring , A. E. , Spackman , J. R. and Fahey , D. W. 2013 . Black Carbon Aerosol Size in Snow . Nature Sci. Rep. , 3 : 1356 doi: 10.1038/srep01356
- Stephens , M. , Turner , N. and Sandberg , J. 2003 . Particle Identification by Laser-Induced Incandescence in a Solid-State Laser Cavity . Appl. Opt. , 42 : 3726 – 3736 .
- Wang , M. , Xu , B. , Zhao , H. , Cao , J. , Joswiak , D. Wu , G. 2011 . The Influence of Dust on Quantitative Measurements of Black Carbon in Ice and Snow When Using a Thermal Optical Method . Aerosol Sci. Technol. , 46 : 60 – 69 .
- Warren , S. G. and Wiscombe , W. J. 1980 . A Model for the Spectral Albedo of Snow, II, Snow Containing Atmospheric Aerosols . J. Atmos. Sci. , 37 : 2734 – 2745 .