Abstract
The growth rates of nonane and D2O nanodroplets produced in supersonic expansions are characterized using small angle X-ray scattering (SAXS) and pressure trace measurements (PTM). The experimental growth rates are compared to the predictions of a Hertz–Knudsen model that assumes either isothermal or nonisothermal droplet growth in the free molecular regime. For nonane, the predicted growth rates are insensitive to both droplet temperature and the evaporation coefficient, and agree well with the experimentally measured growth rates assuming a condensation coefficient of 1. For D2O, droplet growth rates are quite sensitive to droplet temperature, and the best agreement between experiments and theory are achieved for a condensation coefficient of 1 and an evaporation coefficient in the range from 0.5 to 1. Under our experimental conditions, incorporating coagulation is important to match the measured D2O growth rates but not those of nonane.
Copyright 2013 American Association for Aerosol Research
1. INTRODUCTION
The spontaneous condensation of water and n-alkanes in high speed flows is relevant to a broad range of engineering applications from the expansion of steam in low-pressure turbines (Moheban and Young Citation1985; White et al. Citation1996; Bohn et al. Citation2003; Bakhtar et al. Citation2007; Dykas et al. Citation2007; Gerber and Mousavi Citation2007) to the removal of condensible materials from raw natural gas (Rijkers et al. Citation1992; Muitjens et al. Citation1994; Looijmans Citation1995; Luijten et al. Citation1998; Okimoto and Betting Citation2001; Peeters et al. Citation2001). The initial fragments of the new phase arise via homogeneous nucleation, and as these nanodroplets grow they rapidly deplete the vapor phase and add energy to the flow. Both heat addition and vapor depletion quench nucleation, and the coupling between these two processes establishes the initial size distribution of the aerosol. Since, for a fixed amount of condensible vapor, the aerosol number density determines the final average droplet size, droplet growth in the free-molecular regime is critical even when the final droplets are of micron size.
In our earlier work (Sinha et al. Citation2009) modeling the condensation of H2O/D2O in gently diverging supersonic nozzles, we developed a 1-D model to predict the aerosol size distribution near the nozzle exit. The model combined a single nucleation rate expression—calibrated against available rate measurements—with 5 different growth laws including both isothermal and nonisothermal expressions. The results were then compared to the aerosol size distribution parameters determined by in situ small angle X-ray scattering (SAXS) experiments near the nozzle exit. Contrary to our expectations, the isothermal calculations were more successful at predicting the final droplet sizes and number densities. This was because nonisothermal droplet growth did not quench nucleation rapidly enough, the aerosol number density was over predicted and, thus, the average droplet size was under predicted. Our observations were similar to those reported by Young (Citation1982) who modeled pure steam condensation in supersonic nozzles. He found that he could only match both pressure traces and reported droplet sizes if the evaporation rate was lower than that expected under equilibrium conditions; that is, the droplets were growing more quickly than expected.
The goal of the current work is to directly test growth laws in the free molecular regime against a more extensive experimental data set obtained in our supersonic nozzles. In particular, the current experiments follow the condensation of n-nonane or D2O from dilute vapor mixtures with position resolved static pressure trace (PTM) and SAXS measurements. The unique aspects of our work include the position resolved particle size distributions and condensate mass fraction measurements, as well as the self-consistent analysis method that yields more accurate estimates for the flow properties than those based on pressure measurements alone. As discussed in the experimental section, we use these data to directly estimate both the average temperature of the droplets Td and the droplet growth rates. The experimental droplet temperatures Td,exp are compared to the values derived from the implicit solution of the coupled mass and energy fluxes, Td,exact, as well as to explicit approximations based on the Gyarmathy model and 2nd and 3rd order corrections to this model, Td,approx (Smolders Citation1992). The experimental growth rates are compared to those predicted using Td,exp and Td,exact and the Hertz–Knudsen growth law. The role that coagulation plays in our measurements is also considered.
The organization of this article is as follows. We first briefly discuss droplet growth in the free molecular regime, and then describe the experiments and the data analysis methods. We then present and discuss our results.
2 DROPLET GROWTH LAWS
Droplet growth is a dynamic process. The net growth rate is governed by the difference between the rate at which vapor molecules are incorporated into the droplet and the rate with which monomers evaporate from the droplet. Since the condensing vapor releases heat, the temperature of a growing droplet is higher than that of the surrounding gas and the monomer evaporation rate increases above that for a droplet in thermal equilibrium with its surroundings.
The equations used to describe the coupled mass and heat transfer problem depend on the Knudsen number, Kn = l/2⟨r⟩, where l is the mean free path of a vapor molecule and ⟨r⟩ is the average radius of the droplet. In the free molecular regime, ⟨r⟩ is significantly smaller than l and Kn is much larger than 1. In our experiments, Kn is always greater than 10, and thus, droplet growth is always in the free molecular regime.
The Hertz–Knudsen (HK) droplet growth model,
[1] is based on the kinetic theory of gases and describes droplet growth in the free molecular regime (Hill Citation1966). In EquationEquation (1)
[1] , t is time, mv is the mass of a monomer, kB is the Boltzmann constant, T is the temperature of the vapor phase, vl is the molecular volume of the condensate, qc and qe are condensation and evaporation coefficients, respectively, pv is the partial pressure of the vapor, and peq(⟨r⟩, Td) is the equilibrium vapor pressure above the drop of radius ⟨r⟩ at temperature Td. The Kelvin–Helmholtz equation relates peq(⟨r⟩, Td) to the physical properties of the condensate by,
[2] where peq(Td) is the equilibrium vapor pressure over a flat surface at temperature Td, and ζ is the surface tension of the liquid. Finally, Ke = 2ζvl/(kBTd⟨r⟩) is the Kelvin number.
The condensation coefficient qc corresponds to the fraction of molecules impinging on the droplet that are incorporated into the droplet, while the evaporation coefficient is defined as the ratio of the actual evaporation rate to the theoretical evaporation rate. The values of qc and qe are most often taken as unity in order to simplify the analysis although there is no compelling experimental evidence to suggest that this is true for all conditions—especially nonequilibrium conditions—and all substances (Young Citation1982; Marek and Straub Citation2001).
For water, there is still significant uncertainty (Gajewski et al. Citation1974) regarding appropriate values for qc and qe despite extensive experimental and theoretical studies. Experimental values of qc that range over three orders of magnitudes, from 0.001–1, have been reported since Rideal (Rideal Citation1925) conducted the first room temperature experiments in 1925 (Mozurkewich Citation1986; Marek and Straub Citation2001; Davidovits et al. Citation2004). More recent experiments, for temperatures between 238 and 298 K, have reduced the range to 0.01–1 (Beloded et al. Citation1989; Hagen et al. Citation1989; Shaw and Lamb Citation1999; Zagaynov et al. Citation2000; Davidovits et al. Citation2006), and Marek and Straub (Citation2001) suggested that condensation coefficients less than 0.01 indicate contamination of the water surface. Molecular dynamics studies, for temperatures between 300 and 350 K, find the value of qc is close to unity (Morita et al. Citation2004; Tsuruta and Nagayama Citation2004; Vieceli and Tobias Citation2004), a result that is at odds with many experiments. The reported values of qe also range from 0.01 to 1 (Eames et al. Citation1997), and experimental challenges associated with determining qe again include surface contamination as well as accurate surface temperature measurements (Hickman Citation1965, Citation1966). Recent measurements on a train of 12 μm to 15 μm D2O droplets injected into vacuum (1.33 mPa), yielded evaporation coefficients of 0.57 ± 0.06, for droplet surface temperatures 255–295 K, where the latter were determined using Raman thermometry (Drisdell et al. Citation2008). Here, a value of qe less than one was attributed to a kinetic barrier to evaporation that Transition State Theory suggests arises from librational and hindered translational motions at the liquid surface (Drisdell et al. Citation2008).
To our knowledge, there are no experimental measurements of qc and qe for nonane. Molecular dynamics simulations of films (Xia and Landman Citation1994) found qc is ∼0.9 for n-hexane and ∼1 for n-hexadecane.
When vapor condenses on an existing droplet, the heat released due to condensation increases the temperature of the droplet relative to that of its surroundings. The heat dissipation rate from the droplet to the surroundings depends on the temperature difference Td – T, the latent heat of condensation L(Td), and the capacity of gas-vapor mixture to absorb the heat. As the droplet temperature increases, so does the equilibrium vapor pressure and, thus, the evaporation rate relative to a droplet in thermal equilibrium with its surroundings. The net result is that hotter droplets grow more slowly. Thus, growth laws can be broadly divided into two categories: isothermal growth laws—those that assume droplets are at the temperature of the surroundings—and nonisothermal growth laws—those where droplet temperature can differ from that of the surroundings.
For nonisothermal growth laws, calculating the droplet temperature can be critical to predicting the growth rates for droplets. As discussed in more detail in Section 4 of this article, the equations describing heat and mass transfer to growing droplets are strongly coupled, and to determine the droplet temperature Td, these equations should be solved simultaneously. The process is simplified by introducing the wet bulb or quasi-steady state approximation, that is, the assumption that there is an instantaneous balance in the heat and mass transfer processes between the droplet and its surroundings. But even then, there are no exact analytical solutions for the nonisothermal growth equations and numerical methods are required. The computational cost of the numerical solution has motivated researchers to develop explicit approximations of droplet temperatures that relate Td to droplet size and the conditions prevailing in the surroundings. Analytical expressions are derived by linearizing the Clausius–Clapeyron equation (Mason Citation1953; Wagner Citation1982; Mozurkewich Citation1986; Barrett and Clement Citation1988; Vesala et al. Citation1990) or by assuming that there is little difference between the partial pressure of the vapor far away from the droplet and the equilibrium pressure over a drop (Gyarmathy Citation1963).
Finally, we note that the kinetic theory of gases is used to calculate the average molecular velocities when deriving EquationEquation (1)[1] . In a nonequilibrium process the bulk vapor velocity at the interface can affect these average molecular velocity distributions (Scharge Citation1953; Mills and Seban Citation1967) and accounting for this effect modifies the growth rate expressions. Young (Citation1991), however, noted that the effect is easily nullified by small changes in the droplet temperature.
3 EXPERIMENTS
The experiments were conducted using the apparatus and techniques described in detail elsewhere (Laksmono et al. Citation2011) and are summarized only briefly here. A figure of the flow system is available in the online supplementary material as Figure S1.
3.1 Flow Apparatus and Nozzles
In our current continuous flow apparatus, N2(g) is drawn from two liquid nitrogen Dewars and warmed to room temperature by inline heaters. The flow rate of the gas is controlled by two MKS mass flow controllers. One of the N2 streams is heated further and enters the vaporizer to disperse the liquid and provide the energy to evaporate it. The liquid is transferred from a flask to the vaporizer by a peristaltic pump and the liquid flow rate is measured by monitoring the weight of a flask using a balance. The vapor-gas mixture is combined with the second N2(g) stream and the resultant mixture flows through a heat exchanger and into the plenum where the velocity of the mixture is low enough to correspond to stagnation conditions. The stagnation temperature is measured by a resistance temperature detector (RTD). The mixture then enters the supersonic Laval nozzle (Figure S1) and the static pressure is measured in a region of constant cross section upstream of the converging part of the nozzle. The stagnation pressure is determined by correcting the static pressure for the velocity and density of the gas. As the gas flows through the converging/diverging portions of the nozzle, it expands and cools, and downstream of the throat the vapor spontaneously condenses. At the nozzle exit, the mixture is exhausted to the atmosphere by two rotary vane vacuum pumps.
The supersonic nozzles used in this work are machined from aluminum with shaped top and bottom blocks and flat sidewalls. Experiments are conducted in two separate nozzles for each experimental condition, where the nozzles differ in the material used for the sidewall windows. For SAXS experiments, the nozzle has 25 μm thick mica windows; and for PTM, we use a nozzle with 2 mm thick CaF2 windows (Paci et al. Citation2004; Tanimura et al. Citation2005). Since the nozzles throat areas differ slightly, we adjust the flow rates to maintain both the desired stagnation pressure and the partial pressure of the condensible at the nozzle inlet for the corresponding PTM and SAXS experiments.
3.2 Pressure Trace Measurements (PTM)
We follow the expansion of the gas mixture by measuring the static pressure as a function of position using a pressure probe. The pressure is first measured for pure carrier gas (dry trace) in order to determine the effective flow area of the nozzle. The pressure is then measured for the carrier gas—condensible vapor mixture (wet trace). Condensation of the vapor is accompanied by heat release which increases the static pressure above that expected for an isentropic expansion of that mixture through the nozzle. Thus, PTMs rapidly establish the conditions corresponding to the initiation of phase transitions in the supersonic flow.
3.3 Small Angle X-Ray Scattering (SAXS)
SAXS experiments were conducted at the Advanced Photon Source (APS) at the Argonne National Laboratory on beamline 12-ID-C. The 0.2 mm × 0.2 mm beam of 12 keV (wavelength λ = 0.103 nm) X-rays has a wavelength spread, Δλ/λ of 0.01%. The intensity of the scattered X-rays is measured by a 2-D charged-coupled-device (CCD) detector. The nozzle and plenum are mounted on a sliding plate to make the axially resolved SAXS measurements. The relative position of the nozzle is known to better than 0.02 mm. The data reduction program provided by the APS integrates the 2-D data to produce the 1-D spectrum of intensity (I) versus the scattering vector (q) after correcting for spatial inhomogeneties. The scattering vector is defined as
[3] where θ is the scattering angle.
At each nozzle position, scattering is measured as N2 alone flows through the nozzle (background) and as the N2—condensible mixture flow through the nozzle (sample). The background spectrum is subtracted from the sample spectrum to yield the scattering spectrum from the aerosol. We assume that the aerosol is comprised of a polydisperse distribution of spheres that follow a Schulz size distribution (Kotlarchyk and Chen Citation1983). The scattering from this size distribution is well-defined and the APS data analysis program is used to fit the experimental spectra to extract the average radius ⟨r⟩, the spread σ, and the intensity as q goes to 0, I0. The intensity is converted to an absolute scale by matching the D2O volume fractions observed during the SAXS experiments to those obtained from tunable diode laser absorption spectroscopy (TDLAS) experiments conducted under the same conditions (Paci et al. Citation2004; Tanimura et al. Citation2005). The aerosol number density, N is calculated from these fitting parameters using the following formula
[4] Here, Z = (⟨r⟩/σ)2 − 1 and ΔρSLD is the scattering length density difference between the droplets and the surrounding gas mixture. We note that ΔρSLD depends on the density of the condensate ρl and, therefore, depends on the temperature and radius of the droplets through the Laplace effect. Finally, we obtain the mass fraction of the condensate, g from
[5] where ρ is the density of the gas mixture.
3.4 Integrated Data Analysis
Condensing flow in a supersonic nozzle can be described by considering the conservation of mass, momentum, and energy together with an equation of state for the gas mixture. These four equations tie together six variables—the static pressure p, temperature T, density of the mixture ρ, velocity u, mass fraction condensate g, and the flow area A/A* where A* is the area of the throat. We start the integrated data analysis by using the dry pressure trace to estimate the effective flow area of the nozzle, (A/A*)dry. We then integrate the flow equations using the pressure measurement for the condensing flow and (A/A*)dry as input to yield initial estimates for T, ρ, u, and g (i.e., gPTM). This approach assumes that the boundary layers that grow along the nozzle walls are stable against condensation—an assumption that is reasonable up to the region of rapid droplet growth. Beyond this point, the pressure increase associated with heat release can change the boundary layers and the mass fraction of condensate can be significantly underestimated near the nozzle exit (Tanimura et al., Citation2005). In the current experiments, analysis based on pressure measurements alone underestimate the temperatures by up to 10 K and overestimate densities by ∼4%.
The next step in the integrated analysis is to use the initial estimates of T and ρ to determine new values of g based on the SAXS data and EquationEquation (5)[5] . The values of temperature enter the SAXS analysis via the liquid density and, therefore, the scattering length densities of the droplets. The mass fractions obtained from SAXS, gSAXS, are combined with the mass fractions obtained from the PTM, gPTM during the initial stages of condensation and a sigmoidal curve is fit to the combined data set to yield gfit. The flow equations are solved a second time, now using gfit and the condensing flow pressure as input data. This approach provides improved estimates of A/A*, u, T, and ρ. The new values of T and ρ are used to reanalyze the SAXS data and further improve the estimates of g. The process is repeated until the temperature converges to within 0.5 K, and usually requires less than three iterations.
An important aspect of integrating the different techniques is to account for the difference between the location of the actual throat, the minimum in the flow area and the location of the physical throat, the minimum cross-sectional area inside the nozzle. The difference arises because of boundary layer growth along the nozzle walls. In SAXS experiments, positions are measured with respect to the physical throat whereas in PTM positions are measured with respect to the actual throat. In the current experiments the actual throat is located 0.9 mm downstream of the physical throat and all measurements are referenced to the actual throat.
3.5 Materials and Physical Properties
Condensation experiments were conducted using dilute vapor mixtures of D2O and n-nonane in N2. Liquid N2 (Praxair or Airgas) had a minimum purity of 99.99%, D2O (Cambridge Isotopes Labs) had more than 99.9% D substitution, and n-nonane (Sigma Aldrich and Chemsampco) was more than 99% pure. The thermophysical properties used in the analyses are reported in Wölk and Strey (Citation2001) and Sinha et al. (Citation2009) for D2O, and Ghosh et al. (Citation2010) for n-nonane. The compressibility for liquid nonane over the temperature range of interest was estimated as 7 × 10−10 Pa−1 (NIST Citation2002). The isobaric specific heat capacity for D2O vapor is assumed to be constant for our experimental conditions and is set equal to the value at 225 K, 33.55 Joule/mol/K (Abraham and Lester Citation1954).
Table 1 A summary of the position resolved condensation experiments where p0, T0, and pv0 are the pressure, temperature, and partial pressure of condensible vapor at the inlet to the nozzle. The carrier gas in all experiments was N2
4 RESULTS AND DISCUSSION
The D2O and nonane droplet growth experiments are summarized in . PTMs were conducted in nozzle C3 whereas SAXS experiments were conducted in nozzle C2. The expansion rate of Nozzles C3 and C2 is d(A/A*)/dx = 0.076 cm−1. Our experiments are broadly divided into two categories based on the complexity of aerosol evolution. The easiest case is that observed for nonane where, as we will show later, SAXS measurements confirm that droplet growth occurs in the absence of coagulation. In contrast, SAXS experiments indicate that during the D2O droplet growth experiments coagulation was always observed.
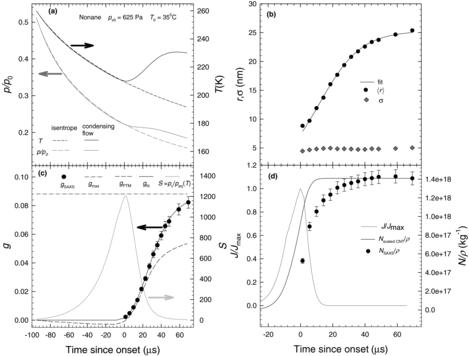
4.1 Nonane
Droplet growth experiments for nonane were conducted with three different inlet condensible partial pressures (pv0). For the highest pv0, summarizes (a) the pressure and temperature profiles together with (b) the droplet radii, (c) the mass fraction of condensate, and (d) the specific number densities and the normalized nucleation rates. All results are presented as a function of time where time is related to position x through the velocity u and the relationship dx = udt. Here, t = 0 corresponds to the onset of condensation that we define as the point in the flow where the nucleation rate is maximized. In the normalized nucleation rates were calculated using Classical Nucleation Theory using the expressions and physical property data presented by Ghosh et al. (Citation2010).
As illustrated in , the pressure and temperature decrease isentropically up to the onset of condensation. Beyond this point, the heat released by condensation increases the pressure and temperature above that expected for the isentropic expansion of a noncondensing gas mixture. In , ∼9 nm radius droplets are first observed about 1 μs after onset. The droplets grow rapidly, more than doubling in size in about 40 μs, before growth slows as the vapor is depleted. The spread of the droplet size distribution increases from ∼4.5 to 5.0 nm for the first 20 μs and then remains almost constant. The aerosol polydispersity (σ/⟨r⟩), therefore, decreases from ∼0.50 near onset to ∼0.20 near the exit. illustrates the changes in mass fraction of condensate g (left axis) and the supersaturation S of nonane in the vapor phase (right axis). The slightly negative values of g prior to onset are thought to arise from nonane vapor phase heat capacities that are slightly lower than those predicted by available correlations (Ghosh et al. Citation2010). Shortly after onset, the estimate for g based on PTM alone are significantly lower those based on SAXS. The deviation reflects the fact that the PTM approach cannot account for compression of the boundary layer due to heat released by condensation. The SAXS estimates are based on the SAXS analysis described in section III.C, whereas the PTM+SAXS estimates are based on the integrated data analysis described in section III.D. The PTM+SAXS results are used to calculate the partial pressure of nonane remaining in the vapor phase, the gas mixture temperature and density, and the supersaturation. The latter increases as the expansion proceeds, reaching a maximum near onset and then decreasing rapidly as the temperature of the gas mixture increases. The values determined by the integrated data analysis, together with the measured droplet sizes and the physical properties of the vapor and liquid, are then used to calculate the droplet temperatures and droplet growth rates. Finally, as illustrated by the symbols in , during the first 30 μs, the experimental specific number densities = N/ρ, increase rapidly from 5 × 1017 kg−1 to a maximum value of 1.4 × 1018 kg−1 and then remain constant. The rapid increase in
indicates that new droplets continue to form even as existing droplets grow. This is consistent with the trends observed for the normalized nucleation rates, as well as the specific number densities calculated by integrating the nucleation rate expression and correcting these to match the experimental number densities near the exit. The constant values of
for t > 30 μs show that droplets do not coagulate during the ∼40 μs available after nucleation stops and before the aerosol leaves the nozzle. A simple estimate of the expected coagulation rate can be made using (Hidy and Brock Citation1970; Pruppacher and Klett Citation1997)
[6]
where the coagulation coefficient Kcoa is given by
[7]
(Seinfeld Citation1986; Seinfeld and Pandis Citation1998). The coalescence efficiency β is taken as 1 because the Van der Waals forces should guarantee that two droplets stick once they collide (Okuyama et al. Citation1984). Solving EquationEquation (6)[6] predicts that in ∼100 microseconds coagulation only decreases the specific number densities by about 3%.
In general, the rate of droplet growth is a strong function of the droplet temperature, Td. One advantage of our data set is that we can directly estimate droplet temperatures based on experimental data alone, Td,exp (Tanimura et al. Citation2010) and compare these to the values calculated based on both an implicit theoretical expression (Td,exact) and explicit approximations (Td,approx) to Td,exact. For our small droplets, droplet temperatures adjust rapidly enough so that we can use the quasi-steady state or wet-bulb approximation to determine the droplet temperature by simultaneously solving the mass flux and heat flux equations. Thus,
[8]
where Jq and Jm are the heat and mass fluxes from a droplet to its surroundings, respectively, and L(Td) is the latent heat of condensation.
In the free molecular regime, the heat flux, due to collision of the carrier gas N2 with a droplet, is given by (Kennard Citation1938).
[9] where
is the mass of a N2 molecule,
is the partial pressure of N2, and
is the specific isobaric heat capacity of N2 in the vapor phase.
To calculate Td,exp, we equate the heat transferred from the growing droplets to the measured latent heat released by condensation while accounting for the temperature change of the vapor (Tanimura et al. Citation2010). Thus,
[10] where cp(v) is the specific isobaric heat capacity of the condensible vapor. Our integrated data analysis yields gfit(t), and L(T) is set equal to the latent heat of condensation of the bulk. Calculations show that droplet curvature effects change the latent heat of the smallest droplets by less than 2% (Tanimura et al., Citation2010) and hence, can be safely ignored. The major contribution to the heat dissipation rate is the first term, L(T)dg/dt, and the other two terms account for less than ∼5% of the total. Thus, dgfit/dt and
are the most important experimental parameters used to determine Td,exp via EquationEquations (9)
[9] and Equation(10)
[10] .
To determine Td,exact, we calculate Jm using EquationEquation (1)[1]
[11] and combined Equations Equation(8)
[8] , Equation(9)
[9] , and Equation(11)
[11] to yield
[12]
This implicit expression for Td,exact is solved numerically.
In computational fluid dynamics codes, solving for Td,exact using EquationEquation (12)[12] can be too time consuming, and, thus, the implicit equation is simplified to yield the droplet temperature explicitly as a function of its size and the surrounding conditions (Mason Citation1953; Gyarmathy Citation1963; Barrett and Clement Citation1988). The most common assumption is that the transport coefficients and the latent heat of condensation are constant in the temperature range (T,Td) and can be evaluated at T. The Clausius–Clapeyron equation, dpeq/dT = Lmvpeq/(RT2), is integrated using this assumption to obtain (Smolders Citation1992) the equation
[13]
Gyarmathy (Citation1963) expressed Jm and Jq in terms of the driving forces and the Nusselt numbers for mass, NuM, and heat, NuH, transfer, that is,
[14]
[15]
The Nusselt numbers are the ratios of the convective to diffusive transport rates, p = is the total pressure, and k is the thermal conductivity of the mixture. Dmod is the modified diffusivity of the binary mixture defined as
[16] where D is the binary diffusion coefficient of the vapor-gas mixture. Both Dmod and k are evaluated at an intermediate temperature Tm between the droplet and the surrounding gas mixture where (Hubbard et al. Citation1975).
[17]
Gyarmathy (Citation1963) then expressed EquationEquation (8)[8] using Equations Equation(14)
[14] and (15), assuming qc = qe = 1, as
[18]) where the saturation ratio S is given by S = pv/peq(T). He also transformed the right hand side of this equation into a logarithm to yield
[19] When peq(⟨r⟩, Td) ≈ pv, ϵ ≈ 1, and Gyarmathy found the following explicit solution for Td (Gyarmathy Citation1963)
[20] where Ke∞ is the Kelvin number evaluated at temperature of the surroundings. For notational convenience, Smolders (Smolders Citation1992) rewrote EquationEquation (20)
[20] as
[21] where
[22]
The exact Nusselt numbers can we obtained by equating EquationEquations (11)[11] and Equation(14)
[14] for NuM or (9) and (15) NuH. In the free molecular regime, the Nusselt numbers are directly proportional to droplet size and when pv ≪
they can be approximated as
[23] where cp − mix and γmix are the specific isobaric heat capacity and the heat capacity ratio of the vapor-gas mixture and mavg is the average molecular weight in the vapor-gas mixture.
When peq(⟨r⟩, Td) ≠ pv, in EquationEquation (19)
[19] can be expanded using a Taylor series around peq(⟨r⟩, Td) = pv to get a more accurate estimate for ϵ. The value of ϵ differs depending on where the Taylor series expansion is truncated. The values for the first three approximations are
[24] Here, the subscript on ϵ refers to the derivative where the Taylor series is truncated.
The final droplet temperature is then calculated using
[25] where
[26]
Smolders (Citation1992) used the second order truncation to calculate the droplet temperatures and also set (1 + δ)−1 in EquationEquation (25)[25] equal to 1 − δ. The latter is a good approximation for his experiments where the saturation ratio of water was always less than 10. In our experiments, S is much larger than 10 and this approximation introduces unnecessary error. Thus, all of our calculations use EquationEquations (25)
[25] and Equation(26)
[26] and the values of ϵ given by in EquationEquation (24)
[24] .
The exact and approximate droplet temperatures are evaluated using the measured average droplet sizes and the conditions prevailing in the gas mixture based on the iterative data analysis.
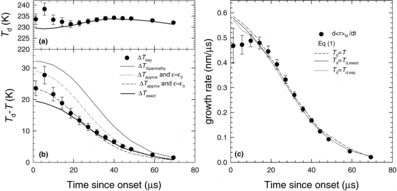
For the nonane experiment conducted at T0 = 308 K and pv0 = 625 Pa nonane, illustrate the experimental droplet temperatures, Td,exp, the droplet temperatures calculated using the implicit method assuming qc = qe = 1, as well as the difference between the droplet temperatures and that of the gas mixture, ΔT = Td – T for all of the methods considered. The hottest droplets are ∼20–30 K warmer than their surroundings, and correspond to the smallest droplets that are growing rapidly during the initial stage of condensation. As droplet growth slows, ΔT decreases to ∼2 K near the nozzle exit. The droplet temperatures calculated using different levels of approximation, Td,approx are ∼2 to ∼10 K higher than Td,exact suggesting that the approximations are not accurate under the current experimental conditions. Indeed, the values of ϵ calculated using EquationEquation (24)[24] are 1.6 to 3.3 times higher than the value ϵ = 1 obtained from EquationEquation (19)
[19] when Td = Td,exact. We therefore conclude that when peq(⟨r⟩, Td) differs significantly from pv, the best way to determine theoretical Td is by using the implicit method. Luo et al. (Luo et al. Citation2006) reached a similar conclusion while modeling water droplet growth in an expansion wave tube at temperatures below 280 K and saturation ratios up to 10.
During the initial stages of condensation, that is, within 10 μs of onset, Td,exp values are almost all ∼5 K higher than Td,exact. During this time the polydispersity of the aerosol is high, that is, greater than 33%. Since Td, exact is calculated using the average droplet size, we investigated whether polydispersity affects Td,exact, by calculating Td,exact for sizes around the average droplet size and determined volume weighted average Td,exact. Including polydispersity changed Td,exact by less than ±1 K from that calculated assuming a monodisperse aerosol. For nonane, this observation is reasonable because the equilibrium vapor pressure over the droplets is always negligible compared to the partial pressure of nonane in the vapor phase. Thus, the vapor impingement rate greatly exceeds the evaporation rate from the droplet, and EquationEquation (12)[12] is essentially independent of droplet size.
Accurate estimates for the mass and heat fluxes are critical for calculating the droplet temperatures, and the ratio of the experimental and theoretical mass, or heat, fluxes is very close to the ratio of ΔTexp and ΔTexact. We estimate that there is an uncertainty of 5% in determining and about 5% uncertainty in determining dgfit/dt based on the subjectivity of the fit to the g(t) data. Together, this results in a 10% uncertainty in ΔTexp. Experiments at the two lower nonane flow rates, Figures S2 and S3 in the online supplementary material, yielded similar trends for ΔT, although the agreement between Td,exp and Td,exact is not quite as good. A more complete understanding of these discrepancies is the subject of future research.
illustrates the nonane droplet growth rates for qc = qe = 1. To determine the experimental growth rates, we can not differentiate the experimental droplet size versus time data directly. Instead, we fit a sigmoidal curve to the ⟨r(t)⟩ data and calculated the experimental droplet growth rates, d⟨r⟩fit/dt from the slope of the fit. We choose a sigmoidal function since, in the absence of coagulation, the radius should not increase indefinitely with time. The sigmoidal fits we chose included a Gompertz function, a logistic function, or any other type of a sigmoidal function with up to five parameters. The goal is to fit the data well over the observed range, not to extrapolate the data. Our approach works well except during the initial stages of condensation where it is often difficult to calculate the slope, and hence the growth rate accurately because of the subjectivity involved in choosing the fit function. This uncertainty is reflected in the error bars illustrated in .
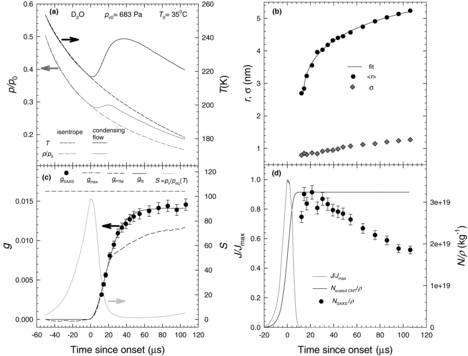
![Figure 4 The droplet temperatures and growth rates for D2O experiments at T0 = 308 K, pv0 = 683 Pa, and p0 = 30.2 kPa are calculated assuming qc = qe = 1. The legend for (a) and (b) are the same. The error bars on Td,exp are based on a 5% uncertainty in calculating the number density and a 5% uncertainty in calculating the condensation rate, dg/dt based on the subjectivity of the sigmoidal fit. (a) The experimental and theoretical implicit droplet temperatures. (b) The temperature difference between the droplets and the surrounding gas mixture. (c) The experimental and theoretical growth rates. Using the values of Td,exp in EquationEquation (1)[1] yields negative growth rates suggesting the droplets are unstable.](/cms/asset/f617dd39-7bd6-4f9b-8cd3-35784dd1580f/uast_a_839980_f0004_b.gif)
In order to calculate the theoretical growth rates, we use EquationEquation (1)[1] with qc = qe = 1, and for the special case of isothermal growth we assume Td = T. shows that despite the wide range in droplet temperatures, all of the nonisothermal growth laws predict essentially the same growth rates and that these rates differ very little from the isothermal growth rate. The reason for this behavior is that even when the nonane droplets are 30 K hotter than the surroundings, the equilibrium vapor pressure over the droplets is still only 5% of the partial pressure of nonane in the vapor phase. Thus, the evaporation rate from the droplets is negligible, and the growth rates are largely determined by the impingement rate. Furthermore, all of the growth laws agree with the experimental data. Since aerosol polydispersity had only a limited effect on Td,exact, it does not affect the growth rates for nonane calculated using Td,exact. Similar results were obtained for the experiments conducted with the other two partial pressures of nonane, and the corresponding figures are available as online supplementary material.
Because the evaporation rates do not affect the nonane droplet growth rates, we cannot constrain the evaporation coefficient for nonane. In contrast, any reduction in the condensation coefficient, qc from 1 would decrease the growth rate proportionally. Thus, our data for nonane are clearly consistent with qc = 1.
4.2 D2O
D2O growth rate experiments are more complex than those involving nonane because, as noted earlier, the D2O aerosols coagulate on the time scale of the experiments. summarizes experimental data for a typical case of D2O condensation.
For the dilute D2O-N2 mixture illustrated in , the pressures and temperatures initially follow the isentropic profile up to the onset of condensation before deviating abruptly as heat is added to the flow by the rapidly growing droplets. The first reliable SAXS measurements are possible ∼12 μs after onset, , and here the average droplet size is only 2.6 nm, that is, significantly smaller than the first nonane droplets we observed. The droplet size increases rapidly to ⟨r⟩ ≈4 nm over the next 12 μs, and then more slowly over the next ∼ 80 μs, reaching 5.3 nm at t = 105 μs. The spread of the size distribution steadily increases from 0.8 to 1.3 nm, while the polydispersity decreases from 0.3 to 0.24. The D2O droplets are significantly smaller than the nonane droplets formed under comparable conditions for two reasons. The first is that the molecular volume of D2O is only 11% of that of nonane, and the second is that the number densities of the water droplets is about 20 times higher than for nonane.
illustrates that the rapid increase in condensate mass fraction mirrors the rapid temperature increase. The values of gPTM merge smoothly with the gSAXS data, but gPTM again underestimates the condensate mass fraction in the later stages of condensation. As growth slows, the values of g stabilize at a value of 0.014, about 14% below the mass fraction of D2O vapor initially entering the nozzle, gmax = 0.0162. The supersaturation S increases rapidly reaching its maximum close to onset before decreasing again. summarizes the measured specific number densities along with the normalized nucleation rates where the theoretical specific number densities are scaled to match the maximum specific number density observed. When the SAXS measurements first detect particles, ∼12 μs after the onset, nucleation is essentially complete. This is consistent with the normalized nucleation rate calculation that finds J/Jmax ≈ 0 for t >10 μs. Over the next ∼100 μs, the specific aerosol number density decreases by 42% from the peak value of 3.2 × 1019 kg−1. Finally, for t > 60 μs, the relatively constant values of g suggest that in this part of the expansion droplets are growing only by coagulation. Thus, droplet temperatures and condensational growth rates will only be calculated for t < 60 μs.
The difference in coagulation rates for nonane and D2O primarily reflects the difference in number densities in the two experiments. In fact, Kcoa calculated from EquationEquation (7)[7] for the small D2O droplets is about 40% of Kcoa for the nonane droplets. The decrease in Kcoa is, however, easily offset by D2O droplet number densities that are an order of magnitude higher than those for nonane, and the fact that coagulation rates are proportional to the square of the number densities and vary only linearly with Kcoa. Finally, the decrease in specific number densities is about three times faster than that expected when Kcoa is estimated using EquationEquation (7)
[7] . The enhanced coagulation rate of water droplets is thought to arise from van der Waals interactions (Alam Citation1987; Kerminen et al. Citation1991; Kerminen Citation1994) and the small size of the droplets (Marlow Citation1980; Kennedy and Harris Citation1990).
summarizes the results of droplet temperature calculations and the condensational droplet growth rates for the D2O experiment illustrated in , assuming qc = qe = 1. As illustrated in , the ΔTexp values decrease from the maximum value of 34 K; for the smallest droplets, we can detect to ∼0 K at ∼106 μs. For the first ∼60 μs after onset, the values of Td,exp are significantly higher than the predicted droplet temperatures as illustrated in . At longer times ΔTexp approaches zero consistent with droplet growth dominated by coagulation.
Unlike the nonane experiments, the Gyarmathy model and the 2nd and 3rd order corrections to this model, all predict the same droplet temperatures, and, furthermore, these all agree with the implicit droplet temperature, Td,exact.
The corresponding growth rates are summarized in . The experimental rates decrease monotonically from 0.11 nm/μs for the smallest droplets to ∼0 at 106 μs. The largest uncertainties again correspond to the smallest droplets where one source of uncertainty stems from the functional form chosen to fit the ⟨r⟩ versus t data. The predictions of the isothermal and nonisothermal growth rates vary significantly, and for 0 μs < t < 30 μs, the experimental growth rates lie between the predictions of the isothermal growth law and the nonisothermal growth law using Td = Td,exact. At intermediate times, these two predictions lie significantly below the experimental rate. Finally, the differences between using Td,exact and Td,exp in EquationEquation (1)[1] are striking. In particular, when Td,exp is used, EquationEquation (1)
[1] suggests that the droplets are not stable and should in fact evaporate.
The reason for the discrepancy between ΔTexp and ΔTexact, and consequently the differences in the predicted growth rates can be traced back to the fact that the heat flux per droplet measured in the experiments (EquationEquation (10[10] )) is ∼30% higher than that predicted by theory. From the experimental viewpoint, uncertainty in
alone cannot account for this difficulty. Increasing
by 30% would increase gSAXS above ginf near the nozzle exit, that is, more D2O would condense in the nozzle exit than entered. Independent FTIR (Fourier-Transform Infrared Spectroscopy) estimates of the vapor phase concentration of D2O suggest that g is underestimated at most by 5% near the nozzle exit, that is,
can only be increased by ∼5%. If we examine the uncertainty in dgfit/dt, reducing this quantity by 30% does not seem reasonable either, since in all cases the estimates of g from SAXS line up consistently with those of the PTMs. For D2O, polydispersity of the aerosol affects the calculated ΔTexact by less than 2%. Given that the uncertainty in the experimental values is not large enough to account for the observed discrepancy we instead re-examine EquationEquation (1)
[1] . One way to shift the mass and energy balances is to vary the values of qc and qe. Since we already assume qc = 1, the net mass flux cannot be increased by changing qc. The net mass flux can, however, be increased if we decrease the value of qe. As illustrated in if we assume qe = 0.5, the experimental and theoretical droplet temperatures and the corresponding growth rates are in much better agreement. This value of qe lies between the value qe = ∼0.1 required by Young (Citation1982) to match steam condensation data in supersonic nozzles, also measured in the free molecular range, and the value qe = 1 expected in the equilibrium state.
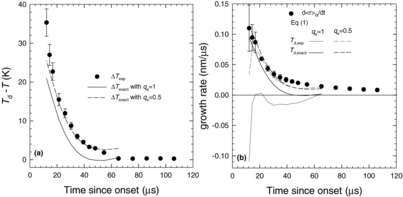
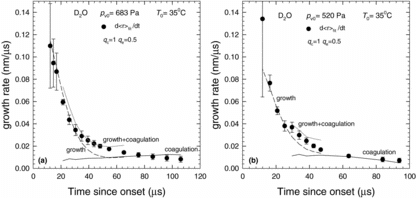
Despite the great improvement, experimental growth rates are still higher than those predicted by theory at intermediate times, that is, 32 μs < t < 60 μs. This discrepancy arises because in the experiment condensation and coagulation are both important but EquationEquation (1)[1] only describes condensation. In order to account for the effect of coagulation on droplet growth, we follow the approach of Alonso et al. (Citation1998) and treat coagulation and growth as independent processes, writing the overall growth rate as
[27] where the first term is growth due to condensation and the second term is growth due to coagulation. In order to estimate the coagulation rate, we start with a volume balance
[28]
where the subscripts 1 and 2 refer to the aerosol prior to and after coagulation, respectively, and where we will ignore the small effect introduced by changes in aerosol polydispersity.
Differentiating EquationEquation (28)[28] with respect to time and recalling that
and ⟨r⟩1 are constant yields
[29] where
is available from the experiments as a function of time and the rate of change in the specific number density ranges from –1.98 × 1023 kg−1 s−1 at ∼20 μs to −1.2 × 1023 kg−1 s−1 near the exit. EquationEquation (27)
[27] can therefore be rewritten in terms of measurable quantities as
[30]
illustrates the droplet growth rates calculated using EquationEquation (30)[30] for qc = 1 and qe = 0.5 and Td = Td,exact. As illustrated in , the modified growth rate better predicts the experimental growth rates than when coagulation is ignored. Likewise, the D2O experiment for pv0 = 520 Pa (), illustrates that incorporating coagulation leads to reasonably good agreement between the experimental data and the predicted growth rates. Nevertheless, to refine the range of qe values consistent with our data we forced the growth rates predicted by EquationEquation (30)
[30] to match the experimental growth rates assuming Td = Td,exp. When 5 < ΔT/K < 25, qe = 0.5 ± 0.1, and the values of qe increased as ΔT decreased. For ΔT < 5 K, qe approached 1.
5 SUMMARY AND CONCLUSIONS
Motivated by the earlier modeling work of Sinha et al. (2009), we directly investigate the growth of water (2.9 < r/nm < 5.2) and nonane (6.2 < r/nm < 25.2) droplets in the free molecular regime. Droplets are produced in a supersonic flow and characterized using SAXS. The SAXS results are combined with PTM in an integrated analysis scheme to determine the other properties of the flow including temperature, density, velocity, and the area of flow. The experimental droplet temperatures are estimated from an energy balance, whereas the experimental growth rates are measured by fitting the ⟨r(t)⟩ data and taking the slope. The theoretical growth rates are calculated using both isothermal and nonisothermal versions of the Hertz–Knudsen model. The droplet temperatures for the nonisothermal growth law are calculated three different ways: by an implicit calculation using EquationEquation (12)[12] that yields Td,exact, or by two approximate explicit expressions, EquationEquations (25)
[25] and Equation(26)
[26] , that yield Td,approx. In our initial analysis, we assumed the evaporation and condensation coefficient were both equal to 1.
For nonane droplet growth, the explicit approximations of Td vary widely and Td is best calculated using the implicit method. Generally, ΔTd,exp is 20–30% higher than the implicit calculations during the initial rapid stage of droplet growth, improving as droplet growth slowed. Under the current experimental conditions, the low equilibrium vapor pressure of nonane results in evaporation rates that are negligible compared to the condensation rates. Consequently, the growth rate is relatively insensitive to Td and qe. For qc = qe = 1, the isothermal and nonisothermal growth rates differ by less than 5% and both agree well with the experimental growth rates. Thus, our data are consistent with qc = 1 but we cannot draw any conclusions regarding qe.
For D2O under our experimental conditions, growth rates are quite sensitive to Td and qe, while the values of Td,approx are within 2 K of Td,exact. During rapid droplet growth, the values of Td,exp are ∼5–10 K higher than Td,exact and using Td,exp in the Hertz–Knudsen growth law suggests the droplets should be unstable. These difficulties can be reconciled by decreasing the value of qe for D2O to 0.5 ± 0.1 when 5 < ΔT /K < 25. Our values of qe lie between the value qe = 0.1 determined by Young in supersonic flow experiments with steam, and the value qe = 1 expected under equilibrium conditions. Incorporating coagulation into the growth calculation is important in the D2O experiments but not in the nonane experiments.
Finally, as in the work of Sinha et al. (Citation2009) D2O droplets grew more quickly than suggested by nonisothermal growth laws with qc = qe = 1. Our current data and analysis, however, confirm that the rapidly growing nanodroplets are significantly hotter than the gas mixture. The latter conflicts with Sinha et al.'s 1-D modeling results for both isotopes of water, where isothermal growth laws yielded the best agreement between the predicted droplet size at the nozzle exit and the corresponding droplet size measurements. However, as already suggested by Sinha et al., more sophisticated modeling effort may be able to resolve this conflict. Models that incorporate 2-D or 3-D effects, tracking subtle changes to the shape of the boundary layer that are induced by condensation, may be able to explain how nucleation is quenched more quickly than expected from heat addition by droplet growth alone.
Supplemental_Files.zip
Download Zip (104.4 KB)REFERENCES
- Abraham, S.F., and Lester, H. (1954). High-Speed Machine Computation of Ideal Gas Thermodynamic Functions. I. Isotopic Water Molecules. J. Chem. Phys., 22:2051–2058.
- Alam, M.K. (1987). The Effect of van der Waals and Viscous Forces on Aerosol Coagulation. Aerosol Sci. Technol., 6:41–52.
- Alonso, M., Hashimoto, T., Kousaka, Y., Higuchi, M., and Nomura, T. (1998). Transient Bipolar Charging of a Coagulating Nanometer Aerosol. J. Aerosol Sci., 29:263–270.
- Bakhtar, F., Zamri, M.Y., and Rodriguez-Lelis, J.M. (2007). A Comparative Study of Treatment of Two-dimensional Two-phase Flows of Steam by a Runge-Kutta and by Denton's Methods. P. I. Mech. Eng. C-J. Mech., 221:689–706.
- Barrett, J.C., and Clement, C.F. (1988). Growth-Rates for Liquid-Drops. J. Aerosol Sci., 19:223–242.
- Beloded, V.V., Kirichewskij, G.A., and Nuzhnyj, V.M. (1989). Condensation Coefficient of Metastable Water. J. Aerosol Sci., 20:1047–1050.
- Bohn, D.E., Surken, N., and Kreitmeier, F. (2003). Nucleation Phenomena in a Multi-stage Low Pressure Steam Turbine. P. I. Mech. Eng. A: J. Pow., 217:453–460.
- Davidovits, P., Kolb, C.E., Williams, L.R., Jayne, J.T., and Worsnop, D.R. (2006). Mass Accommodation and Chemical Reactions at Gas-liquid Interfaces. Chem. Rev., 106:1323–1354.
- Davidovits, P., Worsnop, D.R., Jayne, J.T., Kolb, C.E., Winkler, P., Vrtala, A., et al. (2004). Mass Accommodation Coefficient of Water Vapor on Liquid Water. Geophys. Res. Lett., 31:L22111–L22111.
- Drisdell, W.S., Cappa, C.D., Smith, J.D., Saykally, R.J., and Cohen, R.C. (2008). Determination of the Evaporation Coefficient of D2O. Atmos. Chem. Phys., 8:6699–6706.
- Dykas, S., Wróblewski, W., and Łukowicz, H. (2007). Prediction of Losses in the Flow Through the Last Stage of Low-pressure Steam Turbine. Int. J. Numerical Methods in Fluids, 53:933–945.
- Eames, I.W., Marr, N.J., and Sabir, H. (1997). The Evaporation Coefficient of Water: A Review. Int. J. Heat Mass Transfer, 40:2963–2973.
- Gajewski, P., Kulicki, A., Wisniewski, M., and Zgorzelski, M. (1974). Kinetic Theory Approach to the Vapor-phase Phenomena in a Nonequilibrium Condensation Process. Physics of Fluids, 17:321–327.
- Gerber, A.G., and Mousavi, A. (2007). Application of Quadrature Method of Moments to the Polydispersed Droplet Spectrum in Transonic Steam Flows with Primary and Secondary Nucleation. Appl. Mathematical Modelling, 31:1518–1533.
- Ghosh, D., Bergmann, D., Scwering, R., Wölk, J., Strey, R., Tanimura, S., et al. (2010). Homogeneous Nucleation of a Homologous Series of n-alkanes (CiH2i+2, i = 7–10) in a Supersonic Nozzle. J. Chem. Phys., 132:024307-1–024307-17.
- Gyarmathy, G. (1963). Zur Wachstumsgeschwindigkeit Kleiner Flussigkeitstropfen in Einer Ubersattigten Atmosphare. Zeitschrift Fur Angewandte Mathematik Und Physik 14:280–293.
- Hagen, D.E., Schmitt, J., Trueblood, M., Carstens, J., White, D.R., and Alofs, D.J. (1989). Condensation Coefficient Measurement for Water in the Umr Cloud Simulation Chamber. J. Atmos. Sci., 132:144301-1–144301-22.
- Hickman, K. (1965). Evaporation Coefficient of Liquids, in First International Symposium Water Desalination, Vol. 1, pp. 180–223, U.S. Department of the Interior, Washington, DC.
- Hickman, K. (1966). Reviewing the Evaporation Coefficient. Desalination, 1:13–29.
- Hidy, G.M., and Brock, J.R. (1970). The Dynamics of Aerocolloidal Systems. Pergamon, New York.
- Hill, P.G. (1966). Condensation of Water Vapour During Supersonic Expansion in Nozzles. J. Fluid Mech., 25:593–620.
- Hubbard, G.L., Denny, V.E., and Mills, A.F. (1975). Droplet Evaporation – Effects of Transients and Variable Properties. Int. J. Heat Mass Transfer, 18:1003–1008.
- Kennard, E.H. (1938). Kinetic Theory of Gases, McGraw-Hill, New York, pp. 312–315.
- Kennedy, I.M., and Harris, S.J. (1990). Enhancement of Silica Aerosol Coagulation by Van Der Waals Forces. Aerosol Sci. Technol., 12:869–875.
- Kerminen, V.-M. (1994). Simulation of Brownian Coagulation in the Presence of van der Waals Forces and Viscous Interactions. Aerosol Sci. Technol., 20:207–214.
- Kerminen, V.M., Viisanen, Y., Vesala, T., and Hillamo, R. (1991). Correction for the Brownian Coagulation Coefficient Due to van der Waals Forces between Non-equal Sized Particles. J. Aerosol Sci., 22(Suppl. 1):S105–S107.
- Kotlarchyk, M., and Chen, S.H. (1983). Analysis of Small Angle Neutron Scattering Spectra from Polydisperse Interacting Colloids. J. Chem. Phys., 79:2461–2469.
- Laksmono, H., Tanimura, S., Allen, H.C., Wilemski, G., Zahniser, M.S., Shorter, J.H., et al. (2011). Monomer, Clusters, Liquid: An Integrated Spectroscopic Study of Methanol Condensation. Phys. Chem. Chem. Phys., 13:5855–5871.
- Looijmans, K.N. H. (1995). Homogeneous Nucleation and Droplet Growth in the Coexistence Region of n-alkane/methane Mixtures at High Pressures, in Department of Applied Physics, Eindhoven University of Technology PhD Thesis, Eindhoven.
- Luijten, C.C. M., van Hooy, R.G. P., Janssen, J.W. F., and van Dongen, M.E. H. (1998). Multicomponent Nucleation and Droplet Growth in Natural Gas. J. Chem. Phys., 109:3553–3558.
- Luo, X.S., Prast, B., Van Dongen, M.E. H., Hoeijmakers, H.W. M., and Yang, J.M. (2006). On Phase Transition in Compressible Flows: Modelling and Validation. J. Fluid Mech., 548:403–430.
- Marek, R., and Straub, J. (2001). Analysis of the Evaporation Coefficient and the Condensation Coefficient of Water. Int. J. Heat Mass Transfer., 44:39–53.
- Marlow, W.H. (1980). Lifshitz–van der Waals Forces in Aerosol Particle Collisions. I. Introduction: Water Droplets. J. Chem. Phys., 73:6288–6295.
- Mason, B.J. (1953). The Growth of Ice Crystals in a Supercooled Water Cloud. Quarterly J. Royal Meteorological Soc., 79:104–111.
- Mills, A.F., and Seban, R.A. (1967). Condensation Coefficient of Water. Int. J. Heat Mass Transfer, 10:1815–1827.
- Moheban, M., and Young, J.B. (1985). A Study of Thermal Nonequilibrium Effects in Low-pressure Wet-steam Turbines Using a Blade-to-Blade Time-marching Technique. Int. J. Heat Fluid Flow, 6:269–278.
- Morita, A., Sugiyama, M., Kameda, H., Koda, S., and Hanson, D.R. (2004). Mass Accommodation Coefficient of Water: Molecular Dynamics Simulation and Revised Analysis of Droplet Train/flow Reactor Experiment. J. Phys. Chem. B, 108:9111–9120.
- Mozurkewich, M. (1986). Aerosol Growth and the Condensation Coefficient for Water: A Review. Aerosol Sci. Technol., 5:223–236.
- Muitjens, M., Kalikmanov, V.I., Dongen, v.M. E. H., Hirschberg, A., and Derks, P.A. H. (1994). On Mist Formation in Natural Gas. Revue de l’Institut Francais du Petrole, 49:63–72.
- NIST. (2002). NIST Standard Reference Database REFPROP 23, in Reference Fluid Thermodynamic and Transport Properties. . E. Lemon, M. McLinden, and M. Huber, eds., National Institute of Standards and Technology, Gaithersburg, MD, USA.
- Okimoto, F.T., and Betting, M. (2001). Twister Supersonic Separator, in Proceedings of the 51st Laurance Reid Gas Conditioning Conference, Norman, Oklahoma, USA.
- Okuyama, K., Kousaka, Y., and Hayashi, K. (1984). Change in Size Distribution of Ultrafine Aerosol-Particles Undergoing Brownian Coagulation. J. Colloid Interface Sci., 101:98–109.
- Paci, P., Zvinevich, Y., Tanimura, S., Wyslouzil, B.E., Zahniser, M., Shorter, J., et al. (2004). Spatially Resolved Gas Phase Composition Measurements in Supersonic Flows Using Tunable Diode Laser Absorption Spectroscopy. J. Chem. Phys., 121:9964–9970.
- Peeters, P., Hruby, J., and van Dongen, M.E. H. (2001). High Pressure Nucleation Experiments in Binary and Ternary Mixtures. J. Phys. Chem. B, 105:11763–11771.
- Pruppacher, H.R., and Klett, J.D. (1997). Microphysics of Clouds and Precipitation. Kluwer Academic Publishers, Dordrecht.
- Rideal, E.K. (1925). The Influence of Thin Surface Films on the Evaporation of Water. J. Phys. Chem., 29:1585–1588.
- Rijkers, M.P. W. M., Malais, M., Peters, C.J., and de Swaan Arons, J. (1992). Measurements on the Phase Behavior of Binary Hydrocarbon Mixtures for Modelling the Condensation Behavior of Natural Gas: Part I. The System Methane + Decane. Fluid Phase Equilibria, 71:143–168.
- Scharge, R.W. (1953). A Theoretical Study of Interface Mass Transfer. Columbia University Press, New York.
- Seinfeld, J.H. (1986). Atmospheric Chemistry and Physics of Air Pollution. John Wiley & Sons, Inc., New York.
- Seinfeld, J.H., and Pandis, S.N. (1998). Atmospheric Chemistry and Physics. John Wiley & Sons, New York.
- Shaw, R.A., and Lamb, D. (1999). Experimental Determination of the Thermal Accommodation and Condensation Coefficients of Water. J. Chem. Phys., 111:10659–10663.
- Sinha, S., Wyslouzil, B.E., and Wilemski, G. (2009). Modeling of H2O/D2O Condensation in Supersonic Nozzles, Aerosol Sci. Technol., 43:9–24.
- Smolders, H.J. (1992). Nonlinear Wave Phenomena in a Gas-vapor Mixture with Phase Transition, PhD Thesis, Eindhoven Institute of Technology.
- Tanimura, S., Wyslouzil, B.E., and Wilemski, G. (2010). CH3CH2OD/D2O Binary Condensation in a Supersonic Laval Nozzle: Presence of Small Clusters Inferred from a Macroscopic Energy Balance. J. Chem. Phys., 132:144301–144322.
- Tanimura, S., Zvinevich, Y., Wyslouzil, B.E., Zahniser, M., Shorter, J., Nelson, D., et al. (2005). Temperature and Gas-phase Composition Measurements in Supersonic Flows Using Tunable Diode Laser Absorption Spectroscopy: The Effect of Condensation on the Boundary-layer Thickness. J. Chem. Phys., 122:194304-1–1943041-11.
- Tsuruta, T., and Nagayama, G. (2004). Molecular Dynamics Studies on the Condensation Coefficient of Water. J. Phys. Chem. B, 108:1736–1743.
- Vesala, T., Kulmala, M., and Olin, M. (1990). Condensation and Evaporation of Binary Droplets with Internal Mass-Transfer. J. Aerosol Sci, 21:S7–S10.
- Vieceli, J.S., and Tobias, D.J. (2004). Mass Accomodation Coefficient for Water Vapor on Liquid Water from Computer Simulations. Abstracts of Papers of the American Chemical Society, 227:U1003–U1003.
- Wagner, P.E. (1982). Aerosol Growth by Condensation, in Aerosol Microphysics II:Chemical Physics of Microparticles, W.H. Marlow, ed., Springer-Verlag, Dusseldorf, Germany, pp. 129–178.
- White, A.J., Young, J.B., and Walters, P.T. (1996). Experimental Validation of Condensing Flow Theory for a Stationary Cascade of Steam Turbine Blades. Phil. Trans. R. Soc. Lond. A: Mathematical, Physical and Engineering Sciences, 354:59–88.
- Wölk, J., and Strey, R. (2001). Homogeneous Nucleation of H2O and D2O in Comparison: The Isotope Effect. J. Phys. Chem. B, 105:11683–11701.
- Xia, T.K., and Landman, U. (1994). Molecular Evaporation and Condensation of Liquid n-alkane Films. J. Chem. Phys., 101:2498–2507.
- Young, J.B. (1982). The Spontaneous Condensation of Steam in Supersonic Nozzles. PCH PhysicoChemical Hydrodynamics, 3:57–82.
- Young, J.B. (1991). The Condensation and Evaporation of Liquid Droplets in a Pure Vapour at Arbitrary Knudsen Number. Int. J. Heat Mass Transfer, 34:1649–1661.
- Zagaynov, A.V., Nuzhny, V.M., Cheusova, T.A., and Lushnikov, A.A. (2000). Evaporation of Water Droplet and Condensation Coefficient: Theory and Experiment. J. Aerosol Sci., 31:S795–S796.