Abstract
Accurate exposure assessments are needed to evaluate health hazards caused by airborne microorganisms and require air samplers that efficiently capture representative samples. This highlights the need for samplers with well-defined performance characteristics. While generic aerosol performance measurements are fundamental to evaluate/compare samplers, the added complexity caused by the diversity of microorganisms, especially in combination with cultivation-based analysis methods, may render such measurements inadequate to assess suitability for bioaerosols. Specific performance measurements that take into account the end-to-end sampling process, targeted bioaerosol and analysis method could help guide selection of air samplers.
Nine different samplers (impactors/impingers/cyclones/ electrostatic precipitators/filtration samplers) were subjected to comparative performance testing in this work. Their end-to-end cultivation-based biological sampling efficiencies (BSEs) and PCR-/microscopy-based physical sampling efficiencies (PSEs) relative to a reference sampler (BioSampler) were determined for gram-negative and gram-positive vegetative bacteria, bacterial spores, and viruses.
Significant differences were revealed among the samplers and shown to depend on the bioaerosol's stress–sensitivity and particle size. Samplers employing dry collection had lower BSEs for stress-sensitive bioaerosols than wet collection methods, while nonfilter-based samplers showed reduced PSEs for 1 μm compared to 4 μm bioaerosols. Several samplers were shown to underestimate bioaerosol concentration levels relative to the BioSampler due to having lower sampling efficiencies, although they generally obtained samples that were more concentrated due to having higher concentration factors.
Our work may help increase user awareness about important performance criteria for bioaerosol sampling, which could contribute to methodological harmonization/standardization and result in more reliable exposure assessments for airborne pathogens and other bioaerosols of interest.
Copyright 2014 American Association for Aerosol Research
INTRODUCTION
Accurate and reliable quantification and identification of bioaerosols depends on several factors, including the use of air sampling equipment that efficiently capture representative samples and match the targeted bioaerosol (e.g., concentration level, particle size, and stress–sensitivity), the meteorological conditions (e.g., temperature, humidity, and wind speed) and the employed analysis methods (Alvarez et al. Citation1995). The latter commonly includes microscopy, microbiological, biochemical, immunological, and molecular techniques (Cox and Wathes Citation1995; Eduard and Heederik Citation1998; Buttner et al. Citation2002; Grinshpun and Clark Citation2005; Georgakopoulos et al. Citation2009; Reponen et al. Citation2011; Xu et al. Citation2011). Bioaerosol sampling is usually achieved through common aerosol collection principles such as impaction, impingement, cyclonic separation, filtration, and thermal or electrostatic precipitation (Burge and Solomon Citation1987; Macher and Willeke Citation1992; Nevalainen et al. Citation1992; Cox and Wathes Citation1995; Buttner et al. Citation2002; Grinshpun and Clark Citation2005; Reponen et al. Citation2011). A myriad of air samplers have been developed based on these principles and offer a wide range of different instrument properties with respect to sampling efficiency, collection size range, airflow rate, collection medium type and volume, physical properties (e.g., size, weight, ruggedness, and automation) and inflicted sampling-associated microbial stress (e.g., shear forces and desiccation) (Buttner and Stetzenbach Citation1991; Li Citation1999; Radosevich et al. Citation2002; An et al. Citation2004; Bergman et al. Citation2004; Yao and Mainelis Citation2007; Carvalho et al. Citation2008, Kesavan et al. Citation2008; Citation2010a; McFarland et al. Citation2010). Thus, no single air sampler is likely to be optimal, or even suitable, for all purposes (Macher and Willeke Citation1992; Nevalainen et al. Citation1992).
Different bioaerosol types and sampling applications may be associated with variable inherent challenges and study-specific requirements, which have made equipment and procedural standardization difficult within the bioaerosol community and consequently led to the use of several different air samplers and analysis methods. Interstudy data comparisons are therefore demanding since various methodologies provide different results even when subjected to the same bioaerosol challenge (Shahamat et al. Citation1997; Eduard and Heederik Citation1998; Li Citation1999; Buttner et al. Citation2002; An et al. Citation2004; Yao and Mainelis Citation2007; Griffin et al. Citation2011). Bioaerosol investigations are therefore hampered by the lack of methods that can provide accurate, reliable, and comparable exposure estimates for airborne microorganisms (Macher Citation1999). Taken together, this highlights the need for comparative testing and evaluation (T&E) of air samplers and standardization of sampling procedures (Bartlett et al. Citation2002; An et al. Citation2004; Vitko Jr et al. Citation2005; Millner Citation2009; Xu et al. Citation2011). Such efforts will provide the users with important information upon selection of air samplers in order to meet their specific operational requirements.
Performance measurements for air samplers have been reported using several different, and sometimes redundant, efficiency terms (e.g., aspiration-, inlet-, transmission-, collection-, sampling-, recovery-, retention-, physical-, biological-, total-, and overall efficiency), taking into account different aspects or parts of the end-to-end sampling process (Nevalainen et al. Citation1992; Grinshpun et al. Citation1994; Grinshpun et al. Citation1996; Henningson et al. Citation1997; Li Citation1999; Brixey et al. Citation2002; Kesavan et al. Citation2003; An et al. Citation2004; Bergman et al. Citation2004; Yao and Mainelis Citation2007; Carvalho et al. Citation2008; Kesavan et al. Citation2008; Citation2010a; McFarland et al. Citation2010). The performance information supplied with commercially available samplers is often limited to collection efficiencies for different sizes of inert nonbiological particles (e.g., polystyrene latex spheres), which are used to define the sampler's particle cutoff diameter d 50 at which 50% collection efficiency is observed. Such generic measurements do not, however, incorporate the sampler's propensity to induce sampling stress.
The well-characterized swirling liquid impinger (BioSampler) was used as a reference air sampler in this study (Willeke et al. Citation1998; Lin et al. Citation1999, Citation2000; Hermann et al. Citation2006; Rule et al. Citation2007; Fabian et al. Citation2009; Van Droogenbroeck et al. Citation2009; Chang et al. Citation2010; Kesavan et al. Citation2010b; Chang and Chou Citation2011; Kesavan et al. Citation2011). Compared to the BioSampler, the available performance data for the rest of the involved air samplers were more limited; gelatin filters (Li Citation1999; Lin and Li Citation1999; Tseng and Li Citation2005; Burton et al. Citation2007; Fabian et al. Citation2009; Van Droogenbroeck et al. Citation2009; Chang and Chou Citation2011; Estill et al. Citation2011; Zhao et al. Citation2011), Coriolis FR (or Coriolis μ and δ) (Carvalho et al. Citation2008; Gómez-Domenech et al. Citation2010; Ahmed et al. Citation2013), XMX-CV (or XMX/2L-MIL and XMX/2A) (Cooper Citation2010; Black Citation2011; Kesavan et al. Citation2011; Black and Cooper Citation2012; Enderby Citation2012), BioCapture 650 (Kesavan et al. Citation2011; Enderby Citation2012), OMNI-3000 (Kesavan et al. Citation2011; Zhao et al. Citation2011), SASS 2300 (or SASS 2000) (Kesavan and Stuebing Citation2009; Kesavan et al. Citation2011), and SASS 3100 (Kesavan et al. Citation2010b).
The aim of the present work was to perform aerosol chamber-based comparative T&E of nine air samplers representing different collection principles in order to establish their end-to-end cultivation-dependent biological sampling efficiencies (BSEs) and cultivation-independent physical sampling efficiencies (PSEs) for a selection of aerosol test agents relative to the BioSampler. The BSEs were based on plate count analyses, while the PSEs were based on quantitative real-time PCR (qPCR) or fluorescence microscopy direct count analyses. The aerosol test agents included Gram-positive and Gram-negative vegetative bacteria, bacterial spores and viruses, and also fluorescent polystyrene latex spheres. The T&E scheme was designed to provide the users of air sampling equipment with more specific end-to-end performance measurements for various bioaerosol sampling applications.
MATERIALS AND METHODS
Aerosol Test Agents and Spray Solutions
The biological test agents included Gram-negative (Serratia marcescens, SM) and Gram-positive (Kocuria rhizophila, KR) vegetative bacterial cells, bacterial spores (Bacillus atrophaeus, formerly Bacillus globigii, BG), and viruses (Bacteriophage MS2, non-enveloped ssRNA virus, MS2). A freeze-dried powder containing 2.0 × 108 cfu mg−1 of BG spores (DPG Lot 19076–03268) and a solution containing 3.5 × 1012 pfu ml−1 of MS2 phages (DPG Lot 2011JUN28AKS) in TNME buffer (10 mM Tris-HCl, 100 mM NaCl, 0.1 mM MgSO4, 0.01 mM EDTA) were provided by Dugway Proving Ground (DPG, Dugway, UT, USA). A SM strain (ATCC 274) was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and a KR strain (ATCC 9341, formerly classified as Micrococcus luteus) was provided by the Norwegian School of Veterinary Science (NVH, Oslo, Norway). Fluorescent polystyrene latex spheres (Fluospheres, FS) of two sizes, 1 μm with yellow-green fluorescence (505/515 nm) and 4 μm with red fluorescence (580/605 nm), respectively, were purchased from Invitrogen (Invitrogen, Carlsbad, CA, USA).
BG spores (5 mg ml−1) were suspended in MilliQ water (Millipore, Billerica, MA) assisted by vortexing (5 min). SM and KR were seeded into nutrient broth (Oxoid, Cambridge, UK) or trypticase soy broth (Oxoid), respectively, followed by cultivation (18 h, 200 rpm) at 30°C (SM) or 37°C (KR). The BG, SM, and KR were washed by centrifugation (3,000 × g, 5 min) and re-dissolved in MilliQ water. The optical density (OD) was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA) and adjusted with MilliQ water to OD600 = 1.0 for BG (∼1.0 × 109 cfu ml−1), OD600 = 0.5 for SM (∼5.0 × 109 cfu ml−1), and OD600 = 0.75 for KR (∼3.0 × 109 cfu ml−1). The spray solutions’ concentration levels (cfu ml−1) were determined by plate count analyses before and after aerosol generation. Serial dilutions of the spray solutions were plated in triplicates on trypticase soy agar (TSA) for KR and nutrient agar (NA) for BG and SM, and incubated (18 h) at 37°C (KR) or 30°C (BG and SM). The MS2 stock solution was directly diluted with 12.5% TNME buffer to give a spray solution concentration level of 1.0 × 109 pfu ml−1. The MS2 spray solution's concentration level (pfu ml−1) was determined using a pour-plate method before and after aerosol generation. Briefly, serial dilutions of the spray solution were mixed with 1.0 × 107 cfu of log-phase Escherichia coli (DSM 4230) cells in soft NA (0.7% agar), poured out on NA plates in triplicates, and incubated (18 h) at 37°C. The FS suspensions were directly diluted with MilliQ water to give spray solutions containing 1.0 × 109 spheres ml−1. All spray solutions were prepared fresh each day.
Bioaerosol Test Chamber
The air sampler testing was performed in a 12 m3 (3 × 2 × 2 m) stainless steel aerosol test chamber (ATC, Dycor Technologies, Edmonton, AB, Canada) fitted with external heating, ventilation and air conditioning (HVAC) and high-efficiency particulate air (HEPA)-filtration systems (Figure S1 in the online supplemental information [SI]). The ATC was equipped with two mixing fans (120 mm), meteorology sensors for temperature, humidity and pressure, optical particle counter (Grimm 1.108, Grimm Technologies, Douglasville, GA, USA), aerodynamic particle sizer (APS 3321, TSI, Shoreview, MN, USA), and two slit-to-agar samplers (STA-203, New Brunswick, Edison, NJ, USA). Real-time monitoring of the test aerosol concentration and size distribution was done with the Grimm 1.108 and APS 3321. The agent containing particles per liter of air (ACPLA) levels were monitored using sequentially operated STA-203 samplers (30 lpm, 0.5 rpm). The STA-203s were loaded with TSA (KR) or NA plates (BG and SM), and the plates were incubated (18 h) at 37°C (KR) or 30°C (BG and SM). The STA-203s were not used during MS2 and FS experiments.
Aerosol Generation
Aerosolization of the biological test agents (BG, SM, KR, and MS2) was achieved using a 48 kHz Sono-Tek ultrasonic atomizer nozzle (Sono-Tek, Milton, NY, USA), while FS was aerosolized using a Micro Mist nebulizer (Hudson RCI, Durham, NC, USA). The Sono-Tek nozzle was powered by a broadband ultrasonic generator (Model 06-5108, Sono-Tek) and the spray solution was fed from a syringe feeder (Model 997E, Sono-tek). The Micro Mist nebulizer was operated with N2 gas (2.4 bar). Both dispersion devices were enclosed in an aerosol dilution system (ADS-A20, Dycor Technologies) which offered adjustable dilution of the aerosol with HEPA-filtered air before injection into the ATC. The targeted aerosol particle sizes were 1 and 4 μm mass median aerodynamic diameters (MMAD). The air sampler testing was performed with 4 μm aerosols for all test agents and additionally 1 μm aerosols for FS and BG. Appropriate instrument settings for the ATC and its subsystems were determined during pre-study experiments to generate reproducible concentration levels and size distributions for the test aerosols, and then kept static throughout the study.
Evaluated Air Samplers
Nine different air samplers based on various wet and dry aerosol collection principles, including filtration, impaction, impingement, cyclonic separation, and electrostatic precipitation, were subjected to aerosol chamber-based comparative T&E. The air samplers spanned a wide range of airflow rates (12.5–540 lpm) and ranged in technological sophistication from simple filter cassettes and glassware samplers that require external vacuum sources and manual handling to fully automated and ruggedized systems. The evaluated samplers ( and Figure S2) were SASS 2300, SASS 3100, gelatin filters, Coriolis FR, OMNI-3000, BioCapture 650, Electrostatic precipitator (ESP) prototype, XMX-CV, and BioSampler.
TABLE 1 Air sampling equipment subjected to comparative testing and evaluation (T&E) in this study
The BioSampler was used as a reference and the sampling efficiencies of the other samplers were reported relative to the reference sampler. The samplers were operated according to their respective manufacturers’ instructions-for-use documents. The air flows through the BioSampler and the gelatin filter were monitored using mass flow meters (TopTrak 826, Sierra Instruments, Monterey, CA, USA). The SASS 3100's open-faced filter holder was covered with an aluminum cover cap to avoid deposition of test aerosols on the electret filter during aerosol generation and mixing.
The collection liquids used with Coriolis FR and BioCapture 650 were both phosphate buffered saline (PBS)-based surfactant-containing formulations supplied as single-use consumables. PBS supplemented with 0.05% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) and 0.005% Antifoam A (Sigma-Aldrich, PBSTA) was used as collection liquid with the BioSampler and XMX-CV, and as extraction liquid with the SASS 3100, ESP, and gelatin filters. The OMNI-3000 was operated with collection cartridges containing PBS supplied as single-use consumables and MilliQ water as make-up liquid in the onboard reservoir. The SASS 2300 was operated with PBS as both collection liquid and make-up liquid. The OMNI-3000 and SASS 2300 samples were supplemented with Triton X-100 and Antifoam A to a final concentration of 0.05% and 0.005%, respectively, immediately after sampling.
Air Sampler Testing
The described aerosol chamber-based T&E scheme was consistently used unless otherwise stated. Each air sampler was tested with a minimum of five experimental repetitions for each aerosol test agent and particle size. The samplers were tested in groups with the number of simultaneously tested samplers ranging from 2 to 6. Fixed sampling positions were used in the ATC and the relative positions of the samplers were varied between experiments (Figure S1). Before each experiment, the ATC was sealed and purged using HVAC-conditioned HEPA-filtered air, until the Grimm 1.108 reported background concentration levels (<1 particles liter−1 between 0.8 and 20 μm) and the targeted meteorological conditions were observed (55% relative humidity and 20°C). Test aerosol generation was initiated and continued (∼2 min) until the Grimm 1.108 reached the targeted concentration level (∼160 particles liter−1). The ventilation system, but not the mixing fans, was then switched off and the test aerosol mixed (1 min). The sampling period was 5 min, after which the ATC was purged down to background conditions. The collected samples were immediately transported to the laboratory, and the samplers decontaminated in accordance with their sample-to-sample decontamination procedures. The electret filters from SASS 3100 were extracted with PBSTA (8 ml) using a SASS 3010 extractor instrument (Research International). The collection pipes from the ESP were extracted with PBSTA (10 ml, hand-shaking, 30 s), while the gelatin filters were completely dissolved in PBSTA (10 ml, 37°C, hand-shaking).
Evaluation of Test Aerosol Homogeneity
The homogeneity of test aerosols inside the ATC was evaluated during pre-study experiments by simultaneous sampling with three STA-204 slit-to-agar samplers (30 lpm, 0.5 rpm, New Brunswick) and two STA-203 slit-to-agar samplers. These experiments were performed with 4 μm BG aerosols and repeated while changing the relative positions of the samplers between those used for air sampler testing (Figure S1). Two SASS 3100 electret filter samplers (300 lpm) and two SASS 2000 wetted-wall cyclones (325 lpm, older model of SASS 2300) were used to simulate ongoing air sampler testing during the homogeneity experiments. The ACPLA levels reported by the slit-to-agar samplers were used to assess the homogeneity of test aerosols inside the ATC during simulated air sampler testing. Additionally, wind speed measurements were recorded at multiple heights for each sampling position using a hotwire anemometer (VT200, Kimo Instruments, Montpon, France).
Cultivation Analysis
Plate count analyses (in the [SI]) were used to establish the air samplers’ BSEs for BG, SM, KR, and MS2 aerosols. The BSEs were calculated relative to the reference sampler (BioSampler) using EquationEquation (1) (Henningson et al. Citation1997; An et al. Citation2004; Yao and Mainelis Citation2007; Carvalho et al. Citation2008).
Molecular Analysis
qPCR assays (in the SI) for BG (Buttner et al. Citation2004), SM (Saikaly et al. Citation2007) and KR (this study), and a reverse transcriptase qPCR (qRT-PCR) assay for MS2 (O’Connell et al. Citation2006), were used to determine the air samplers’ PSEs. The PSEs were calculated relative to the reference sampler using EquationEquation (1).
Direct Count Analysis
Fluorescence microscopy-based direct count analyses (in the SI) were used to determine the air samplers’ PSEs for FS aerosols. The concentration levels of FS in the collected air samples were calculated using EquationEquation (2). The PSEs were calculated relative to the reference sampler using EquationEquation (1).
Statistical Analysis
The results were subjected to statistical analyses using SigmaPlot (Systat Software, San Jose, CA, USA). Normality checking was done with the Shapiro–Wilk test and depending on whether the normality and equal variance criteria were fulfilled or not, significance testing was performed with the Student's t-test or the Mann–Whitney rank sum test, respectively. The significance level was set at p < 0.05 for all statistical tests.
RESULTS AND DISCUSSION
Spray Solutions and Test Aerosols
The spray solutions showed less than ±10% variability in cultivable counts (BG, KR, SM, and MS2) or direct microscopy counts (FS) throughout the period they were used for aerosol generation. Particle size calculations based on APS 3321 measurements from all experiments showed re-producible size distributions for all test aerosols with median sizes close to the targeted 1 and 4 μm MMAD (Table S2). The homogeneity of test aerosols in the ATC was evaluated to ensure that all samplers were exposed to the same challenge. Five simultaneously operated slit-to-agar samplers showed similar recoveries (±2%) of 4 μm BG aerosols at each sampling position, irrespectively of whether or not four high-volume air samplers were operated at the same time. The airflow velocities in the ATC were determined to evaluate the risk of observing over- or undersampling through the air samplers’ inlets. Airflow velocities below 1 m s−1 were consistently observed at all sampling positions and inlet heights, suggesting that substantial over- or undersampling were unlikely for the involved particle sizes and inlet velocities (Grinshpun et al. Citation1994; Baron Citation1998; Li et al. Citation2000).
T&E Results (Test Agent Categorized)
The following sections present statistical interpretations of the T&E results assisted by two figures (Figures and ). A consolidated result summary showing BSEs and PSEs for each air sampler and test agent is provided as SI (Table S3).
FIG. 1 The evaluated air samplers’ end-to-end cultivation-based biological sampling efficiencies (BSEs) and qPCR- or fluorescence microscopy-based physical sampling efficiencies (PSEs) relative to a reference sampler (BioSampler) for 1 and 4 μm mass median aerodynamic diameter (MMAD) aerosols of FluoSpheres (FS) and B. atrophaeus spores (BG). The evaluated air samplers’ sampling efficiencies were significantly different from the BioSampler's sampling efficiency (gray dotted line) except when specified with an asterisk (*). The evaluated air samplers’ sampling efficiencies were significantly different for 1 μm aerosols compared to 4 μm aerosols when specified with the same letter.
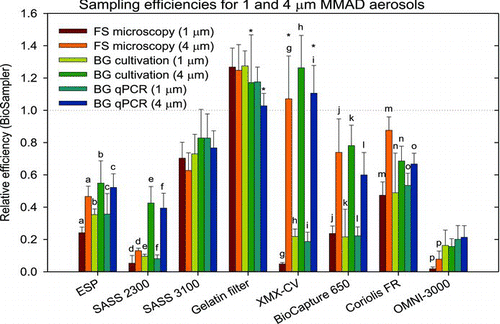
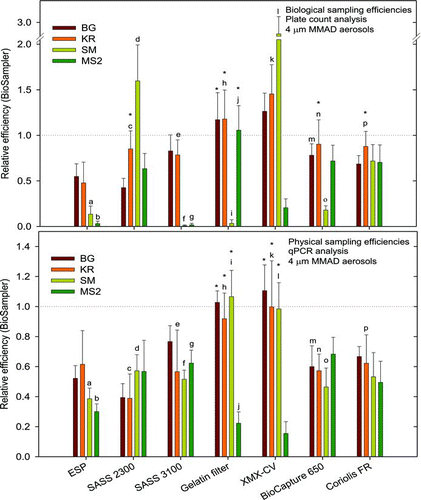
FIG. 2 The evaluated air samplers’ end-to-end cultivation-based biological sampling efficiencies (BSEs, upper panel) and qPCR-based physical sampling efficiencies (PSEs, lower panel) relative to the reference sampler (BioSampler) for 4 μm mass median aerodynamic diameter (MMAD) aerosols of B. atrophaeus spores (BG), Kocuria rhizophila (KR), Serratia marsescens (SM), and bacteriophage MS2 (MS2). The evaluated air samplers’ sampling efficiencies were significantly different from the BioSampler's sampling efficiency (gray dotted line) except when specified with an asterisk (*). The evaluated air samplers’ BSEs and PSEs were significantly different from each other when specified with the same letter.
FluoSpheres (FS)
The air samplers’ PSEs relative to the reference sampler were determined for 1 and 4 μm FS aerosols (). The filter-based samplers (SASS 3100 and gelatin filters) did not show significantly different PSEs for 1 μm compared to 4 μm. The other samplers showed significantly lower PSEs for 1 μm compared to 4 μm FS, although the reductions were smaller for ESP and Coriolis FR than for SASS 2300, XMX-CV, BioCapture 650, and OMNI-3000. The largest sampling efficiency reduction for 1 μm compared to 4 μm FS was observed for XMX-CV (∼26-fold).
In general, the evaluated samplers showed significantly lower PSEs for both sized FS compared to the reference sampler, except for: (i) gelatin filters that had significantly higher PSEs for both sized FS, and (ii) XMX-CV that did not have a significantly different PSE for 4 μm FS. Low PSEs (≤0.13) were observed for both sized FS with SASS 2300 and OMNI-3000.
B. atrophaeus spores (BG)
The air samplers’ BSEs and PSEs relative to the reference sampler were determined for 1 and 4 μm BG aerosols (). The filter-based samplers (SASS 3100 and gelatin filters) and OMNI-3000 did not show significantly different BSEs or PSEs for 1 μm compared to 4 μm BG. The other samplers had significantly lower BSEs and PSEs for 1 μm compared to 4 μm BG, although the efficiency reductions were smaller for ESP and Coriolis FR compared to SASS 2300, XMX-CV, and BioCapture 650. The largest sampling efficiency reductions for 1 μm compared to 4 μm BG was observed for XMX-CV (∼6-fold) and SASS 2300 (∼5-fold).
The evaluated samplers had significantly lower BSEs and PSEs for both sized BG compared to the reference sampler, except for: (i) gelatin filters that had a significantly higher BSE and PSE for 1 μm BG and not significantly different BSE and PSE for 4 μm BG, and (ii) XMX-CV that had a significantly higher BSE and not significantly different PSE for 4 μm BG only.
For both sized BG, no significant differences were observed between the BSEs and PSEs, except for 4 μm BG with BioCapture 650 that had a significantly lower PSE (∼1.5-fold).
Low BSEs and PSEs (≤0.22) were observed for both sized BG with OMNI-3000 and for 1 μm BG with SASS 2300, XMX-CV, and BioCapture 650. Since OMNI-3000 showed low PSEs for both sized BG and FS, this sampler was excluded from further testing.
Kocuria rhizophila (KR)
The air samplers’ BSEs and PSEs relative to the reference sampler were determined for 4 μm KR aerosols (). The evaluated samplers had significantly higher BSEs compared to PSEs, except for ESP that had a lower but not significantly different BSE.
The evaluated samplers also had significantly lower PSEs compared to the reference sampler, except for XMX-CV and gelatin filters that did not have significantly different PSEs. XMX-CV had a significantly higher BSE compared to the reference sampler, while ESP and SASS 3100 had significantly lower BSEs. The gelatin filters, SASS 2300, BioCapture 650, and Coriolis FR did not have significantly different BSEs compared to the reference sampler.
Serratia marcescens (SM)
The air samplers’ BSEs and PSEs relative to the reference sampler were determined for 4 μm SM aerosols (). Air samplers collecting directly into liquid had significantly higher (SASS2300 and XMX-CV) or not significantly different (Coriolis FR) BSEs compared to PSEs, while samplers employing dry collection principles (ESP, SASS 3100, gelatin filters, and BioCapture 650) had significantly lower BSEs compared to PSEs. The largest reductions in BSEs compared to PSEs were observed for the filter-based samplers, SASS 3100 (∼52-fold) and gelatin filters (∼36-fold), that both had very low BSEs (≤0.03).
The evaluated samplers had significantly lower BSEs and PSEs compared to the reference sampler, except for: (i) XMX-CV that had significantly higher BSE and not significantly different PSE, (ii) SASS 2300 that had a significantly higher BSE, and (iii) gelatin filters that did not have a significantly different PSE.
Bacteriophage MS2
The air samplers’ BSEs and PSEs relative to the reference sampler were determined for 4 μm MS2 aerosols (). SASS 3100 and ESP had significantly lower BSEs than PSEs, while gelatin filters had a significantly higher BSE than PSE. The other samplers (SASS 2300, XMX-CV, BioCapture 650, and Coriolis FR) did not have significantly different BSEs compared to PSEs. Low BSEs and PSEs (≤0.21) were observed with XMX-CV.
The evaluated air samplers had significantly lower BSEs and PSEs compared to the reference sampler, except for gelatin filters that did not have a significantly different BSE.
T&E Results (Test Agent- and Particle Size-Consolidated)
The following sections present and discuss the consolidated T&E results to identify and highlight performance differences and/or similarities between the evaluated air samplers.
Particle size
The particle size of the targeted bioaerosol is an important parameter to consider upon selection of air sampler. Our results showed that the BSEs and PSEs for BG and FS aerosols with the filter-based samplers (SASS 3100 and gelatin filters) were not affected by reducing the particle size from 4 μm to 1 μm, while the XMX-CV, SASS 2300, and BioCapture 650 showed strongly reduced BSEs and PSEs for 1 μm compared to 4 μm aerosols (). The ESP and Coriolis FR also showed reduced BSEs and PSEs for 1 μm aerosols, but the reductions were only moderate compared to those observed for the XMX-CV, SASS 2300 and BioCapture 650 (). These results are in agreement with previous studies showing that the d 50 cutoff diameters are above 1 μm for XMX-CV, SASS 2300 and BioCapture 650, and below 1 μm for the BioSampler (Kesavan and Stuebing Citation2009; Kesavan et al. Citation2011).
Physical sampling efficiency (PSE)
The PSEs for 1 and 4 μm FS aerosols based on microscopy and 1 and 4 μm BG aerosols based on qPCR corresponded with each other (). The observation that similar PSEs could be reproduced for two test agents even when based on different analysis methods, suggested that the PSEs were accurate and representative for the respective samplers. However, two minor exceptions were observed; SASS 2300 and OMNI-3000 had about two-fold and six-fold lower PSEs, respectively, for FS compared to similar sized BG. These air samplers had the largest internal surface areas and tubing coming in contact with the collection liquid during sampling. This could possibly lead to internal loss of sampled material due to adherence to the samplers’ walls and tubing, thus having an impact on the end-to-end sampling efficiency. Observations also indicated that FS were more hydrophobic than BG, which could contribute to increase the internal loss of FS in the SASS 2300 and OMNI-3000. The OMNI-3000 was excluded from further testing due to its very low PSE for both sized FS and BG aerosols. It should however be noted that technical issues with this sampler's fluid monitoring system led to abnormal replenishment of collection liquid on some occasions.
When comparing the air samplers’ PSEs for 4 μm BG, KR, SM, and MS2 aerosols consistency was observed, suggesting that the PSEs were similar for all test agents (). However, for MS2, the gelatin filters and XMX-CV both had ≥5-fold lower PSEs for MS2 compared to BG, KR, and SM. A corresponding BSE result was seen for XMX-CV which had ≥5-fold lower BSE for MS2 compared to the other test agents, but this was not observed for the gelatin filters.
When the two discrepancies observed for MS2 were discarded, the PSEs for BG, KR, SM, and MS2 showed that gelatin filters and XMX-CV had an averaged PSE (1.01 ± 0.06 and 1.03 ± 0.07, respectively) close to the reference sampler, while ESP (0.46 ± 0.14), SASS 3100 (0.62 ± 0.11), SASS 2300 (0.48 ± 0.10), BioCapture 650 (0.58 ± 0.09), and Coriolis FR (0.58 ± 0.08) had an averaged PSE that was lower than the reference sampler.
Biological sampling efficiency (BSE)
The targeted bioaerosol's sensitivity to sampling stress and the sampler's propensity to induce such effects are important parameters to consider when cultivation-dependent analysis methods are needed for quantification and identification of airborne microorganisms. By comparing the cultivation-dependent BSEs to the cultivation-independent PSEs, our results showed that the evaluated air samplers differed substantially regarding their propensity to induce sampling stress. The evaluated air samplers’ BSEs for 4 μm BG, KR, SM, and MS2 aerosols differed more between the test agents than the PSEs (), although this was not surprising since the test agents were selected to provide microorganisms with variable sensitivities to sampling stress.
The observation that the BSEs and PSEs for BG aerosols corresponded with each other provided additional support to the accuracy of the PSEs since Bacillus spores are known to be highly tolerant to microbial stress (Sinclair et al. Citation2008). These results also suggested that the BSEs and PSEs were comparable measurements that could be used to assess sampling-associated stress. The BSEs and PSEs for KR aerosols also corresponded with each other and with BG aerosols. The BSEs for SM and MS2 aerosols were however more variable, showing both lower and higher BSEs compared to PSEs. Our results () therefore suggested that BG (bacterial spores) and KR (gram-positive vegetative bacteria) were much more resistant to sampling-inflicted microbial stress than SM (gram-negative vegetative bacteria) and MS2 (non-enveloped ssRNA viruses). It should however be taken into account that these results were obtained with 4 μm aerosols consisting of cell/spore aggregates, which may respond differently to stresses than aerosols consisting of single cells/spores.
Coriolis FR was the only sampler that had similar BSEs for BG, KR, SM, and MS2, with an averaged BSE (0.75 ± 0.09) corresponding to the averaged PSE for the same test aerosols (0.58 ± 0.08), with a small offset (+30%).
BioCapture 650 had similar BSEs for BG, KR, and MS2, with an averaged BSE (0.80 ± 0.09) corresponding to the averaged PSE (0.58 ± 0.09) with a small offset (+38%). The BSE for SM was >4-fold reduced compared to that obtained for BG, KR and MS2, suggesting that that BioCapture 650 injured SM more than the reference sampler, but that the stress did not affect BG, KR, or MS2.
XMX-CV had similar BSEs for BG and KR, with an averaged BSE (1.36 ± 0.13) corresponding to the averaged PSE (1.03 ± 0.07) with a small offset (+32%). The BSE for SM was about two-fold higher compared to that obtained for BG and KR, suggesting that the reference sampler injured SM more than XMX-CV, but that the stress did not affect BG and KR. The BSE for MS2 was >6-fold lower compared to that obtained for BG and KR, but since the PSE for MS2 also showed a similarly reduced efficiency (>5-fold) compared to FS, BG, KR, and SM, these results could not be attributed to differences in sampling stress.
Gelatin filters had similar BSE for BG, KR, and MS2, with an averaged BSE (1.14 ± 0.07) corresponding to the averaged PSE (1.01 ± 0.06) with a small offset (+13%). The BSE for SM was about 38-fold lower compared to that obtained for BG, KR, and MS2, suggesting that the gelatin filters injured SM more than the reference sampler, but that the stress did not have an impact on BG, KR, and MS2.
SASS 3100 had similar BSEs for BG and KR, with an averaged BSE (0.80 ± 0.04) corresponding to the averaged PSE (0.62 ± 0.11) with a small offset (+30%). The BSEs for SM and MS2 were about 80-fold and 40-fold lower, respectively, compared to that obtained for BG and KR, suggesting that the SASS 3100 injured both SM and MS2 more than the reference sampler, but that no such effect was seen for BG and KR.
SASS 2300 had similar BSEs for BG, KR, and MS2, with an averaged BSE (0.64 ± 0.21) corresponding to the averaged PSE (0.48 ± 0.10) with a small offset (+33%). The BSE for SM was about 2.5-fold higher compared to that obtained for BG, KR, and MS2, suggesting that the reference sampler injured SM more than the SASS 2300, but that the stress did not have an impact on BG, KR, and MS2.
ESP had similar BSEs for BG and KR, with an averaged BSE (0.52 ± 0.05) corresponding to the averaged PSE (0.46 ± 0.14) with a small offset (+13%). The BSEs for SM and MS2 were about 4-fold and 17-fold lower, respectively, compared to the averaged BSE for BG and KR, suggesting that the ESP injured both SM and MS2 more than the reference sampler, but that the stress did not affect BG and KR.
The consistently observed positive offset (13%–38%) between BSEs and PSEs suggested that there was a small bias toward overestimating the BSEs, or underestimating the PSEs, of the evaluated samplers relative to the reference sampler.
Taken together our results suggested that samplers employing dry collection principles (ESP, SASS 3100, gelatin filters, and BioCapture 650) had a more dramatic impact on the cultivability/viability of SM than those employing wet collection (SASS 2300, XMX-CV, and Coriolis FR). The same pattern was not observed with MS2, for which two dry collection samplers (ESP and SASS 3100) seemed to injure MS2 more than the wet collection samplers (SASS 2300 and Coriolis FR), while the other two dry collection samplers (gelatin filters and BioCapture 650) did not seem to influence MS2 differently from the wet collection samplers. A possible explanation could be that MS2 was less sensitive to desiccation than SM, and remained infective with the gelatin filters and BioCapture 650 but not with the ESP and SASS 3100. The manufacturer's instructions-for-use document for the gelatin filters states that they contain 46%–49% residual dampness, while the BioCapture 650 automatically rinses the sampled material from its spinning disk impactor at the end of the sampling period. Thus, the sampled material had a short holding time in a desiccated state before transfer into liquid, in contrast to the ESP and SASS 3100 where the samples had to be manually extracted into liquid after sampling (i.e., the sampled material had a longer holding time in a dry state before transfer to liquid). For BG and KR, no differences were observed between samplers that could be linked to specific collection principles, suggesting that both these test agents were resistant to the sampling stress inflicted by the evaluated air samplers, independent of whether they employed wet or dry collection principles.
Concentration Factor
In addition to the importance of the sampling efficiency, which is fundamental to ensure accurate quantification of the bioaerosols, the air sampler's concentration factor may also be important since it impacts the amount and concentration of sampled material than can be obtained and subjected to analysis. An air sampler's concentration factor will depend on its airflow rate, collection medium volume, and sampling efficiency. An air sampler with a relative concentration factor of 100 compared to the BioSampler will have a 100-fold lower detection limit for the same sampling operation, and which could be an important performance criterion when the targeted bioaerosol's concentration level is low. Similarly, for sampling applications requiring high temporal resolutions, or when other factors limit the sampling time, employing an air sampler with a high concentration factor may be crucial to ensure that sufficient sampled material is present.
The evaluated air samplers’ theoretical concentration factors relative to the reference sampler showed that while the gelatin filters had a theoretical concentration factor of 2, the other samplers had much higher theoretical concentration factors ranging from 46 to 201 (). The theoretical relative concentration factors were transformed into end-to-end relative biological concentration factors (BCFs) and physical concentration factors (PCFs), respectively, by multiplying them with the BSEs and PSEs obtained in the current work (Table S3). A consolidated summary showing the BCFs and PCFs for each air sampler and test agent is provided as SI (Table S4).
The results showed that the BCFs and PCFs for the evaluated samplers were consistently higher than for the reference sampler, except the BCFs for 4 μm SM with SASS 3100 and gelatin filters, and the PCFs for 1 μm FS with OMNI-3000 and 4 μm MS2 with gelatin filters. The highest BCFs and PCFs were observed with XMX-CV for all test aerosols (≥200), except 4 μm MS2, 1 μm BG, and 1 μm FS. For 4 μm MS2, the SASS 2300 had the highest BCF (∼93) and PCF (∼84). For 1 μm BG and 1 μm FS, the SASS 3100 had the highest BCF (BG; ∼44) and PCF (BG; ∼50, and FS; ∼43). In comparison to the other evaluated samplers, low BCFs and PCFs were consistently observed for the gelatin filters (≤3) and OMNI-3000 (≤10).
In summary, the observed results showed that while several of the evaluated air samplers may underestimate bioaerosol concentration levels due to having lower sampling efficiencies (BSEs/PSEs) than the reference sampler (Table S3), they would generally obtain samples that contained higher concentrations of sampled material due to having higher concentration factors (BCFs/PCFs) than the reference sampler (Table S4).
Air Sampler Suitability Assessments
Based on the demonstrated performance of the evaluated air samplers (Figures and , and Tables S3 and S4), generalized features regarding their suitability for various bioaerosol sampling applications were assessed ().
TABLE 2 Generalized features regarding the evaluated air samplers’ suitability for various bioaerosol sampling applications
The suitability features were separated into quantitative and qualitative sampling applications because the fundamental air sampler performance requirements may be different depending on whether the objective is to accurately determine concentration levels (quantify) or to detect/identify (qualify).
Since the sampling efficiency directly impacts bioaerosol concentration level estimates (e.g., an absolute sampling efficiency of 50% results in a two-fold underestimation of the true level), it may be considered a fundamental performance requirement for quantitative bioaerosol sampling. The evaluated air samplers’ BSEs and PSEs were therefore used to assess their suitability for quantitative sampling applications. An air sampler was considered suitable if the relative sampling efficiency was ≥0.5, thus implying less than twofold underestimation of the bioaerosol level compared to the reference sampler ().
While a direct quantitative relationship between air and sample is essential for accurate quantification, qualitative sampling applications are, however, not dependent on the existence of such a direct relationship.
For qualitative applications, obtaining sufficient material (i.e., above the analysis method's detection limit) may be considered the primary sampling objective, and the sampler's ability to concentrate bioaerosols from the air and into a sample may therefore be considered a fundamental performance requirement. The evaluated air samplers’ BCFs and PCFs were therefore used to assess their suitability for qualitative sampling applications. An air sampler was considered suitable if the relative concentration factor was ≥1.0, thus implying similar or higher concentration levels of sampled material compared to the reference sampler ().
It should however be noted that because the T&E results obtained in the current work were relative to the BioSampler, the employed suitability criteria were also inherently relative. For the purpose of this study, the PSEs and BSEs and concentration factors of the BioSampler were therefore considered as “performance benchmarks,” although this should not be taken to suggest that the BioSampler has 100% absolute PSEs and BSEs for all bioaerosols or that its concentration factors will be suitable for all bioaerosol sampling purposes.
The BioSampler was chosen as a reference sampler because it has been shown to have sampling efficiencies ≥90% for the particle sizes involved in this study (Willeke et al. Citation1998; Kesavan et al. Citation2011). The BioSampler has also been shown to inflict limited sampling stress allowing retained cultivability of stress-sensitive airborne microorganisms (Lin et al. Citation1999, Citation2000). However, some of the T&E results obtained in the current study, and especially the BSEs for 4 μm SM aerosols observed with XMX-CV and SASS 2300 which both were >1.5, could suggest that the BioSampler inflicted sampling stress that reduced the cultivability of SM more than some of the evaluated samplers. While these are interesting observations, further studies will be needed to verify and investigate these results. Still, they may be seen as an interesting reminder about that in all likelihood no single air sampler will be universally optimal, or even suitable, for all bioaerosols and bioaerosol sampling purposes.
CONCLUSION AND IMPLICATIONS
Selecting an air sampler for bioaerosol sampling applications is a nontrivial task that must be performed with great care to ensure that the obtained results accurately and reliably describe the studied bioaerosol. The current work contributes to increase user awareness about important factors that should be considered during air sampler selection. Our results highlight the importance of harmonizing air sampler performance to the targeted bioaerosol and the downstream analysis method, and provide several generalized features regarding the evaluated air samplers’ suitability for various bioaerosol sampling applications ().
Although this study involved a diverse selection of test agents, several other types of commonly studied bioaerosols were not specifically addressed (e.g., fungi, fungal spores, protists, pollen, and microbial fragments such as endotoxins and mycotoxins). However, since we established PSEs and BSEs for aerosol test agents with highly variable morphologies and sensitivities to sampling stress, practitioners interested in other types of bioaerosols may still be able to extract relevant performance information, at least within the evaluated particle size range. Extrapolations of the results to include other types of bioaerosols should in any case only be carefully attempted and mindfully considered, even within the microorganism classes that were specifically evaluated.
We believe that the performance measurements presented in this work could contribute to a more well-harmonized selection of air sampling equipment, and thereby lead to more accurate, reliable, comparable, and relevant exposure assessments for airborne pathogens and other bioaerosols of interest.
FUNDING
This work was funded by the Norwegian Defence Research Establishment.
SUPPLEMENTAL MATERIAL
Supplemental data for this article can be accessed on the publisher's website.
Supplemental_Information.zip
Download Zip (234.3 KB)REFERENCES
- Ahmed , M. F. , Schulz , J. and Hartung , J. 2013 . Air Samplings in a Campylobacter Jejuni Positive Laying Hen Flock . Ann. Agr. Env. Med. , 20 : 16 – 20 .
- Alvarez , A. J. , Buttner , M. P. and Stetzenbach , L. D. 1995 . PCR for Bioaerosol Monitoring: Sensitivity and Environmental Interference . Appl. Environ Microbiol. , 61 : 3639 – 3644 .
- An , H. R. , Mainelis , G. and Yao , M. 2004 . Evaluation of a High-Volume Portable Bioaerosol Sampler in Laboratory and Field Environments . Indoor Air , 14 : 385 – 393 .
- Baron , P. A. 1998 . Personal Aerosol Sampler Design: A Review . Appl. Occupat. Environ. Hyg. , 13 : 313 – 320 .
- Bartlett , K. H. , Lee , K. S. , Stephens , G. , Black , W. , Brauer , M. and Copes , R. 2002 . Evaluating Indoor Air Quality: Test Standards for Bioaerosols, School of Occupational and Environmental Hygiene , University of British Columbia, Vancouver, Canada .
- Bergman , W. , Shinn , J. , Lochner , R. , Sawyer , S. , Milanovich , F. and Jr , R. M. 2004 . High Volume, Low Pressure Drop, Bioaerosol Collector Using a Multi-slit Virtual Impactor . J. Aerosol Sci. , 36 : 619 – 638 .
- Black , J. E. 2011 . Evaluation of XMX/2L-MIL Virtual Impactor Performance and Capture and Retention of Aerosol Particles in Two Different Collection Media , DTIC Document .
- Black , J. E. and Cooper , C. W. 2012 . Interinstrument Variability and Validation Study for the XMX/2L-MIL Biological Air Sampler , DTIC Document .
- Brixey , L. A. , Paik , S. Y. , Evans , D. E. and Vincent , J. H. 2002 . New Experimental Methods for the Development and Evaluation of Aerosol Samplers . J.Environ. Monitor. , 4 : 633 – 641 .
- Burge , H. A. and Solomon , W. R. 1987 . Sampling and Analysis of Biological Aerosols . Atmos. Environ. (1967) , 21 : 451 – 456 .
- Burton , N. C. , Grinshpun , S. A. and Reponen , T. 2007 . Physical Collection Efficiency of Filter Materials for Bacteria and Viruses . Ann. Occup. Hyg. , 51 : 143 – 151 .
- Buttner , M. P. , Cruz , P. , Stetzenbach , L. D. , Klima-Comba , A. K. , Stevens , V. L. and Cronin , T. D. 2004 . Determination of the Efficacy of Two Building Decontamination Strategies by Surface Sampling with Culture and Quantitative PCR Analysis . App. Environ. Microbiol. , 70 : 4740 – 4747 .
- Buttner , M. P. and Stetzenbach , L. D. 1991 . Evaluation of Four Aerobiological Sampling Methods for the Retrieval of Aerosolized Pseudomonas Syringae . Appl. Environ. Microbiol. , 57 : 1268 – 1270 .
- Buttner , M. , Willeke , K. and Grinshpun , S. 2002 . “ Sampling and Analysis of Airborne Microorganisms ” . In Manual of Environmental Microbiolgy , Edited by: Hurst , C. J. , Crawford , R. L. , Knudsen , G. , McInerney , M. and Stetzenbach , L. D. 814 – 826 . Washington, DC : ASM Press .
- Carvalho , E. , Sindt , C. , Verdier , A. , Galan , C. , O’Donoghue , L. Parks , S. 2008 . Performance of the Coriolis Air Sampler, a High-Volume Aerosol-Collection System for Quantification of Airborne Spores and Pollen Grains . Aerobiologia , 24 : 191 – 201 .
- Chang , C.-W. and Chou , F. C. 2011 . Methodologies for Quantifying Culturable, Viable, and Total Legionella Pneumophila in Indoor Air . Indoor Air , 21 : 291 – 299 .
- Chang , C.-W. , Chou , F.-C. and Hung , P.-Y. 2010 . Evaluation of Bioaerosol Sampling Techniques for Legionella Pneumophila Coupled with Culture Assay and Quantitative PCR . J. Aerosol Sci. , 41 : 1055 – 1065 .
- Cooper , C. W. 2010 . High Volume Air Sampling for Viral Aerosols: A Comparative Approach , DTIC Document .
- Cox , C. S. and Wathes , C. M. 1995 . Bioaerosols Handbook Boca Raton, FL : Lewis Publishers, .
- Eduard , W. and Heederik , D. 1998 . Methods for Quantitative Assessment of Airborne Levels of Noninfectious Microorganisms in Highly Contaminated Work Environments . Am. Ind. Hyg. Assoc. J. , 59 : 113 – 127 .
- Enderby , J. C. 2012 . Comparative Analysis of Two Biological Warfare Air Samplers Using Live Surrogate Agents , DTIC Document .
- Estill , C. F. , Baron , P. A. , Beard , J. K. , Hein , M. J. , Larsen , L. D. Deye , G. J. 2011 . Comparison of Air Sampling Methods for Aerosolized Spores of B. Anthracis Sterne . J. Occup. Environ. Hyg. , 8 : 179 – 186 .
- Fabian , P. , McDevitt , J. J. , Houseman , E. A. and Milton , D. K. 2009 . Airborne Influenza Virus Detection with Four Aerosol Samplers using Molecular and Infectivity Assays: Considerations for a New Infectious Virus Aerosol Sampler . Indoor Air , 19 : 433 – 441 .
- Georgakopoulos , D. G. , Després , V. , Fröhlich-Nowoisky , J. , Psenner , R. , Ariya , P. A. Pósfai , M. 2009 . Microbiology and Atmospheric Processes: Biological, Physical and Chemical Characterization of Aerosol Particles . Biogeosciences , 6 : 721 – 737 .
- Gómez-Domenech , M. , García-Mozo , H. , Alcázar , P. , Brandao , R. , Caeiro , E. Munhoz , V. 2010 . Evaluation of the Efficiency of the Coriolis air Sampler for Pollen Detection in South Europe . Aerobiologia , 26 : 149 – 155 .
- Griffin , D. W. , Gonzalez , C. , Teigell , N. , Petrosky , T. , Northup , D. and Lyles , M. 2011 . Observations on the Use of Membrane Filtration and Liquid Impingement to Collect Airborne Microorganisms in Various Atmospheric Environments . Aerobiologia , 27 : 25 – 35 .
- Grinshpun , S. A. , Chang , C.-W. , Nevalainen , A. and Willeke , K. 1994 . Inlet Characteristics of Bioaerosol Samplers . J. Aerosol Sci. , 25 : 1503 – 1522 .
- Grinshpun , S. A. and Clark , J. M. 2005 . Measurement and Characterization of Bioaerosols . J. Aerosol Sci. , 36 : 553 – 555 .
- Grinshpun , S. A. , Willeke , K. , Ulevicius , V. , Donnelly , J. , Lin , X. and Mainelis , G. 1996 . Collection of Airborne Microorganisms: Advantages and Disadvantages of Different Methods . J. Aerosol Sci. , 27 ( Supplement 1 ) : S247 – S248 .
- Henningson , E. W. , Lundquist , M. , Larsson , E. , Sandström , G. and Forsman , M. 1997 . A Comparative Study of Different Methods to Determine the Total Number and the Survival Ratio of Bacteria in Aerobiological Samples . J. Aerosol Sci. , 28 : 459 – 469 .
- Hermann , J. R. , Hoff , S. J. , Yoon , K. J. , Burkhardt , A. C. , Evans , R. B. and Zimmerman , J. J. 2006 . Optimization of a Sampling System for Recovery and Detection of Airborne Porcine Reproductive and Respiratory Syndrome Virus and Swine Influenza Virus . Appl. Environ. Microbiol. , 72 : 4811 – 4818 .
- Kesavan , J. S. , Bottiger , J. R. and McFarland , A. R. 2008 . Bioaerosol Concentrator Performance: Comparative Tests with Viable and with Solid and Liquid Nonviable Particles . J. Appl. Microbiol. , 104 : 285 – 295 .
- Kesavan , J. S. , Doherty , R. W. and Bottiger , J. R. 2003 . Performance Characterization Methods of Aerosol Samplers, Edgewood Chemical Biological Center (ECBC) , MD : Aberdeen Proving Ground .
- Kesavan , J. S. , Schepers , D. and Bottiger , J. 2011 . Characteristics of Twenty-Nine Aerosol Samplers Tested at US Army Edgewood Chemical Biological Center (2000–2006) , DTIC Document .
- Kesavan , J. , Schepers , D. and McFarland , A. R. 2010a . Sampling and Retention Efficiencies of Batch-Type Liquid-Based Bioaerosol Samplers . Aerosol Sci. Technol. , 44 : 817 – 829 .
- Kesavan , J. , Schepers , D. , Sutton , T. , Deluca , P. , Williamson , M. and Wise , D. 2010b . Characteristics Sampling Efficiency and Battery Life of Smart Air Sampler System (SASS) 3000 and SASS 3100 , DTIC Document .
- Kesavan , J. and Stuebing , E. 2009 . “ Aerosol Sampling Efficiency Evaluation Methods at the US Army Edgewood Chemical Biological Center ” . In Atmospheric and Biological Environmental Monitoring , Edited by: Kim , Y. , Platt , U. , Gu , M. and Iwahashi , H. 83 – 103 . Netherlands : Springer .
- Li , C.-S. 1999 . Evaluation of Microbial Samplers for Bacterial Microorganisms . Aerosol Sci. Technol. , 30 : 100 – 108 .
- Li , S. N. , Lundgren , D. A. and Rovell-Rixx , D. 2000 . Evaluation of Six Inhalable Aerosol Samplers . Am. Ind. Hyg. Assoc. J. , 61 : 506 – 516 .
- Lin , W. H. and Li , C. S. 1999 . Evaluation of Impingement and Filtration Methods for Yeast Bioaerosol Sampling . Aerosol Sci. Technol. , 30 : 119 – 126 .
- Lin , X. , Reponen , T. , Willeke , K. , Grinshpun , S. A. , Foarde , K. K. and Ensor , D. S. 1999 . Long-Term Sampling of Airborne Bacteria and Fungi into a Non-Evaporating Liquid . Atmos. Environ. , 33 : 4291 – 4298 .
- Lin , X. , Reponen , T. , Willeke , K. , Wang , Z. , Grinshpun , S. A. and Trunov , M. 2000 . Survival of Airborne Microorganisms During Swirling Aerosol Collection . Aerosol Sci. Technol. , 32 : 184 – 196 .
- Macher , J. , ed. 1999 . Bioaerosols: Assessment and Control Cincinnati, OH : American Conference of Governmental Industrial Hygienists (ACGIH), .
- Macher , J. M. and Willeke , K. 1992 . Performance Criteria for Bioaerosol Samplers . J. Aerosol Sci. , 23 ( Suppl. 1 ) : 647 – 650 .
- McFarland , A. R. , Haglund , J. S. , King , M. D. , Hu , S. , Phull , M. S. Moncla , B. W. 2010 . Wetted Wall Cyclones for Bioaerosol Sampling . Aerosol Sci. Technol. , 44 : 241 – 252 .
- Millner , P. D. 2009 . Bioaerosols Associated with Animal Production Operations . Bioresource Technol. , 100 : 5379 – 5385 .
- Nevalainen , A. , Pastuszka , J. , Liebhaber , F. and Willeke , K. 1992 . Performance of Bioaerosol Samplers: Collection Characteristics and Sampler Design Considerations . Atmos. Environ. Part A. General Topics , 26 : 531 – 540 .
- O’Connell , K. P. , Bucher , J. R. , Anderson , P. E. , Cao , C. J. , Khan , A. S. Gostomski , M. V. 2006 . Real-Time Fluorogenic Reverse Transcription-PCR Assays for Detection of Bacteriophage MS2 . Appl. Environ. Microbiol. , 72 : 478 – 483 .
- Radosevich , J. L. , Wilson , W. J. , Shinn , J. H. , DeSantis , T. Z. and Andersen , G. L. 2002 . Development of a High-Volume Aerosol Collection System for the Identification of Air-Borne Micro-Organisms . Lett. Appl. Microbiol. , 34 : 162 – 167 .
- Reponen , T. , Willeke , K. , Grinshpun , S. and Nevalainen , A. 2011 . “ Biological Particle Sampling ” . In Aerosol Measurement , 549 – 570 . New York : John Wiley & Sons, Inc .
- Rule , A. M. , Kesavan , J. , Schwab , K. J. and Buckley , T. J. 2007 . Application of Flow Cytometry for the Assessment of Preservation and Recovery Efficiency of Bioaerosol Samplers Spiked with Pantoea agglomerans . Environ. Sci. Technol. , 41 : 2467 – 2472 .
- Saikaly , P. E. , Barlaz , M. A. and de los Reyes , F. L. 2007 . Development of Quantitative Real-Time PCR Assays for Detection and Quantification of Surrogate Biological Warfare Agents in Building Debris and Leachate . Appl. Environ. Microbiol. , 73 : 6557 – 6565 .
- Shahamat , M. , Levin , M. , Rahman , I. , Grim , C. , Heidelberg , J. , Stelma , G. and Colwell , R. 1997 . Evaluation of Media for Recovery of Aerosolized Bacteria . Aerobiologia , 13 : 219 – 226 .
- Sinclair , R. , Boone , S. A. , Greenberg , D. , Keim , P. and Gerba , C. P. 2008 . Persistence of Category A Select Agents in the Environment . Appl. Environ. Microbiol. , 74 : 555 – 563 .
- Tseng , C.-C. and Li , C.-S. 2005 . Collection Efficiencies of Aerosol Samplers for Virus-Containing Aerosols . J. Aerosol Sci. , 36 : 593 – 607 .
- Van Droogenbroeck , C. , Van Risseghem , M. , Braeckman , L. and Vanrompay , D. 2009 . Evaluation of Bioaerosol Sampling Techniques for the Detection of Chlamydophila Psittaci in Contaminated Air . Vet. Microbiol. , 135 : 31 – 37 .
- Vitko , J. Jr. , Franz , D. , Alper , M. , Biggins , P. , Brandt , L. Bruckner-Lea , C. 2005 . Committee on Materials and Manufacturing Processing for Advanced Sensors. The National Research Council of the National Academies, The National Academic Press, Washington DC. Sensor Systems for Biological Agent Attacks: Protecting Buildings and Military Bases .
- Willeke , K. , Lin , X. and Grinshpun , S. A. 1998 . Improved Aerosol Collection by Combined Impaction and Centrifugal Motion . Aerosol Sci. Technol. , 28 : 439 – 456 .
- Xu , Z. , Wu , Y. , Shen , F. , Chen , Q. , Tan , M. and Yao , M. 2011 . Bioaerosol Science, Technology, and Engineering: Past, Present, and Future . Aerosol Sci. Technol. , 45 : 1337 – 1349 .
- Yao , M. and Mainelis , G. 2007 . Analysis of Portable Impactor Performance for Enumeration of Viable Bioaerosols . J. Occup. Environ. Hyg. , 4 : 514 – 524 .
- Zhao , Y. , Aarnink , A. J. A. , Doornenbal , P. , Huynh , T. T. T. , Koerkamp , P. W. G. G. de Jong , M. C. M. 2011 . Investigation of the Efficiencies of Bioaerosol Samplers for Collecting Aerosolized Bacteria Using a Fluorescent Tracer. I: Effects of Non-Sampling Processes on Bacterial Culturability . Aerosol Sci. Technol. , 45 : 423 – 431 .
