Abstract
The refractive index is a fundamental property controlling aerosol optical properties. Secondary organic aerosols have variable refractive indices, presumably reflecting variations in their chemical composition. Here, we investigate the real refractive indices (mr) and chemical composition of secondary organic aerosols (SOA) generated from the oxidation of α-pinene and limonene with ozone and NOx/sunlight at different HC/NOx ratios. Refractive indices were retrieved from polar nephelometer measurements using parallel and perpendicular polarized 532-nm light. Particle chemical composition was monitored with a high-resolution time-of-flight aerosol mass spectrometer (HR-Tof-AMS). For photochemically generated SOA, the values of refractive indices are consistent with prior results, and ranged from about 1.34 to 1.55 for limonene and from 1.44 to 1.47 for α-pinene, generally increasing as the particles grew. While AMS fragments are strongly correlated to the refractive index for each type of SOA, the relationships are in most cases quite different for different SOA types. Consistent with its wide range of refractive index, limonene SOA shows larger variations compared to α-pinene SOA for most parameters measured with the AMS, including H:C, O:C, f43 (m/z 43/organic), fC4H7 +, and others. Refractive indices for α-pinene ozonolysis SOA also fell in narrow ranges; 1.43–1.45 and 1.46–1.53 for particles generated at 19–22 and 23–29°C, respectively, with corresponding small changes of f43 and H:C ratio and other parameters. Overall, H:C ratio, m/z 43 and 55 (C2H3O+, C4H7 +) were the best correlated with refractive index for all aerosol types investigated. The relationships between mr and most fragments support the notion that increasing condensation of less oxygenated semivolatile species (with a possible role for a concomitant decrease in low refractive index water) is responsible for the increasing mrs observed as the experiments progress. However, the possibility that oligomerization reactions play a role cannot be ruled out.
1. INTRODUCTION
Atmospheric aerosols strongly influence direct radiative transfer by scattering and absorbing solar radiation (Intergovernmental Panel on Climate Change, 2007). Refractive indices are fundamental to aerosol optical parameters such as the single scatter albedo, asymmetry factor, and specific absorption, which in turn are necessary to estimate radiative transfer. Additionally, refractive indices are needed to estimate reliable phase function and polarization information, which are essential to infer aerosol optical depth, size, and single scatter albedo from satellite data and aircraft measurements (Mishchenko et al. Citation2007). Aerosol refractive index is fundamentally the result of a combination of particle chemical composition and internal mixing.
Organic compounds are ubiquitous in lower tropospheric aerosols, comprising 20–70% of aerosol mass depending on location and time (Zhang et al. Citation2007). Of this, (Hallquist et al. Citation2009) estimated that roughly 70–90% of the organic material is secondary. The contribution of SOA to the global radiation balance depends on their concentration, size distributions, interactions with other radiatively important atmospheric constituents, and on their direct interactions with solar insolation and upwelling terrestrial infrared radiation. Because of both the chemical and physical complexity of the organic aerosols as well as the small number of measurements of their optical properties the radiative properties of SOA are poorly understood.
Recently several methods to measure or estimate optical properties of particles have been developed, and some laboratory studies have begun to derive refractive indices from SOA (summarized in Kim and Paulson Citation2013). These studies show that mrs span a reasonably wide range from 1.35 to 1.62, depending on precursors, oxidation chemistry, temperature, diameter, mass concentration. Under longer time scales, other factors such as in-particle reactions and heterogeneous aging may also play a role (Kim et al. Citation2012). For application to atmospheric models and satellite retrievals, a better understanding of factors controlling SOA optical properties is desirable. In addition, because the refractive index is a property that arises from molecular composition as well as supramolecular interactions, it provides information about the composition of particles that is not accessible with other methods.
In this study, we present measurements that directly connect composition and refractive index of SOA generated from limonene and α-pinene, using ozone and photochemical oxidation. Photooxidation experiments were performed at different HC/NOx ratios. Because earlier studies showed refractive indices of limonene SOA to be more variable (Kim et al. Citation2012), we have placed more emphasis on limonene in this study. Here, we measure angular scattering with a second-generation polar nephelometer (Kim et al. Citation2010), and characterize the chemical composition of SOA particles using a high-resolution time-of-flight aerosol mass spectrometer (HR-ToF-AMS).
2. EXPERIMENTAL
Secondary organic aerosol was generated via homogeneous nucleation in six experiments performed in an outdoor 24 m3 Teflon chamber at UCLA. Real refractive indices were retrieved by combining data from a polar nephelometer and a scanning mobility particle sizer and applying a genetic algorithm retrieval scheme (Barkey et al. [Citation2007]; Kim et al. [Citation2010]). Details on the chamber, its physical parameters, aerosol generation, measurements to characterize the gas phase, and aerosol optical properties are described in the online supplementary information (SI). summarizes initial conditions and results for the experiments.
TABLE 1 Initial conditions, temperatures, RH, and results of the experiments
An Aerodyne high-resolution time-of-flight aerosol mass spectrometer (HR-ToF-AMS; Aerodyne, Billerica, MA, USA) was used to measure chemical composition and mass size distribution of SOA components. The HR-ToF-AMS is detailed in DeCarlo et al. (Citation2006). Briefly, the instrument consists of five major components: aerosol sampling inlet (aerodynamic lens), particle time-of-flight chamber, particle vaporization and ionization chamber, and a high-resolution time-of-flight mass spectrometer. Particles entering the instruments are focused by the aerodynamic lens, forming a highly collimated particle beam. The particles impact a heated surface (600°C) and the nonrefractory components are flash vaporized. The vapors are ionized by electron impaction and simultaneously fragmented, followed by quantification with a time-of-flight mass spectrometer. Particle vacuum aerodynamic diameter is determined by particle time-of-flight (PToF) between a mechanical rotating chopper (at the front of the particle time-of-flight chamber) and the vaporizer using a calibration curve, which is derived from PToF of polystyrene latex particles of known sizes. During the measurements, the mass spectrometer (MS) mode (including the high S/N V mode and the high mass-resolution W mode) and the PToF mode alternated with ∼5-min time resolution. Chemical composition of ensemble particles, including organic mass (OM), nitrate, and oxygen-to-carbon ratio (O:C) and hydrogen-to-carbon ratio (H:C) are derived from the V-mode measurements. Ionization efficiency of the AMS was calibrated by 350-nm ammonium nitrate particles on 16 January and 26 January, respectively, before each set of the (limonene and α-pinene) experiments. Size calibration of the AMS was conducted with PSLs with known sizes.
3. RESULTS AND DISCUSSION
Six experiments were performed, three each with limonene and α-pinene, using photooxidation and ozonolysis (). The photooxidation experiments were performed at low, intermediate, and high NOx (initial HC/NOx = 32 ± 1, 14, and 6.6 ppbC/ppb, respectively). Ozonolysis experiments were performed with and without a scavenger (Paulson et al. Citation1998). Earlier experiments and chemical kinetics modeling studies have shown that for limonene, HC/NOx ratios produce aerosol that should be dominated by RO2 + HO2/RO2 chemistry in the low NOx case, with only a minor contribution for the high NOx case (Kim et al. Citation2012). Precursor concentrations ranged from 170 to 200 ppb for limonene, 134–149 ppb for α-pinene, and 0–290 ppb for NO. RH ranged from 13 to 29%, and temperatures from 16 to 29°C, with the exception of the α-pinene photooxidation experiment (29 January, 6% RH, 33–37°C). For five of the six experiments, temperatures were lower than the summer experiments described in Kim et al. (Citation2012) (31–39°C).
Throughout the discussions presented here, we interpret some behaviors as being related to a shift in condensation of more polar, low volatility, and higher O:C compounds to less polar higher volatility and higher H:C compounds, and relate this to changing mr values. The possibility that some of the behaviors are due to oligomerization reactions is discussed in detail in Section 3.4, and it also seems likely that especially for the limonene case, water uptake by the more polar compounds, decreasing as particles become less polar, plays a role in the observed mr's, especially at the low end (Kim et al. Citation2012).
3.1. SOA Refractive Indices
In , SOA refractive indices are plotted versus particle diameter. The particle diameter axis is similar to a reaction time axis, as particle diameter continuously increases throughout each experiment, but it makes the initial growth phase when particles are growing rapidly much easier to visualize. For individual experiments, particle diameter is consistently the independent physical variable best correlated with refractive index (Kim et al. Citation2012).
FIG. 1 Retrieved refractive indices for SOA formed by (a) photooxidation of limonene and α-pinene at different HC/NOx ratios and (b) ozonolysis of α-pinene with and without scavenger as a function of particle diameter.
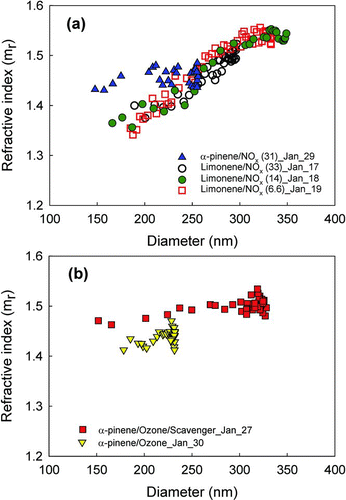
For photochemically generated SOA, limonene SOA mrs increase from 1.34 to 1.55 with increasing diameter (166–331 nm). α-Pinene SOA mrs appear to increase, but only slightly from 1.44 to 1.47 as particles grow from 148 to 256 nm. Both the ranges and values are consistent with previous studies (Kim et al. Citation2010, Citation2012) although the dependence on HC/NOx for limonene is different from observations by Kim et al. (Citation2010). This may be because current experiments were performed during winter when light intensity and temperatures were low, so that chemical reactions and particle growth is slower, changing particle dynamics, particularly the balance between nucleation versus condensation and growth. Differences in this balance appear to be responsible for much of the size difference between higher and lower NOx experiments, especially in summer experiments (Kim et al. Citation2012). Additionally, the balance of chemical pathways can shift. Lower insolation shifts the balance between NO2 and NO in favor of NO2; consistent with this, Leungsakul et al. (Citation2005) found that ozone formation is most sensitive to the changes of light intensity, and decreasing the actinic flux (photon cm2 s−1) by 30% (from September to winter solstice) (Finlayson-Pitts and Pitts Citation1999), decreased the ozone concentration by 13%. Here, although the dependence is much weaker than that in Kim et al. (Citation2012), there is a slight increasing trend of maximum size with decreasing HC/NOx (). The overall range and trend of mrs is consistent with previous studies; smallest, newest particles have significantly lower refractive indices than slightly older, larger particles, and after the particles grow to approximately 90% of their maximum size, their mrs level out and then start to decrease (Kim et al. Citation2012). The lowest values of α-pinene mr, 1.44, are significantly higher than those of limonene SOA at similar particle sizes, a trend that was also observed by Kim et al. (Citation2012). This may be due to a smaller water fraction in very fresh α-pinene SOA compared to limonene SOA, as observed by VanReken et al. (Citation2005).
shows the size-dependent trend of mr. The level of mrs increased for both limonene and α-pinene with decreasing HC/NOx ratio, although the dependence was much stronger for limonene SOA. The HC/NOx ratio has the same effect on the size; the maximum particle size increases as HC/NOx ratio is decreased, although the trend is not as pronounced as it was in summer experiments by Kim et al. (Citation2012), as noted above.
shows refractive indices of SOA generated from α-pinene ozonolysis performed with and without an OH scavenger, as a function of particle diameter. The two experiments have significantly different ranges for mr, 1.46–1.52 and 1.43–1.45, respectively. In previous studies, a scavenger has not produced a discernible effect on the refractive index (Kim et al. Citation2010). However, we have repeatedly observed that significantly lower oxidation temperatures (∼14°C vs. 26°C from Kim et al. [Citation2010] and ∼19°C vs. 24°C for Kim et al. [Citation2012]) had a substantial effect on the refractive index; lower temperatures in this range lower mr by ∼0.05. Several literature studies have reported that in some ranges, the temperature at which particles are generated can affect the chemical composition of SOA; Warren et al. (Citation2009) found evidence for a nonreversible difference in the chemical composition of α-pinene aerosol formed at ∼5°C versus at 28°C, and Salo et al. (Citation2012) suggest that between 20°C and 40°C there may be a transition temperature that changes the chemical composition of α-pinene SOA. The ranges of mrs retrieved here (1.43–1.45 at 19–22°C and 1.46–1.52 at 23–29°C) are consistent with previous results (1.4–1.44 at ∼14°C and 1.44–1.48 at 26–28°C (Kim et al. Citation2010); 1.39–1.45 at 18–20°C and 1.39–1.52 at 23–28°C (Kim and Paulson Citation2013)). Kim et al.'s (Citation2010) mr values are lower by ∼0.02–0.03 due to the longer wavelength of laser used in that study (670 nm) than the light used in the later retrievals (532 nm) consistent with the wavelength dependence for α-pinene SOA (Yu et al. Citation2008).
3.2. Chemical Composition of SOA
For all SOA examined here, the most intense peaks measured with the AMS occur at m/z 27 (C2H3 +), 29 (CHO+), 41 (C3H5 +), 43 (C2H3O+, C3H7 +), 44 (CO2 +), 55 (C3H3O+, C4H7 +), and 57 (C3H5O+, C4H9 +). Of these, m/z 43 and 44 are the largest. The contributions from the different m/z signals vary with organic loading. shows a triangle plot of f44 versus f43 (e.g., m/z 43/org) for all experiments. The portion of the experiments that had retrievable mrs is indicated with filled symbols, and the direction of the trends with time is indicated with arrows in the figure. The two dotted lines in the figures outline the space into which ambient SOA measurements typically fall (Ng et al. Citation2010). Limonene SOA () reside at higher f44 and lower f43 (open symbols) when particles first nucleate, after which f43 increases and f44 decreases. α-Pinene () has a somewhat different trend; f44 first decreases slightly and then increases again; f43 also changes direction in the middle of the experiment.
FIG. 2 Triangle plot for photooxidation of (a) limonene at different HC/NOx ratios and (b) ozonolysis of α-pinene with and without scavenger. Outline of triangle shown in gray line indicate the space where ambient OOA components fall (Ng et al. Citation2010). Arrows represent the time progression of experiments.
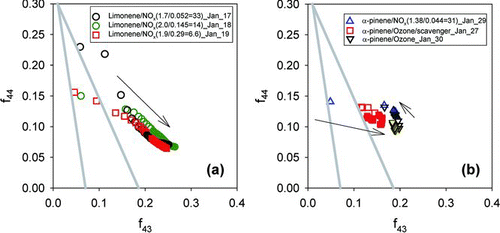
Particles large enough to have a refractive index retrieved all fall in a fairly narrow range of f44 and f43, although α-pinene SOA () has somewhat higher f44 and lower f43 than limonene (). All of the data, for which mr was retrieved, reside outside of the dotted lines, indicating that the laboratory SOA has somewhat different range of f43 and f44 than typical for ambient aerosol. Possible reasons for laboratory SOA being different from ambient aerosols include higher loadings employed in laboratory experiments; these favor partitioning of less oxidized species (which should remain in the gas phase under atmospheric conditions) (Duplissy et al. Citation2008; Shilling et al. Citation2009) and the limited residence time (5–6 h) in chambers, much shorter than the atmospheric lifetime (∼4–5 days) of ambient aerosol, leading to less oxidative processing.
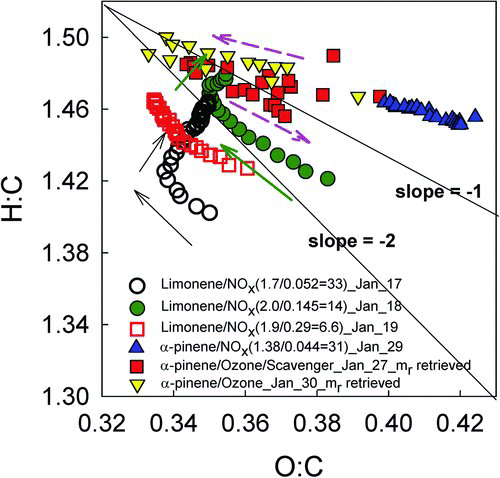
A Van Krevelen diagram is shown in . For limonene SOA, the earliest aerosol formed has an O:C ratio of 0.4. This decreases as the experiment progresses and increases again slightly later in the experiment (17 January, 18 January), reaching a final value of approximately 0.3. In contrast, H:C ratio (), f43 and f44 () did not reverse direction; H:C ratio increases from about 1.4 to 1.5 and f44 and f43 both consistently decrease. This may indicate that for these experiments f44 is not a good proxy for O:C, in contrast to the results for ambient aerosol found by Aiken et al. (Citation2008). In our experiments, initial O:C is dominated by f44(CO2 +) and then f43(C2H3O+, C3H7 +; note that C2H3O+ is dominant by a factor of 20) takes over. This behavior has been explained by increased partitioning of less oxidized semivolatile compounds as the mass concentration increases (Shilling et al. Citation2009; Chan et al. Citation2010; Kim et al. Citation2012; Kim and Paulson Citation2013). In contrast, photochemically generated α-pinene SOA O:C starts at 0.4 and then increases to 0.42 and H:C changes modestly throughout the experiment (1.45–1.5). This is similar to α-pinene SOA generated by ozonolysis, although this SOA had slightly lower O:C ratios of 0.34–0.38. Although the trends and ranges of O:C and H:C ratios are slightly different depending on the type of SOA, the overall ranges are in agreement with results for biogenic SOA in previous studies (Aiken et al. Citation2008; Heaton et al. Citation2009; Shilling et al. Citation2009; Chhabra et al. Citation2011).
FIG. 3 Van Krevelen diagram for limonene SOA at different HC/NOx ratios and ozonolysis of α-pinene SOA with and without scavenger. Line with slopes with -1 and -2 are represented by black lines. Arrows indicate the directions of experiments. The dashed arrows indicate the overall directions for the α-pinene ozonolysis experiments.
The dependence of O:C on aerosol mass concentration (in the chamber) is shown in . Higher O:C is believed to be related to the mole fraction of low volatility material (Shilling et al. Citation2009). Particles for which refractive index data were retrieved are shown with filled symbols, others with open symbols. Missing data for 30 January is due to an instrument problem at that time. In all cases, O:C decreases rapidly initially, and then begins to level out as particle growth slows. In some cases, late in the experiments, O:C trends upward again. This is consistent with the notion that initial nucleation and growth is due to the most highly oxygenated and least volatile species, which produce a higher O:C. These highly oxygenated species are later diluted in the particle phase by the partitioning of less oxygenated semivolatile species with lower O:C.. This explanation is more strongly supported by the SI Figures S1a–c, which show O:C with time as the experiments progress. Such behavior has been observed in several previous studies (Bahreini et al. Citation2005; Kostenidou et al. Citation2007; Shilling et al. Citation2009).
FIG. 4 Change of O:C as a function of organic loading in the chamber over the course of (a) ozonolysis of α-pinene with and without scavenger, (b) photooxidation of α-pinene, and (c) photooxidation of limonene at different HC/NOx ratio.
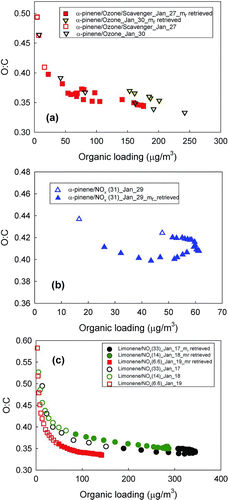
For both α-pinene ozonolysis with and without scavenger, O:C is very similar. As shown in Figures and , at the beginning of mr retrieval, O:C decreases while H:C increases () slightly from 1.5 to 1.45, presumably a result of increased partitioning of less oxidized semivolatile compounds, which cause SOA growth. Later, O:C increases, possibly due to oxidation processes that overtake partitioning of semivolatile species leading to an increase in the oxidation state of SOA (Chhabra et al. Citation2011). The increase in O:C is not obvious in because after the SOA mass peaks and the organic loadings start to decrease owing to wall loss. The late increase in O:C is the most easily recognized in the SI, Figures S1e and f, showing O:C versus time.
3.3. The Relationship Between Refractive Index and Chemical Composition of SOA
In an effort to relate SOA optical properties to chemical composition, the retrieved refractive indices of SOA are plotted versus several indicators of chemical composition, including f44, f43, and the O:C and H:C ratios (Figures and ).
FIG. 5 Retrieved refractive index of ozonolysis of α-pinene with and without scavenger and photooxidation of α-pinene and limonene at different HC/NOx ratio with the variation of (a) O:C, (b) H:C, (c) f44, and (d) f43.
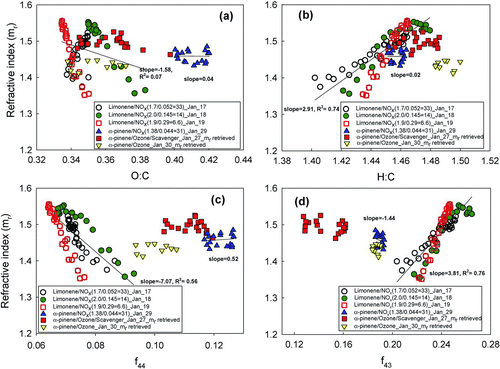
3.3.1. α-Pinene Ozonolysis SOA
As shown in , the slight increases in f44 for α-pinene ozonolysis SOA coincide with small increases in refractive indices, the latter of which rise from 1.43 to 1.45 and 1.46 to 1.52 for the low temperature 30 January experiment and higher temperature 27 January experiment, respectively, during the growth of particles. 30 January shows lower O:C and f44 and higher H:C and f43 than the 27 January experiment, indicating that SOA from the lower temperature 30 January experiment may contain higher volatility, higher H:C species than 27 January SOA, as might be expected due to the difference in temperature of the two experiments (19–22 and 23–29°C, for 30 January and 27 January, respectively).
3.3.2. Photochemically Generated α-Pinene SOA
illustrates the mass loading effect on O:C for photochemically generated α-pinene SOA. For particles with sufficient scattering for mr retrievals (filled symbols), O:C stays constant, indicating that there is little change in partitioning or level of oxidation for the more volatile material that condenses later in the experiments. The portion of the experiments with constant O:C in the lower middle of coincides with a period where α-pinene SOA mr is flat (). Toward the end of the experiment, f44 increases and f43 decreases slightly (, see arrows), indicating further changes in SOA composition, in the direction of increasing oxidation state. At this point, there is also little associated change in refractive index, suggesting relatively little optical sensitivity to this shift. f44, f43, H:C, and O:C and other fragments (not shown) fall in much narrower ranges than the limonene SOA fragments (below; ), consistent with the small variations of mrs observed for α-pinene compared to limonene SOA.
3.3.3. Photochemically Generated Limonene SOA
In contrast to α-pinene, limonene produces products with wide ranges of mrs (1.34–1.55) and AMS values (f43: 0.2–0.26, f44: 0.065–0.09, H:C: 1.40–1.48, and O:C: 0.34–0.38). Limonene is expected to produce products with a wider range of volatility due to its two double bonds. For the intermediate HC/NOx experiment (18 January, HC/NOx = 14), a slight decreasing trend of mr is observed for particles larger than 330 nm, reproducing results from (Kim et al. Citation2012). We note that while there is no direct evidence available indicating low mr's for limonene SOA partly due to a contribution from water uptake (in AMS measurements, particles are expected to desiccate in the vacuum of the inlet), it is very difficult to explain the low mr's without a contribution from water. The reader is referred to Kim et al. (Citation2012) for more discussion. Chemical composition and refractive index data for photochemically generated limonene SOA are shown in Figures , , and . In contrast to α-pinene SOA, both mr and f44 change continuously throughout the experiments (). Correlations between mr and chemical parameters are much clearer (slopes range from −7.07 to 3.81 within R 2 values of 0.56–0.74. Mrs vs. O:C is an exception; here O:C changes direction in the middle/end of 17 January experiment, as discussed in Sections 3.2 and 3.3.3.1).
FIG. 6 Retrieved refractive index of limonene SOA versus the fraction of each fragment. fCO2 + is not shown here because it is identical to f44 in .
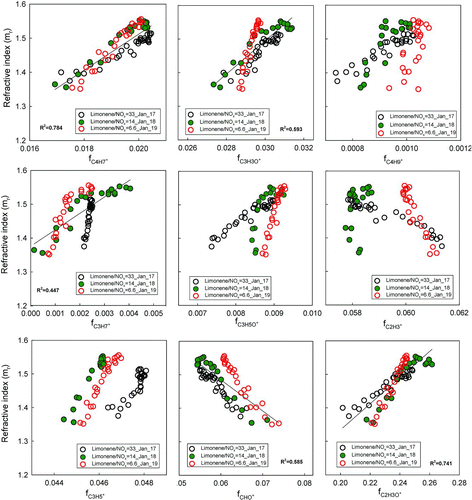
The decreases in O:C suggest continuing condensation of less oxygenated species throughout the experiments, which are accompanied by continuous increases in the refractive index (). H:C () and f43 () as well as many other fragments () also change continuously with refractive index, some with positive and others with negative correlations. Shifts are generally consistent with the shift to less oxygenated condensate as the experiments progress (possibly with a concomitant decrease in water content). With the exception of C2H3 +, which has a complex relationship, all of the hydrocarbon fragments, including C4H7 +, C3H7 +, C4H9 +, and C3H5 +, are positively correlated with refractive index. The oxygenated fragments are mixed; some are positively correlated, including C2H3O+, C3H3O+, C3H5O+, while the smaller oxygenated fragments CO2 + and HCO+ are anticorrelated.
The fragments most strongly correlated with refractive index are fC2H3O + (m/z 43) and the minor peak for fC4H7 + (m/z 55, R2 = 0.78). fC3H3O + (m/z 55) and fCHO + (m/z 29) are also correlated with refractive index (R 2 = 0.59). m/z 55 is a marker for hydrocarbon-like aerosol (HOA) (Zhang et al. Citation2007). This comparison adds further credence to the notion that shifting chemical composition due to condensation of species with different volatility/hygroscopicity is responsible for shifting refractive indices.
Taken together, shows that the H:C ratio has the best correlation with mr. As the H:C ratio increases, mr increases until the H:C ratio is about 1.47. However, after this point, mr starts to decrease, indicating that other factors influence mrs. Toward the end of the limonene/NOx = 14 experiment (18 January, Figure S1b), mr starts to decrease. This coincides with the O:C ratio starting to increase. The continuously increasing H:C ratio after this point seems to imply continued partitioning of semivolatile species, but either condensing species are more oxygenated or species already incorporated in the particles are becoming oxidized.
3.3.3.1Effect of the HC/NOx ratio
Three limonene photooxidation experiments were performed at low, intermediate, and high initial HC/NOx ratios, respectively. mr is not differentiable among the three experiments, in contrast to our earlier results (Kim et al. Citation2012). This earlier study, performed with higher insolation and temperatures, showed a marked dependence of both particle size and mr on the HC/NOx ratio. We note that lower wintertime insolation results in higher partitioning of NOx toward NO2, and thus less available NO, thus even the experiment with highest initial NOx may be dominated by RO2/HO2 self and crossreactions later in the experiment, making the chemistry fairly similar among all three limonene photochemical experiments.
For all three HC/NOx ratios, the ranges of all parameters (f43, f44 H:C and O:C, ) are similar for similar ranges of mrs. mr versus f43, f44, and H:C also all follow the same trends with slightly different slopes. However, O:C versus mr trends in one direction for the two lower HC/NOx ratios; mrs increase with decreasing O:C whereas the highest HC/NOx ratio (lowest NOx) has the opposite trend. Cappa et al. (Citation2011) showed in a previous study that heterogeneous OH oxidation of the squalene and azelaic acid increases refractive index with addition of oxygen (increasing O:C). However, our other cases (HC/NOx = 6.6, 14) show the opposite relationship, suggesting that O:C ratio is not the only factor controlling the refractive index.
Chemical characterizations of SOA typically find significant concentrations of peroxides from RO2 + HO2 under high HC/NOx conditions. At low HC/NOx ratios, organic nitrates and other nonperoxide products from RO2 + NO2 and RO2 + RO2 reactions dominate (Presto et al. Citation2005; Kroll and Seinfeld Citation2008). Fragmentation of organic compounds in the AMS, however, may mask differences between the dominant reaction pathways for HC/NOx; RO2 + NO versus RO2 + HO2 and RO2 + RO2. Difficulty differentiating chemical composition of high versus low HC/NOx SOA with an AMS has been discussed in many previous studies (Chhabra et al. Citation2010; Farmer et al. Citation2010; Rollins et al. Citation2010).
3.4. Oligomerization and Aging Reactions
The presence of oligomeric species is well documented, especially as particles grow and as oxidation progresses (Surratt et al. Citation2006; Heaton et al. Citation2009; Gao et al. Citation2010). The AMS does not probe oligomers directly, however, increasing H:C ratios are consistent with oligomerization, as many oligomers are formed via reactions that release oxygen (such as condensation reactions) (Reinhardt et al. Citation2007). Refractive indices of oligomeric compounds are expected to be at the upper end of the observed values, in the 1.55–1.60 range, because of their higher polarizability (Reinhardt et al. Citation2007; Redmond and Thompson Citation2011). As a result, data presented here relating increases in H:C and mr are consistent with a role for oligomerization. However, there are several aspects of the results that are more consistent with increasing condensation of lower volatility, more hydrocarbon-like species later in the experiments: First, this is the simplest explanation. Second, the fragments with the strongest positive correlations with mr, such as C4H7 + have been previously shown to be associated with hydrocarbon-like species (Zhang et al. Citation2007). Third, Kim and Paulson (Citation2013) showed that most limonene and α-pinene particles shrank upon heating, and in the process returned to the mr corresponding to the smaller particle size as observed earlier in the same experiment. This is most consistent with either simple evaporation of recently condensed material or rapidly reversible oligomerization reactions. Fourth, the time scale of our experiments is relatively short, suggesting oligomerization reactions may be modest. Next, α-pinene refractive indices change little throughout experiments, suggesting that at least for α-pinene, if oligomerization reactions happen to an appreciable extent, they do not change the refractive index much. A counter argument is as follows: given that at lower temperatures, higher volatility material should condense, given the arguments above, lower temperature experiments might be expected to produce SOA with higher refractive indices. The results for the 30 January experiment follow the expected trend with somewhat lower O:C and higher H:C ratios than α-pinene SOA from warmer experiments. Because oligomerization reactions should be somewhat slower at lower temperatures, the argument could be made that there might be less oligomers at lower temperatures. On the other hand, as described above, a small handful of studies have found that α-pinene SOA generated at lower temperatures have different chemical composition than SOA generated at higher temperatures, potentially due to temperature-dependent differences in the gas phase reactions (Warren et al. Citation2009; Salo et al. Citation2012). None of these arguments eliminates a role for oligomerization in explaining the changes observed here, but they do suggest that oligomerization may not play a dominant role.
Aging, in contrast, should increase O:C and increase hygroscopicity (Hall et al. Citation2013), potentially enhancing water uptake and lowering the refractive index. Aging is consistent with the decrease in mr that is sometimes observed toward the end of experiments (18 January and data in Kim and Paulson [Citation2013]).
4. SUMMARY AND ATMOSPHERIC IMPLICATIONS
The refractive index and chemical composition of different types of SOA generated under different oxidation chemistries and precursors are investigated in an effort to connect optical properties to chemical composition. Both limonene refractive index and AMS parameters vary widely, in contrast to the same values for α-pinene, which vary little. Strong correlations are observed between limonene refractive indices and particular fragment ions, and other chemical properties such as the O:C ratio. The same relationships are difficult to discern for α-pinene SOA. Unlike other parameters measured with the HR-ToF-AMS, H:C ratio versus refractive index shows a strong, reasonably consistent positive relationship for all experiments with the exception of one of the α-pinene ozonolysis experiments. This is consistent with the notion that condensation of less oxygenated, more volatile species increases the refractive index. However, there are some cases for which H:C cannot explain the variations of mrs, suggesting that there are additional factors controlling the mrs, such as O:C ratio. For limonene, the best correlation with refractive indices is with the hydrocarbon-like minor peak at m/z 55 (C4H7 +). Most results are more consistent with condensation of less polar, more hydrocarbon-like species as the experiments progress (especially for limonene), but roles for oligomerization and aging cannot be ruled out. Finally, as previously noted (Kim et al. Citation2012), a single value for SOA refractive index does not appear to be sufficient to accurately model radiative transfer.
FUNDING
Hwajin Kim was supported with a UCLA dissertation year fellowship. Hwajin Kim also received support from the Korea Institute of Science and Technology. Lynn M. Russell and Shang Liu received support from NSF grant ATM0904203 for AMS, FTIR, and NEXAFS measurements and analysis.
SUPPLEMENTAL MATERIAL
Supplemental data for this article can be accessed on the publisher's website.
Supplemental_Files_for_Publication.zip
Download Zip (189 KB)ACKNOWLEDGMENTS
The authors gratefully acknowledge assistance with experiments from Ms. Michelle Kuang.
REFERENCES
- Aiken , A. C. , Decarlo , P. F. , Kroll , J. H. , Worsnop , D. R. , Huffman , J. A. Docherty , K. S. 2008 . O/C and OM/OC Ratios of Primary, Secondary, and Ambient Organic Aerosols with High-Resolution Time-of-Flight Aerosol Mass Spectrometry . Environ. Sci. Technol. , 42 : 4478 – 4485 .
- Bahreini , R. , Keywood , M. D. , Ng , N. L. , Varutbangkul , V. , Gao , S. Flagan , R. C. 2005 . Measurements of Secondary Organic Aerosol from Oxidation of Cycloalkenes, Terpenes, and m-Xylene Using an Aerodyne Aerosol Mass Spectrometer . Environ. Sci. Technol. , 39 : 5674 – 5688 .
- Barkey , B. , Paulson , S. E. and Chung , A. 2007 . Genetic Algorithm Inversion of Dual Polarization Polar Nephelometer Data to Determine Aerosol Refractive Index . Aerosol Sci. Technol. , 41 : 751 – 760 .
- Cappa , C. D. , Che , D. L. , Kessler , S. H. , Kroll , J. H. and Wilson , K. R. 2011 . Variations in Organic Aerosol Optical and Hygroscopic Properties upon Heterogenous OH Oxidation . J. Geophys. Res. , 116 : 12
- Chan , A. W. H. , Chan , M. N. , Surratt , J. D. , Chhabra , P. S. , Loza , C. L. Crounse , J. D. 2010 . Role of Aldehyde Chemistry and NOx Concentrations in Secondary Organic Aerosol Formation . Atmos. Chem. Phys. , 10 : 7169 – 7188 .
- Chhabra , P. S. , Flagan , R. C. and Seinfeld , J. H. 2010 . Elemental Analysis of Chamber Organic Aerosol Using an Aerodyne High-Resolution Aerosol Mass Spectrometer . Atmos. Chem. Phys. , 10 : 4111 – 4131 .
- Chhabra , P. S. , Ng , N. L. , Canagaratna , M. R. , Corrigan , A. L. , Russell , L. M. Worsnop , D. R. 2011 . Elemental Composition and Oxidation of Chamber Organic Aerosol . Atmos. Chem. Phys. , 11 : 8827 – 8845 .
- DeCarlo , P. F. , Kimmel , J. R. , Trimborn , A. , Northway , M. J. , Jayne , J. T. Aiken , A. C. 2006 . Field-Deployable, High-Resolution, Time-of-Flight Aerosol Mass Spectrometer . Anal. Chem. , 78 : 8281 – 8289 .
- Duplissy , J. , Gysel , M. , Alfarra , M. R. , Dommen , J. , Metzger , A. Prevot , A. S. H. 2008 . Cloud Forming Potential of Secondary Organic Aerosol Under Near Atmospheric Conditions . Geophys. Res. Lett. , 35 L03818, DOI: 10.1029/2007GL031075
- Farmer , D. K. , Matsunaga , A. , Docherty , K. S. , Surratt , J. D. , Seinfeld , J. H. Ziemann , P. J. 2010 . Response of an Aerosol Mass Spectrometer to Organonitrates and Organosulfates and Implications for Atmospheric Chemistry . Proc. Natl. Acad. Sci. USA , 107 : 6670 – 6675 .
- Finlayson-Pitts , B. J. and Pitts , J. N. 1999 . Chemistry of the Upper and Lower Atmosphere; Theory, Experiments, and Applications , 1040 San Diego : Academic Press .
- Gao , Y. Q. , Hall , W. A. and Johnston , M. V. 2010 . Molecular Composition of Monoterpene Secondary Organic Aerosol at Low Mass Loading . Environ. Sci. Technol. , 44 : 7897 – 7902 .
- Hall , W. A. , Pennington , M. R. and Johnston , M. V. 2013 . Molecular Transformations Accompanying the Aging of Laboratory Secondary Organic Aerosol . Environ. Sci. Technol. , 47 : 2230 – 2237 .
- Hallquist , M. , Wenger , J. C. , Baltensperger , U. , Rudich , Y. , Simpson , D. Claeys , M. 2009 . The Formation, Properties and Impact of Secondary Organic Aerosol: Current and Emerging Issues . Atmos. Chem. Phys. , 9 : 5155 – 5236 .
- Heaton , K. J. , Sleighter , R. L. , Hatcher , P. G. , Hall , W. A. and Johnston , M. V. 2009 . Composition Domains in Monoterpene Secondary Organic Aerosol . Environ. Sci. Technol. , 43 : 7797 – 7802 .
- Intergovernmental Panel on Climate Change . 2007 . Climate Change 2007: The Physical Science Basis. In: The Fourth Assessment of the Intergovernmental Panel on Climage Change . S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, and H. L. Miller, eds. Cambridge University Press, Cambridge, UK, and NY, USA
- Kim , H. , Barkey , B. and Paulson , S. E. 2010 . Real Refractive Indices of alpha- and beta-Pinene and Toluene Secondary Organic Aerosols Generated from Ozonolysis and Photo-Oxidation . J. Geophys. Res.-Atmos. , 115 : 10
- Kim , H. , Barkey , B. and Paulson , S. E. 2012 . Real Refractive Indices and Formation Yields of Secondary Organic Aerosol Generated from Photooxidation of Limonene and α-Pinene: The Effect of the HC/NOx Ratio . J. Phys. Chem. A , 116 : 6059 – 6067 .
- Kim , H. and Paulson , S. E. 2013 . Real Refractive Indices and Volatility of Secondary Organic Aerosol Generated from Photooxidation and Ozonolysis of Limonene, α-Pinene and Toluene . Atmos. Chem. Phys. , 13 : 7711
- Kostenidou , E. , Pathak , R. K. and Pandis , S. N. 2007 . An Algorithm for the Calculation of Secondary Organic Aerosol Density Combining AMS and SMPS Data . Aerosol Sci. Technol. , 41 : 1002 – 1010 .
- Kroll , J. H. and Seinfeld , J. H. 2008 . Chemistry of Secondary Organic Aerosol: Formation and Evolution of Low-Volatility Organics in the Atmosphere . Atmos. Environ. , 42 : 3593 – 3624 .
- Leungsakul , S. , Jeffries , H. E. and Kamens , R. M. 2005 . A Kinetic Mechanism for Predicting Secondary Aerosol Formation from the Reactions of d-Limonene in the Presence of Oxides of Nitrogen and Natural Sunlight . Atmos. Environ. , 39 : 7063 – 7082 .
- Mishchenko , M. I. , Cairns , B. , Kopp , G. , Schueler , C. F. , Fafaul , B. A. Hansen , J. E. 2007 . Accurate Monitoring of Terrestrial Aerosols and Total Solar Irradiance: Introducing the Glory Mission . Bull. Amer. Meteor. Soc. , 88 : 677 – 691 .
- Ng , N. L. , Canagaratna , M. R. , Zhang , Q. , Jimenez , J. L. , Tian , J. Ulbrich , I. M. 2010 . Organic aerosol Components Observed in Northern Hemispheric Datasets from Aerosol Mass Spectrometry . Atmos. Chem. Phys. , 10 : 4625 – 4641 .
- Paulson , S. E. , Chung , M. , Sen , A. D. and Orzechowska , G. 1998 . Measurement of OH Radical Formation from the Reaction of Ozone with Several Biogenic Alkenes . J. Geophys. Res.-Atmos. , 103 : 25533 – 25539 .
- Presto , A. A. , Hartz , K. E. H. and Donahue , N. M. 2005 . Secondary Organic Aerosol Production from Terpene Ozonolysis. 2. Effect of NOx Concentration . Environ. Sci. Technol. , 39 : 7046 – 7054 .
- Redmond , H. and Thompson , J. E. 2011 . Evaluation of a Quantitative Structure-Property Relationship (QSPR) for Predicting Mid-Visible Refractive Index of Secondary Organic Aerosol (SOA) . Phys. Chem. Chem. Phys. , 13 : 6872 – 6882 .
- Reinhardt , A. , Emmenegger , C. , Gerrits , B. , Panse , C. , Dommen , J. Baltensperger , U. 2007 . Ultrahigh Mass Resolution and Accurate Mass Measurements as a Tool to Characterize Oligomers in Secondary Organic Aerosols . Anal. Chem. , 79 : 4074 – 4082 .
- Rollins , A. W. , Smith , J. D. , Wilson , K. R. and Cohen , R. C. 2010 . Real Time In Situ Detection of Organic Nitrates in Atmospheric Aerosols . Environ. Sci. Technol. , 44 : 5540 – 5545 .
- Salo , K. , Hallquist , M. , Jonsson , A. M. , Saathoff , H. , Naumann , K. H. Spindler , C. 2012 . Volatility of Secondary Organic Aerosol During OH Radical Induced Ageing . Atmos. Chem. Phys. , 11 : 11055 – 11067 .
- Shilling , J. E. , Chen , Q. , King , S. M. , Rosenoern , T. , Kroll , J. H. Worsnop , D. R. 2009 . Loading-Dependent Elemental Composition of alpha-Pinene SOA Particles . Atmos. Chem. Phys. , 9 : 771 – 782 .
- Surratt , J. D. , Murphy , S. M. , Kroll , J. H. , Ng , N. L. , Hildebrandt , L. Sorooshian , A. 2006 . Chemical Composition of Secondary Organic Aerosol Formed from the Photooxidation of Isoprene . J. Phys. Chem. A , 110 : 9665 – 9690 .
- VanReken , T. M. , Ng , N. L. , Flagan , R. C. and Seinfeld , J. H. 2005 . Cloud Condensation Nucleus Activation Properties of Biogenic Secondary Organic Aerosol . J. Geophys. Res.-Atmos. , 110 : 9
- Warren , B. , Austin , R. L. and Cocker , D. R. 2009 . Temperature Dependence of Secondary Organic Aerosol . Atmos. Environ. , 43 : 3548 – 3555 .
- Yu , Y. , Ezell , M. J. , Zelenyuk , A. , Imre , D. , Alexander , L. Ortega , J. 2008 . Photooxidation of alpha-Pinene at High Relative Humidity in the Presence of Increasing Concentrations of NO(x) . Atmos. Environ. , 42 : 5044 – 5060 .
- Zhang , Q. , Jimenez , J. L. , Canagaratna , M. R. , Allan , J. D. , Coe , H. Ulbrich , I. 2007 . Ubiquity and Dominance of Oxygenated Species in Organic Aerosols in Anthropogenically-Influenced Northern Hemisphere Midlatitudes . Geophys. Res. Lett. , 34 : 6
