Abstract
Agar plate volume in bioaerosol impactors affects collection efficiency, but it is often overlooked in practice. This study investigated the effect of agar volume (20, 35, and 50 mL) and, consequently, jet-to-plate distance on accuracy of culturable impactors. Laboratory experiments investigated sensitive Escherichia coli and hardy Bacillus atrophaeus bacteria with a BioStage impactor. Outdoors bacterial and fungal sampling assessed effects of varying agar volume in BioStage, Sampl’air Lite, and SAS Super 180 multinozzle impactors relative to a reference BioStage with 35 mL agar. The results demonstrate that agar plate volume affects not only overall collection efficiency, but also species selection and colony masking. Culturable concentrations of E. coli in laboratory were underestimated by 35% when using 20 versus 35 mL agar volume (p < 0.001). However, data indicate selection of healthier bacteria, as E. coli colonies were significantly larger on 50 versus 20 mL agar plates (p < 0.001). For outdoors, lower agar volume significantly improved accuracy of Sampl’air relative to the reference BioStage for bacterial (p < 0.001) and fungal (p = 0.03) aerosols. Changes for other samplers were not statistically significant, likely due to wide variability in microbial profiles. Outdoors data indicate that culturable concentrations may be positively correlated with increasing dimensionless jet-to-plate distance, especially for bacteria (p = 0.04). This effect may be attributable to sampler jet dissipation with lower nozzle number impactors (i.e., the Sampl’air) being more sensitive. This study demonstrates that bioaerosol impactor agar plate volume should be considered prior to sampling.
Copyright 2013 American Association for Aerosol Research
1. INTRODUCTION
Bioaerosol is comprised of airborne particles of biological origin, such as viable and nonviable bacteria and fungi, viruses, pollens, and any toxins or fragments associated with organisms. Thus, bioaerosol composition is complex with a wide range of potential health effects following exposure (Douwes et al. Citation2003). Current bioaerosol concerns broadly range from issues like the increased threat of mold contamination and exposure following hurricane coastal flooding (Mousavi et al. Citation2011; Hoppe et al. Citation2012) to the risk for airborne transmission of multidrug resistant bacteria like Mycobacterium tuberculosis (Cegielski Citation2010). These complexities have contributed to interrelated challenges in bioaerosol sampling including a lack of standardized methods, inadequate exposure-response data and few health-based exposure limits (Douwes et al. Citation2003; Eduard et al. Citation2012).
Various bioaerosol collection methods exist, such as inertial and centrifugal impaction, liquid impingement, filtration, and electrostatic precipitation; however, no single method can fully characterize all bioaerosol components (Reponen et al. Citation2011). For the quantification and identification of culturable microorganisms, agar impactors are among the most commonly used. The popularity of these samplers stems from their ease of use for estimating culturable bioaerosol concentrations without the need for post-collection sample processing and the availability of not only stationary, but also portable (battery-powered) impactors. Stationary and portable agar impactors have been evaluated in numerous studies (Lembke et al. Citation1981; Jones et al. Citation1985; Li Citation1999a; Yao and Mainelis Citation2006a,Citationb; Yao and Mainelis Citation2007a,Citationb; Zhen et al. Citation2009). Also, agar impaction sampling protocols have been developed by the National Institute for Occupational Safety and Health (NIOSH Method 0800) and the American Conference of Governmental Industrial Hygienists (ACGIH) for evaluation of indoor bioaerosols (Macher Citation1999; United States Department of Health and Human Services (DHHS) Citation2003).
At the same time, limitations to the use of bioaerosol impactors, similar to regular impactors, can include particle bounce from the collection surface, loss of particles to internal surfaces of the impactor other than the collection surface, and overloading of collection surfaces (Marple and Olson Citation2011). Specifically, for agar impactors, overloading of agar plates with colonies can lead to counting errors and microbial growth inhibition effects; therefore, use of agar impactors is typically restricted to environments with low bioaerosol concentrations and/or to short sampling durations (Reponen et al. Citation2011).
As one of the most widely used aerosol sampling methods, inertial impaction has been extensively studied. An important parameter characterizing impactor design is the cutoff diameter, d50, which represents particle aerodynamic diameter for which the impactor's collection efficiency is 50% (Hinds Citation1982; Marple and Olson Citation2011)
[1] where μg is air viscosity, W is diameter of a round jet nozzle or width of a rectangular nozzle, ρP is particle density, U0 is air velocity through an impactor nozzle, CC is Cunningham slip correction factor and
is square root of Stokes number yielding the cutoff diameter. Operational parameters such as the jet Reynolds number and the nondimensional jet-to-plate distance (S/W)—impactor jet-to-plate (agar) distance divided by nozzle diameter—also influence an impactor's collection efficiency (Marple and Olson Citation2011). For round nozzle impactors, S/W should be greater than unity to avoid sensitivity of d50 to small changes in S/W, and impactors have been shown to operate as expected with S/W as high as five to ten (Marple and Olson Citation2011). However, in multinozzle impactors, there is some evidence that even slightly changing S/W may influence collection efficiency even when the ratio remains within the recommended range (Kwon et al. Citation2002). At the same time, by using a much lower S/W than the recommended values, one can collect particles with aerodynamic diameters smaller than would have been achieved at the originally intended S/W, as was demonstrated by decreasing S/W with an adjustable, adhesive-coated impaction slide (Grinshpun et al. Citation2005; Grinshpun et al. Citation2007).
Despite dependence of impactor collection efficiency on these design and operational parameters, as of yet there is limited analysis on how agar plate volume in bioaerosol impactors can simultaneously affect physical (S/W and consequently d50) and biological collection efficiency. While staying within the recommended S/W range of about one to five (Marple and Olson Citation2011), Yao and Mainelis (Citation2006a) have demonstrated improved physical collection efficiency in multinozzle portable agar impactors by increasing agar plate volume and thus decreasing S/W. However, in addition to physical collection of particles, biological collection efficiency also needs to be considered when collecting culturable samples with different agar volumes. Increasing S/W may increase dissipation of impactor jets (Yao and Mainelis Citation2006a) resulting in gentler sampling; however, insufficient agar embedding at lower impaction velocity may decrease culturability due to desiccation and lack of access to nutrients (Stewart et al. Citation1995). Alternatively, decreasing S/W may improve physical collection efficiency as long as particle bounce is negligible; but, impaction at higher jet velocities could potentially reduce culturability due to greater stress to sampled microorganisms (Stewart et al. Citation1995).
Bioaerosol impactor manufacturers typically provide some guidance on optimal agar fill volumes to maintain the intended d50 of the sampler (Lacey and Venette Citation1995). Regardless, the guidance is often overlooked in practice (Scott et al. Citation2011) and can be unclear. For example, a jet-to-plate distance of 1.0 to 1.5 mm is recommended by the manufacturer when using the BioStage impactor (SKC Inc., Eighty Four, PA, USA), a single-stage Andersen N6-equivalent impactor (Dillon et al. Citation1996). Andersen (Citation1958), on the other hand, argues that a jet-to-plate distance of 1 mm in an Andersen type impactor is too difficult to control in agar plates, may increase the airstream velocity too much near the horizontal periphery of the agar plate, and is unnecessarily small. For another type of Andersen impactor, the N6 Single-Stage Viable Andersen Cascade Impactor (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA), a minimum agar fill height of 5.2 mm is recommended to achieve the theoretical d50 of 0.65 μm (Scott et al. Citation2011). Even though both the BioStage and the N6 Single-Stage Viable Andersen Cascade Impactor share the same design, the agar fill height recommendations of the two manufacturers are not consistent. Summation of a fill height of 5.2 mm and a maximum recommended jet-to-plate distance of 1.5 mm (Dillon et al. Citation1996) indicates a total sampler head space of 6.7 mm in single-stage Andersen N6 impactors; but, agar plates with fill heights greater than 6.7 mm can fit inside these samplers without contacting the sampler head and a user would not be aware that sampling parameters are outside the recommended range.
Given the potential effect of S/W on culturable impactor measurement accuracy and a lack of guidance on the topic, the goal of this study is to investigate the effect of agar plate volume in bioaerosol impactors on culturable bioaerosol sampling through laboratory and outdoor field experiments. We believe this is the first study to focus on culturable bioaerosol samples quantified with varying agar volume. Understanding the relationship between agar plate volume and accuracy of estimated bioaerosol concentrations may lead to improved interpretation of sampling data, evidence for more standardized impaction sampling, and improved bioaerosol impactor design. Additionally, this study presents relative performance data on the Sampl’air Lite (AES-Chemunex Inc., Princeton, NJ, USA), a relatively new bioaerosol impactor with limited performance information available in the literature.
2 MATERIALS AND METHODS
2.1 Summary
Two different bacterial species were aerosolized in a laboratory and sampled using different agar volumes (20, 35, and 50 mL) in the BioStage impactor (SKC, Inc., Eighty Four, PA). To account for daily variation in bioaerosol concentration and culturability, samples collected with 35 mL agar volume on a particular day were used as the reference. Outdoor field sampling of culturable bacteria and fungi was also performed with these agar volumes in three single stage multinozzle impactors: BioStage, Sampl’air Lite (AES-Chemunex Inc., Princeton, NJ) and SAS Super 180 (Bioscience International, Rockville, MD). A separate (second) BioStage with 35 mL of agar was used as a reference in field sampling. The BioStage is a single-stage Andersen N6-equivalent impactor (Andersen Citation1958) and has been used as a reference sampler in other bioaerosol studies (Yao and Mainelis Citation2006b; Yao and Mainelis Citation2007a). Preliminary laboratory data when sampling E. coli indicated that using 35 mL agar volume in the BioStage provided the most colony forming units when comparing across 20, 35, and 50 mL agar volumes. Laboratory experiments were conducted prior to field sampling, so 35 mL was chosen as the reference agar volume in the reference BioStage for field and laboratory sampling.
2.2 Agar Plate Volumes
The agar volumes used in this study were chosen for the following reasons: (1) the manufacturer recommended agar plate volumes for the BioStage, Sampl’air and SAS180 are within the range or close to the tested agar volumes; (2) the graduated cylinder used to measure and pour agar has an accuracy of ±1 mL (ASTM International Citation2012) thus necessitating that tested agar volumes were sufficiently different from one another; and (3) equally spaced apart values were used to investigate general trends in impactor performance across samplers.
Adjustment of agar fill volumes to accommodate differences in agar plate dimensions is essential for achieving recommended jet-to-plate distances (Scott et al. Citation2011). The agar plates used in this study were 100 × 15 mm polystyrene Petri dishes (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA, catalog number FB0875712). These agar plates have tapered walls with a bottom inner diameter of 84 mm and a top inner diameter of 87 mm. An average plate diameter of 85.5 mm was used to calculate agar fill heights, agar plate volumes and jet-to-plate distances. The manufacturer of the BioStage recommends a jet-to-plate distance of 1.0 to 1.5 mm. Based on the jet-to-plate distance of 1.7 mm reported by Yao and Mainelis (Citation2006b) when using the BioStage with 50 mL agar, the manufacturer recommended agar fill volume ranges from 51 to 54 mL for the agar plates used in this study. For the SAS180 and Sampl’air, the manufacturer recommended agar volumes were 20 to 22 mL and 14 to 20 mL, respectively. An accuracy of ± 1 mL for the agar fill volumes was verified by measuring the fill heights for three dried, prepared agar plates of each volume (20, 35, and 50 mL) and comparing to the calculated fill height expected for each. Thus, the tested agar volume range includes or is very close to manufacturers’ recommended volumes for all three samplers.
2.3 Experimental Methods—Laboratory Sampling
Two bacterial species were investigated—Bacillus atrophaeus, a hardy, gram-positive bacterium, and Escherichia coli, a sensitive, gram-negative bacterium; these species have been recommended and commonly used in bioaerosol research (Jensen et al. Citation1992; Macher Citation1997; Li Citation1999b; Hospodsky et al. Citation2010). Bacteria of the genus Bacillus are some of the most commonly found bacterial species in indoor and outdoor environments (Zhu et al. Citation2003; Fang et al. Citation2007; Stanley et al. Citation2008). As a nonspore-forming bacteria, E. coli is often used as a representative sensitive bacteria to investigate the effects of microorganism hardiness during bioaerosol sampling in contrast to B. atrophaeus (Li Citation1999b). B. atrophaeus vegetative cells and spores have reported aerodynamic diameters of 0.86 μm and 0.9 μm, respectively, and E. coli cells have an aerodynamic diameter of 0.78 μm (Aizenberg et al. Citation2000; Yao and Mainelis Citation2007b). Stock cultures of B. atrophaeus (ATCC 49337) and E. coli (ATCC 15597) were obtained from American Type Culture Collection Inc. (ATCC, Rockville, MD). Active B. atrophaeus cultures were inoculated in nutrient broth (Thermo Fisher Scientific Inc., Remel Products, Lenexa, KS, USA) and incubated at 30°C for 18 h; active E. coli cultures were inoculated in liquid trypticase soy broth (Becton, Dickson and Company, Sparks, MD) and incubated at 37°C for 18 h. Cells were harvested from microbial suspensions by centrifugation for five minutes at 7000 rpm (BR-4 centrifuge, Jouan, DEC Inc., Lorton, VA, USA). Cells were then washed three times by resuspending pellets in sterile deionized water (EMD Millipore Corporation, Milli-Q Direct 8, Billerica, MA, USA) and repeating centrifugation. Using the staining procedure described by Seshadri et al. (Citation2009), acridine orange-based epifluorescence microscopy determined that the final, washed B. atrophaeus suspension contained approximately 80% vegetative cells and 20% spores.
For a given day on which sampling was performed, the same bacterial suspension, which was prepared on that day, was used throughout the experiment. Diluted bacterial suspensions were stored in the refrigerator at 4°C until being used in the experiment; this storage time for a given bacterial suspension was a maximum of 90 min. To estimate changes in viability that may have occurred, a serial dilution of prepared E. coli suspension was spread plated in triplicate prior to and after storage in refrigerator for 90 min and incubated for 72 h. NCFU prerefrigeration were 174 ± 7 and NCFU postrefrigeration were 160 ± 9. This difference was not statistically significant (p > 0.05).
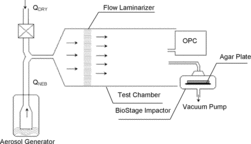
illustrates laboratory setup. Bioaerosols were generated with a six-jet Collison nebulizer (BGI Inc., Waltham, MA, USA) at a flow rate of QNEB = 4 L/min using 10 psig dry, compressed air and carried into the chamber by dry, filtered air at QDRY = 40 L/min. Low aerosolization air pressure was used to minimize damage to the bacteria. The sampling chamber was 10 cm in diameter and had a total flow rate of approximately 44 L/min corresponding to still air sampling conditions; the sampling probe diameter (1 cm) satisfied Davies’ criterion to ensure negligible sampling error from particle settling and inertia during still air sampling (Davies Citation1968; Hinds Citation1982). Chamber relative humidity and temperature were monitored with a Traceable Hydrometer (Fisher Scientific, Pittsburgh, PA, USA). An OPC (optical particle counter, model 1.108 Grimm Technologies, Inc., Douglasville, GA, USA) monitored particle number concentrations within the chamber throughout sampling and allowed us to determine dilution factors for initial bacterial suspensions to prevent overloading sampling plates. The BioStage impactor sampled from the chamber through its calibration adaptor at 28.3 L/min with each sampling repeat being performed for 1 min. The calibration adaptor was used due to limited chamber diameter. Fresh microbial suspension (10 mL) was replaced in the nebulizer before each sampling repeat for each new agar plate to minimize aerosolization stress (Yao and Mainelis Citation2006b). Sampling lines were as short as feasible and conductive tubing was used to minimize aerosol electrostatic charge effects.
Agar plates were prepared with trypticase soy agar (Beckton, Dickson and Company, Sparks, MD). Before each repeat, a new agar plate was loaded in the BioStage. Agar volume chosen for each repeat was randomized and tested in sets of three so that each set contained one of each volume—a 20, 35, and 50 mL plate. Each of these sets was completed within 15 min. This randomized variability, such as changes in E. coli viability or aerosolized particle size distribution, across all tested agar volumes throughout the experiment so that comparative data across the tested agar volumes would be valid. Furthermore, sporulation of B. atrophaeus is not expected to have influenced results due to short storage time of suspension. According to the OPC data, bacterial suspensions of the same concentration on the same day had similar aerosolized particle size distributions. For example, for a B. atrophaeus suspension aerosolized multiple times after replacement in the Collison Nebulizer, the arithmetic mean diameters across sample runs ranged from 0.85 to 0.99 μm, count median and mode diameters all were in the 1.0–1.6 μm OPC measurement bin, and total particle number concentrations ranged from 1.2 × 106 to 2.1 × 106 /m3. Repeats were performed until there were at least ten full sets of these tested volumes for each tested bacteria. After sampling, plates were incubated for 72 h at room temperature (E. coli) or 48 h at 37°C (B. atrophaeus). The number of colony forming units (NCFU) were counted every 24 h until end of incubation time and corrected for coincidence using positive-hole correction method for multiple jet impactors (Macher Citation1989).
At the end of incubation time, a photograph was taken of each plate (Canon PowerShot ELPH 110 HS, Canon USA Inc., Lake Success, NY) illuminated on a light box (Bel-Art Products, Wayne, NJ) for colony size analysis with ImageJ software version 1.45 (Rasband Citation2013). About half of the photographs were chosen randomly by sampling date to be used for colony size analysis. Briefly, using the diameter of the plate as a reference distance, images were size-calibrated from pixels to micrometers, background noise was decreased, color threshold was adjusted to distinguish colonies, and total area of the agar plate covered by colonies was measured (ATotal) (i.e., the individual colonies were not measured separately). Average colony size for each plate was then determined:
[2]
where Uncorrected NCFU represents an individual plate's raw number of colony forming units.
2.4 Experimental Methods—Field Sampling
Outdoors field sampling was performed near an office building on an agricultural campus. Samplers were placed approximately 0.3 m from each other, 1 m above the ground. Bacteria and fungi were sampled throughout August–September, 2012. provides operational details of field samplers. The S/W of the impactors was determined by measuring the height from bottom of agar plates to jets and then subtracting different agar fill heights. All samplers were operated at their standard flow rate and started simultaneously for each repeat. To avoid overloading plates, however, sampling times were established on each sampling date based on flow rate, particle concentrations monitored by OPC, and relative humidity/temperature. Due to shorter headspace in the Sampl’air and SAS Super 180, these samplers could not accommodate 50 mL plates and 35 mL of agar was the highest volume used in these two samplers. In addition to the investigated BioStage impactor, a separate (second) BioStage served as a reference sampler and was loaded with a new 35 mL plate for each repeat. Use of a separate BioStage impactor with a fixed agar volume as a reference accounted for daily variability in bioaerosol concentration and culturability. Repeats and randomization of agar volumes in the tested samplers was performed the same way as for laboratory sampling. Sampling plates were prepared using malt extract agar for fungi sampling and trypticase soy agar for bacteria sampling (Beckton, Dickson and Company, Sparks, MD, USA). Fungicide was used for bacteria sampling in the ratio of 200 mg cycloheximide (Sigma-Aldrich Co., St. Louis, MO, USA) per liter agar.
Table 1 Characteristics of samplers used in field sampling
After sampling, all plates were incubated for 72 h at room temperature and NCFU were counted and corrected same as for laboratory sampling. When the reference BioStage plate and/or all experimental plates of a sampling set were overloaded, then this particular set was excluded from analyses. Culturable bacterial and fungal concentrations were determined:
[3] where CCFU is the culturable concentration; Q and t are sampler flow rate and sampling time, respectively.
2.5 Statistical Analysis
For laboratory data, all NCFU from the BioStage were normalized by average NCFU across 35 mL plates on each respective sampling date to reduce variability resulting from preparing bacteria and sampling on multiple dates. For field sampling, data obtained from the samplers were normalized to the results from the reference BioStage for each repeat. Residual plots assessed statistical model assumptions for normality and homogeneity of variance. All analyses were performed using log-transformed data because data were approximately lognormal. Due to unequal residual variances for some log-transformed datasets, Welch's one-way analysis of variance (ANOVA) and t-tests were performed followed by Games-Howell multiple comparison of means. The Welch procedure is generally applicable for simple comparisons, such as those performed in this study (Oehlert Citation2000). All analyses were performed using SPSS Statistics for Windows, v20 (IBM Corporation Citation2011) with α = 0.05.

3 RESULTS AND DISCUSSION
3.1 Laboratory Sampling—Colony Forming Unit Enumeration
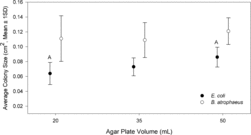
Normalized microorganism concentrations measured in laboratory experiments are presented in . For experiments with E. coli, 22 sets of 20, 35, and 50 mL agar plates comprised the results for a total of 66 plates with E. coli colonies. For B. atrophaeus, 13 sets of 20, 35, and 50 mL agar plates were completed for a total of 39 plates with B. atrophaeus colonies. Across the testing days and all agar plate volumes, measured E. coli concentrations ranged from 500 to 23,000 NCFU/m3 and measured B. atrophaeus concentrations ranged from 400 to 28,000 NCFU/m3. Analysis of the results by one-way ANOVA revealed a statistically significant effect of agar volume for E. coli (Welch's F (2, 39.86) = 14.68, p < 0.001) with 20 mL plates having significantly fewer E. coli NCFU than 35 mL plates (p < 0.001) and 50 mL plates (p < 0.001) as indicated by Games-Howell post hoc test. Thus, the results suggest that for sensitive microbial species, 20 mL agar volume may provide decreased overall collection efficiency and approximately 35% underestimation of bioaerosol concentrations as compared to collection using 35 mL plates. These results are consistent with previous findings that increasing agar volume and decreasing S/W can improve collection efficiency and lower an impactor's d50 (Yao and Mainelis Citation2006a). The B. atrophaeus normalized NCFU data follow a similar trend as the E. coli data, but findings were not statistically significant (Welch's F (2, 23.45)) = 1.98, p = 0.16), which was likely due to greater variability in the data. Additionally, E. coli cells have a smaller aerodynamic diameter than B. atrophaeus vegetative cells and spores; thus, when using 20 mL agar, the jet-to-plate distance may have been large enough, or d50 high enough, to allow for substantial loss of E. coli in the sampling stream.
Grinshpun et al. (Citation2005) suggested that decreasing S/W may result in excessive shear force in the impaction zone resulting in increased particle deaggregation and bounce, particularly for spore agglomerates. Increasing agar volume may have increased particle bounce and loss from the sampler at 50 mL agar volume, but the results suggest that this will not lead to significant and substantial underestimation of concentrations as compared to 35 mL agar volume. Future studies could perform similar experiments with an optical particle counter or a filter both upstream and downstream of the impactor to investigate particle bounce as a function of agar volume.
3.2 Laboratory Sampling—Colony Size
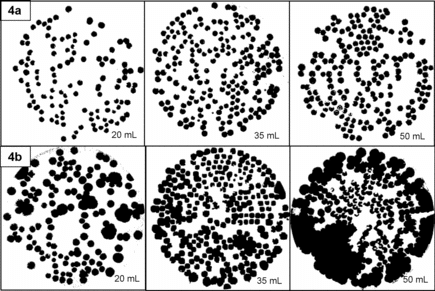
presents average colony size compared across agar plate volumes. At the end of incubation time, average colony size was calculated using EquationEquation (2)[2] and ImageJ Software (Rasband Citation2013) for half the photographs chosen at random by sampling date. and illustrates representative examples of ImageJ processed photographs for E. coli colonies and B. atrophaeus colonies, respectively. demonstrates a statistically significant effect of agar plate volume on average E. coli colony size (Welch's F (2, 21.45) = 9.32, p < 0.001) with 50 mL plates having significantly larger colonies than 20 mL plates (p = 0.001). This may be due to increased physical collection efficiency resulting in a higher number of bacteria deposited under each nozzle with increasing agar volume eventually resulting in larger colony forming units. However, dividing by positive hole corrected NCFU in EquationEquation (2)
[2] rather than raw NCFU resulted in a similar trend with 50-mL plates having the largest average E. coli colony size (Welch's F(2, 21.84) = 2.13, p = 0.14; data not shown). In addition to less efficient physical collection efficiency at 20 mL agar volume, smaller colony size might indicate insufficient particle embedding resulting in nutrient limitation, desiccation, and a significant decrease in NCFU (Stewart et al. Citation1995).
Results were not statistically significant for B. atrophaeus data (Welch's F (2, 9.43) = 0.71, p = 0.51). However, results illustrate how NCFU counting error due to indistinguishable colony overlap (Chang et al. Citation1994; Chang et al. Citation1995) needs to be considered conjointly with analysis method when choosing agar volume for impaction sampling. Since B. atrophaeus colonies were counted at 24 and 48 h post sampling, it was possible to discern individual colonies by manual counting. However, B. atrophaeus colony masking at 35 and 50 mL agar volume was a major limitation in the image analysis. This was largely due to the lighter hue of B. atrophaeus colonies against the agar color; in striving to maintain a standardized image processing procedure, there was increasing difficulty for the software to detect colony edges as these edges overlapped. This may have caused errors in colony size calculation for B. atrophaeus. Since investigation of colony growth with ImageJ software is a new approach to analysis of agar impactor data, conclusions should be interpreted as preliminary with wider application of ImageJ software in future experiments.
3.3 Field Sampling—Bacterial and Fungal Culturable Concentrations
Outdoor field sampling of culturable bacteria and fungi was performed with three different single-stage multinozzle bioaerosol impactors. Normalized culturable concentrations versus agar plate volume are presented in and for bacteria and fungi, respectively. Eighteen sets of agar plate volumes were attempted across 4 days for bacteria sampling and 14 sets were attempted for fungi sampling across 3 days in August/September 2012. Of these, 17 (17/18, 94%) complete sets of different agar plate volumes were countable for bacteria for each of the samplers. For fungi sampling, 11 (11/14, 79%) complete sets of different agar plate volumes were countable for the Sampl’air and 12 (12/14, 86%) complete sets of data were countable for the BioStage and SAS180. Incomplete sets of agar plate volumes indicate overloading of a particular plate and/or overloading of the reference BioStage plate and thus exclusion from further analysis. Culturable concentrations estimated by the Sampl’air were statistically significantly greater for 20 mL versus 35 mL plates for both bacteria (Welch's t (1, 32.94) = 21.81, p < 0.001) and fungi (Welch's t (1, 19.09) = 5.93, p = 0.03). Sampl’air measured culturable concentrations ranged from 10 to 1200 NCFU/m3 for bacteria and from 200 to 2500 NCFU/m3 for fungi across all agar volumes. Compared to using 20 mL agar volume, using 35 mL in the Sampl’air resulted in approximately a 65% and 25% reduction in mean, normalized bacterial and fungal culturable concentrations, respectively. Culturable concentrations estimated by the BioStage impactor were not significantly different across agar volumes (bacteria: Welch's F (2, 30.46) = 1.07, p = 0.36; fungi: Welch's F (2, 21.11) = 0.15, p = 0.86). BioStage measured concentrations ranged from 40 to 4000 NCFU/m3 for bacteria and from 700 to 5000 NCFU/m3 for fungi across all agar volumes. A similar outcome of insignificant differences across agar volumes was observed for the SAS Super 180 (bacteria: Welch's t (1, 32.83) = 1.18, p = 0.29; fungi: Welch's t (1, 21.85) = 0.33, p = 0.57) with measured bacteria concentrations ranging from 40 to 1000 NCFU/m3 and measured fungi concentrations ranging from 500 to 3000 NCFU/m3 across all agar volumes.
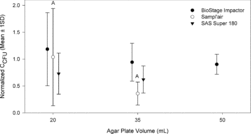

Each of these impactors may have an optimal agar volume for culturable sampling accuracy based on species-specific resistivity to different types of sampling stress, such as changes in impaction jets due to changes in S/W. If so, then the lack of significant findings for two out of three investigated samplers might indicate that these preferences become obscured due to diverse outdoor microbial profiles. All outdoors sampling was performed in one location; but, across sampling dates, wide variability in sampling conditions, including relative humidity ranging 50–98% and temperature ranging 20–37°C, most likely resulted in varying bioaerosol composition and concentrations potentially masking the effect of agar volume. The size distribution of airborne microbial particles also could have been changing over time.
All S/W values tested in this study were above the recommended minimum of unity (). However, in most cases, the tested S/W values were high compared to the upper range of S/W values (5 to 10) reported by Marple and Olson (Citation2011). S/W values less than unity (1) were not attainable due to limited headspace in the samplers. So, the effect achieved by Grinshpun et al. (Citation2005) of increased collection efficiency with impaction plates and very small S/Ws could not be evaluated with agar plates. Regardless, culturable sampling of bioaerosols may be most accurate with optimized sampler-specific parameters, including S/W values, that provide sufficient physical collection and preservation of culturability. When the outdoor sampling data from the three different samplers of this study are pooled together, they provide moderate evidence that a positive correlation exists between mean, normalized CCFU and S/Ws, particularly for outdoor bacterial species (bacteria: Spearman's correlation coefficient = 0.75, p = 0.04; fungi: Spearman's correlation coefficient = 0.5, p = 0.24; data not shown). A trend of increasing mean, normalized CCFU with increasing S/W seems contradictory to laboratory evidence of potenitally larger, healthier colonies with decreasing S/W ( and ). As interpreted from and , the bacteria that survive embedding into agar plates with smaller S/W may result in healthier colonies, but there may be an increase in the number of surviving bacteria when using agar plates with greater S/W due to impaction jet dissipation. For example, for the Sampl’air Lite, a greater S/W value of 11.2 at 20 mL agar volume significantly increased culturable concentration estimates; the Sampl’air may have been most sensitive to this effect since it has the lowest number of nozzles (258 versus 400 for BioStage and 401 for SAS180). Dissipation of the jets may have increased the number of particles depositing in positions not directly under the jets and resulted in less dessication to already collected microorganisms.
3.4 Field Sampling—Relative Sampler Performances
Pooling CCFU results across 20 and 35 mL agar volumes, differences in normalized, outdoor culturable concentrations by sampler type (BioStage, Sampl’air and SAS180) were assessed using Welch's one-way ANOVA. Mean bacterial CCFU results were significantly different across samplers (Welch's F (2, 64.25) = 15.55, p < 0.001). The Sampl’air and SAS180 were significantly different from the BioStage (p < 0.001), but were not significantly different from one another (p = 0.42). The same trend was seen for fungal CCFU. In this case, statistically significant differences were detected across the samplers (Welch's F (2, 44.25) = 10.93, p < 0.001) with the Sampl’air and SAS180 being significantly different from the BioStage (p ≤ 0.001), but not different from one another (p = 0.99).
Relative sampler efficiency, REFF, may be used to compare sampler performance in field studies by calculating the ratio of CCFU estimated by a test sampler, CTest, to CCFU of a reference BioStage impactor using the same agar volume, CBioStage (Yao and Mainelis Citation2007a):
[4]
For mean, normalized CCFU, REFF's of the SAS180 fell within the range of 66–77% for both bacterial and fungal bioaerosols regardless of agar volume. These results are similar to those obtained by Yao and Mainelis (Citation2007a) for outdoor bacteria and fungi. For the Sampl’air, REFF for bacterial CCFU ranged from 30 to 88% for 35 and 20 mL agar volume, respectively, while REFF for fungal CCFU ranged from 64 to 86% for these two agar volumes. The REFF of the Sampl’air compared to SAS180 instead of BioStage was 142% and 58% for bacterial CCFU at 20 and 35 mL agar volume, respectively, and 121% and 83% for fungal CCFU at 20 and 35 mL agar volumes. Thus, the Sampl’air tended to perform better than the SAS180 at 20 mL agar volume for both bacterial and fungal sampling and worse for 35 mL agar volume. Since the Sampl’air is a relatively new sampler, these data provide important guidance in suggesting that lower agar volumes may significantly and substantially improve the accuracy of culturable samples obtained with the Sampl’air. At the same time, the Sampl’air underestimated airborne microorganism concentrations relative to the BioStage impactor, despite the BioStage being operated for longer periods of time, which is known to substantially and significantly decrease culturable bioaerosol counts (Mainelis and Tabayoyong Citation2010).
4 CONCLUSIONS
Adjusting agar volume, and therefore S/W ratio, has already been shown to influence physical collection efficiency and impactor d50 (Yao and Mainelis Citation2006a). This study demonstrates for the first time how agar plate volume can also affect impactor measurement accuracy for culturable samples by influencing culturability, colony masking, and possibly preferential species selection (based on laboratory results). The extent of these effects seems to depend on microbial resistivity to sampling stress and impactor type. For the BioStage impactor, laboratory data suggest colony forming unit concentration for microorganisms sensitive to desiccation and nutrient limitation may be significantly underestimated when using low agar volume (i.e., 20 mL) while colony masking may increase with increasing agar volume. Thus, bacteria that can survive embedding in agar plates with lower S/W may result in larger, healthier colonies. Also, laboratory sampling indicated that there might be an impactor-specific upper limit to which S/W can be increased before significant physical particle loss occurs. This could result in significant preferential sampling of biological species with larger aerodynamic diameters.
Field sampling data indicated that overall colony forming unit concentrations, especially for bacteria, could be positively correlated with S/W. This may possibly be due to the presence of hardier, more desiccation resistant species outdoors and sampler jet dissipation with lower nozzle number impactors being more sensitive to this effect. Results obtained with the BioStage and SAS Super 180 seemed to be less sensitive to S/W, while greater S/W substantially improved accuracy of estimated culturable concentrations when using Sampl’air. As the Sampl’air is a relatively new sampler, this study offers performance data and guidance on the use of the Sampl’air relative to the BioStage impactor, an Andersen N6 type of sampler often used as a reference. The BioStage impactor appeared to provide the most accurate culturable concentration data overall compared to the Sampl’air and SAS180.
Results of field sampling may reflect differences in microbial sampling stress related to sampler design and variable outdoor bioaerosol composition. Laboratory data indicate that the use of different agar volumes in bioaerosol impactors may potentially result in preferential sampling and detection of certain types of bioaerosol species. Therefore, choice of agar volume in bioaerosol impactors and understanding how it will influence results should be considered prior to sampling. This study highlights the potential for complications when comparing data across different impactors or across data collected with the same impactor, using different agar volumes. Agar impaction sampling for bioaerosols could be further improved through the use of such evidence to derive standardized guidance on agar plate volumes depending on impactor type, sampling conditions, and sampling purpose.
REFERENCES
- Aizenberg, V., Reponen, T., Grinshpun, S.A., and Willeke, K. (2000). Performance of Air-O-Cell, Burkard, and Button Samplers for Total Enumeration of Airborne Spores.Am. Ind. Hyg. Assoc. J., 61:855–864.
- Andersen, A.A. (1958). New Sampler for the Collection, Sizing, and Enumeration of Viable Airborne Particles. J. Bacteriol., 76:471–484.
- ASTM International (2012). Standard Specification for Laboratory Glass Graduated Cylinders, ASTM International, West Conshohocken, PA.
- Cegielski, J.P. (2010). Extensively Drug-Resistant Tuberculosis: “There Must be Some Kind of Way Out of Here.” Clin. Infect. Dis., 50(Suppl 3):S195–S200.
- Chang, C.W., Grinshpun, S.A., Willeke, K., Macher, J.M., Donnelly, J., Clark, S., et al. (1995). Factors Affecting Microbiological Colony Count Accuracy for Bioaerosol Sampling and Analysis. Am. Ind. Hyg. Assoc. J., 56:979–986.
- Chang, C.W., Hwang, Y.H., Grinshpun, S.A., Macher, J.M., and Willeke, K. (1994). Evaluation of Counting Error Due to Colony Masking in Bioaerosol Sampling. Appl. Environ. Microbiol., 60:3732–3738.
- Davies, C.N. (1968). The Entry of Aerosols into Sampling Tubes and Heads. J. Phys. D: Appl. Phys., 1:921–932.
- Dillon, H.K., Heinsohn, P.A., and Miller, J.D. (1996). Field Guide for the Determination of Biological Contaminants in Environmental Samples. AIHA Publications, Fairfax, VA.
- Douwes, J., Thorne, P., Pearce, N., and Heederik, D. (2003). Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg., 47:187–200.
- Eduard, W., Heederik, D., Duchaine, C., and Green, B.J. (2012). Bioaerosol Exposure Assessment in the Workplace: The Past, Present and Recent Advances. J. Environ. Monit., 14:334–339.
- Fang, Z., Ouyang, Z., Zheng, H., Wang, X., and Hu, L. (2007). Culturable Airborne Bacteria in Outdoor Environments in Beijing, China. Microb. Ecol., 54:487–496.
- Grinshpun, S.A., Adhikari, A., Cho, S.-H., Kim, K.-Y., Lee, T., and Reponen, T. (2007). A Small Change in the Design of a Slit Bioaerosol Impactor Significantly Improves Its Collection Characteristics. J. Environ. Monit., 9:855–861.
- Grinshpun, S.A., Mainelis, G., Trunov, M., Górny, R.L., Sivasubramani, S.K., Adhikari, A., et al. (2005). Collection of Airborne Spores by Circular Single-Stage Impactors with Small Jet-to-Plate Distance. J. Aerosol Sci., 36:575–591.
- Hinds, W.C. (1982). Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. Wiley-Interscience, New York.
- Hoppe, K.A., Metwali, N., Perry, S.S., Hart, T., Kostle, P.A., and Thorne, P.S. (2012). Assessment of Airborne Exposures and Health in Flooded Homes Undergoing Renovation. Indoor Air, 22:446–456.
- Hospodsky, D., Yamamoto, N., and Peccia, J. (2010). Accuracy, Precision, and Method Detection Limits of Quantitative PCR for Airborne Bacteria and Fungi. Appl. Environ. Microbiol., 76:7004–7012.
- IBM Corporation (2011). IBM SPSS Statistics for Windows. IBM Corp., Armonk, NY.
- Jensen, P.A., Todd, W.F., Davis, G.N., and Scarpino, P.V. (1992). Evaluation of Eight Bioaerosol Samplers Challenged with Aerosols of Free Bacteria. Am. Ind. Hyg. Assoc. J., 53:660–667.
- Jones, W., Marring, K., Morey, P., and Sorenson, W. (1985). Evaluation of the Andersen Viable Impactor for Single Stage Sampling. Am. Ind. Hyg. Assoc. J., 46:294–298.
- Kwon, S.B., Kim, M.C., and Lee, K.W. (2002). Effects of Jet Configuration on the Performance of Multi-Nozzle Impactors. J. Aerosol Sci., 33:859–869.
- Lacey, J., and Venette, J. (1995). Outdoor Air Sampling Techniques, in Bioaerosols Handbook, C.S. Cox, and C.M. Wathes, eds., CRC Press, Lewis Publishing, New York, p. 419.
- Lembke, L.L., Kniseley, R.N., Van Nostrand, R.C., and Hale, M.D. (1981). Precision of the All-Glass Impinger and the Andersen Microbial Impactor for Air Sampling in Solid-Waste Handling Facilities. Appl. Environ. Microbiol., 42:222–225.
- Li, C.-S. (1999a). Sampling Performance of Impactors for Bacterial Bioaerosols. Aerosol Sci. Technol., 30:280–287.
- Li, C.-S. (1999b). Evaluation of Microbial Samplers for Bacterial Microorganisms. Aerosol Sci. Technol., 30:100–108.
- Macher, J.M. (1989). Positive-Hole Correction of Multiplejet Impactors for Collecting Viable Microorganisms. Am. Ind. Hyg. Assoc. J., 50:561–568.
- Macher, J.M. (1997). Evaluation of Bioaerosol Sampler Performance. Appl. Occup. Environ. Hyg., 12:730–736.
- Macher, J.M., ed. (1999). Bioaerosols: Assessment and Control. American Conference of Governmental Industrial Hygienists, Cincinnati, Ohio.
- Mainelis, G., and Tabayoyong, M. (2010). The Effect of Sampling Time on the Overall Performance of Portable Microbial Impactors. Aerosol Sci. Technol., 44:75–82.
- Marple, V.A., and Olson, B.A. (2011). Sampling and Measurement Using Inertial, Gravitational, Centrifugal and Thermal Techniques, in Aerosol Measurement: Principles, Techniques and Applications, P.A. Baron, K. Willeke, and P. Kulkarni, eds., John Wiley, New York.
- Mousavi, M., Irish, J., Frey, A., Olivera, F., and Edge, B. (2011). Global Warming and Hurricanes: The Potential Impact of Hurricane Intensification and Sea Level Rise on Coastal Flooding. Clim. Change, 104:575–597.
- Oehlert, G.W. (2000). A First Course in Design and Analysis of Experiments. W. H. Freeman, New York.
- Rasband, W.S. (2013). ImageJ, U.S. National Institutes of Health, Bethesda, MD.
- Reponen, T., Willeke, K., Grinshpun, S., and Nevalainen, A. (2011). Biological Particle Sampling, in Aerosol Measurement: Principles, Techniques and Applications, P. Kulkarni, P. Baron, and K. Willeke, eds., John Wiley & Sons, Inc., Hoboken, NJ, pp. 549–570.
- Scott, J.A., Summerbell, R.C., and Green, B.J. (2011). Detection of Indoor Fungi Bioaerosols, in Fundamentals of Mold Growth in Indoor Environments and Strategies for Healthy Living, O.C. G. Adan, and R.A. Samson, eds., Wageningen Academic Publishers, Wageningen, The Netherlands.
- Seshadri, S., Han, T., Krumins, V., Fennell, D.E., and Mainelis, G. (2009). Application of ATP Bioluminescence Method to Characterize Performance of Bioaerosol Sampling Devices. J. Aerosol Sci., 40:113–121.
- Stanley, N.J., Kuehn, T.H., Kim, S.W., Raynor, P.C., Anantharaman, S., Ramakrishnan, M.A., et al. (2008). Background Culturable Bacteria Aerosol in Two Large Public Buildings Using HVAC Filters as Long Term, Passive, High-Volume Air Samplers. J. Environ. Monit., 10:474–481.
- Stewart, S.L., Grinshpun, S.A., Willeke, K., Terzieva, S., Ulevicius, V., and Donnelly, J. (1995). Effect of Impact Stress on Microbial Recovery on an Agar Surface. Appl. Environ. Microbiol., 61:1232–1239.
- United States Department of Health and Human Services (DHHS) (2003). NIOSH Manual of Analytical Methods, P.C. Schlecht, and P.F. O’Connor, eds., DHHS, Cincinnati, OH.
- Yao, M., and Mainelis, G. (2006a). Investigation of Cut-Off Sizes and Collection Efficiencies of Portable Microbial Samplers. Aerosol Sci. Technol., 40:595–606.
- Yao, M., and Mainelis, G. (2006b). Effect of Physical and Biological Parameters on Enumeration of Bioaerosols by Portable Microbial Impactors. J. Aerosol Sci., 37:1467–1483.
- Yao, M., and Mainelis, G. (2007a). Analysis of Portable Impactor Performance for Enumeration of Viable Bioaerosols. J. Occup. Env. Hyg., 4:514–524.
- Yao, M.S., and Mainelis, G. (2007b). Use of Portable Microbial Samplers for Estimating Inhalation Exposure to Viable Biological Agents. J. Expo. Sci. Environ. Epidemiol., 17:31–38.
- Zhen, S., Li, K., Yin, L., Yao, M., Zhang, H., Chen, L., et al. (2009). A Comparison of the Efficiencies of a Portable BioStage Impactor and a Reuter Centrifugal Sampler (RCS) High Flow for Measuring Airborne Bacteria and Fungi Concentrations. J. Aerosol Sci., 40:503–513.
- Zhu, H., Phelan, P., Duan, T., Raupp, G., Fernando, H.S., and Che, F. (2003). Experimental Study of Indoor and Outdoor Airborne Bacterial Concentrations in Tempe, Arizona, USA. Aerobiologia, 19:201–211.