ABSTRACT
This article presents a new way to model the narrow band ultraviolet (UV-C, 254.7 nm) radiation dose–response behavior of Bacillus spores in clusters based on knowledge of the UV-C dose–response behavior of the single spores. The approach is demonstrated using experimentally obtained survival rates for Bacillus atrophaeus var. globigii (BG) spores and BG spore clusters of several sizes exposed to UV-C fluence when aerosolized and when deposited on dry surfaces. These results are modeled to derive predicted survival rates for spores in clusters of arbitrary cluster diameter under similar conditions. The essential feature of the approach is accurate accounting for attenuation of incident UV fluence within the spore cluster to derive the fluence incident on individual spores within the cluster. The results suggest that UV fluence attenuation by bacterial spores may increase with increasing fluence, a phenomenon that has not been previously reported for bacterial spores. The results are of utility in estimating dispersed biological hazards and evaluating the effectiveness of ultraviolet germicidal irradiation (UVGI) systems.
Copyright © 2016 American Association for Aerosol Research
EDITOR:
Introduction
The release of pathogenic Bacillus spores into the environment, either indoors or outdoors, can adversely affect living organisms. Bacillus anthracis (Ba) can be released purposefully as a biological warfare agent (BWA), as a terrorist weapon, or accidentally as occurred in 1979 at Sverdlosk (Meselson et al. Citation1994). Exposure to ultraviolet radiation (UV), either solar UV during outdoor transport and dispersion or UV-C in ultraviolet germicidal irradiation (UVGI) systems, has been demonstrated to reduce the potential adverse effects by inducing damage to DNA that renders Bacillus spores such as Ba inactive. The mechanism of UV inactivation of spores has been investigated extensively (Riesenman and Nicholson Citation2000; Sinha and Hader Citation2002; Weber Citation2005; Pattison and Davies Citation2006; Setlow Citation2006; Moeller et al. Citation2009; Kowalski Citation2011) and involves species, strain, and preparation dependent (1) spore protective mechanisms, (2) photochemistry of the UV interactions with the DNA, and (3) repair mechanisms available to the spores, typically during and after germination. A variety of photochemical reactions leading to inactivation involve UV photon-induced breakage of the hydrogen bonds that link DNA base pairs and formation of a variety of photoproducts that result in lesions in the DNA.
Experimental data has been obtained to characterize the UV-C dose–response behavior of a variety of Bacillus strains dispersed as single spores in air (Nicholson and Galeano Citation2003; Blatchley et al. Citation2005; Lim and Blatchley Citation2012). Such data can be used to estimate dispersed single spore hazards generated by computer models that simulate transport and dispersion of releases as well as to evaluate the effectiveness of UVGI systems. In addition, benign or less pathogenic organisms such as Bacillus subtilis, Bacillus cereus, and Bacillus atrophaeous have been experimentally characterized to use as surrogates for B. anthracis (Lim and Blatchley Citation2012) or as solar spore dosimeters (Munakata et al. Citation2000). To support hazard estimation and UVGI system design and evaluation, analytical models have been developed that allow accurate predictions of single spore inactivation dose–response, given the magnitude and spectral composition of the UV fluence on the spore (Kowalski and Bahnfleth Citation2000). Currently, hazard prediction computer codes use simple exponential decay models to reduce computational burden (Sykes et al. Citation2011). For UVGI system design and evaluation, models for single spore dose–response have been developed and show significant utility including the multi-hit model and the series-event model (Severin et al. Citation1983; Pennel et al. Citation2008).
However, modeling UV inactivation of spores to assess environmental releases is problematic since measurements have generally been performed on single spores, whereas environmental releases involve spore clusters (Lighthart, et al. Citation1991; Matsumoto Citation2003). Bacillus spores (including Ba) tend to clump when released due to sticky chains of proteins and sugar molecules on their surfaces and the attractive influence of van der Waals forces, and sophisticated preparation techniques are required to avoid such clumping (Matsumoto Citation2003; Duncan and Ho Citation2008). Liquid slurry aerosolization produces drop sizes ranging from a few microns to several hundreds of microns, and the resulting dry cluster diameters range between a few and a few tens of microns (Lighthart et al. Citation1991). Release of dry BG has been reported to produce cluster sizes from two to nine microns (Holler et al. Citation1998; Duncan and Ho Citation2008). Clusters larger than about ten microns are too heavy to remain airborne to present an inhalation hazard or to reach the narrowest passages in the lung and cause infection (Hofmann and Koblinger Citation1992; Hofmann Citation2011). Therefore, realistic hazard assessments and UVGI effectiveness evaluation require understanding the danger posed by clusters of spores in the size range from one to ten microns. Clustering of spores can protect the inner spores from the effects of the UV radiation and enhance survivability of the spores and thus affect hazard estimates for outdoor releases as well as effectiveness of UVGI system designs (Kesavan et al. Citation2014). The net impact of clustering of spores (such as anthrax) on hazards presented to humans by outdoor releases depends on a number of factors in addition to just UV survivability. These factors include meteorological conditions such as wind speed and stability class, variation with particle size of the rate of cluster deposition onto the ground, variation of the intensity of solar irradiance, variation with particle size of the infectivity of inhaled spores, and downwind distance from the initial release. A previous paper (Handler and Edmonds Citation2015) considered the net impact of all of the above factors on hazards presented by outdoor releases of clustered spores of various diameters up to 10 μm and compared the results to hazards presented by single spores. A simplified form of the model presented in this article was used, also based on the data from Kesavan et al. Citation(2014), and only aerosolized UV decay was considered. The result was that release of clustered spores can present significantly greater hazards to humans, depending on the meteorological conditions, downwind distance, and amount of solar irradiance. The work presented here provides a more detailed model for UV-C decay of aerosolized clusters and extends the model to UV-C decay of clusters on surfaces. The focus of the work presented here is on the model of the decay process itself and net impacts on hazard estimates are not examined.
The dose–response of spores within clusters is affected by the attenuation of fluence as it propagates through spores (and possibly other material) within the cluster. Experiments performed on Mycobacterium terrae in water found that aggregates of organisms showed evidence of reduced inactivation due to shielding of some of the organisms by the extended aggregate (Bohrerova and Linden Citation2006). This was achieved by spiking water samples with M. terrae aggregates distributed over sizes ranging from single organisms to aggregates over 100 mm. The spiked sample was then filtered sequentially through 100-, 41-, and 20-μm nylon net filters and the filtrates were subsequently exposed to UV-C irradiance. Analysis of that data in Pennel et al. Citation(2008) applied a series-event model with a shielding factor multiplying the fluence term and found that doing so found 7.3%, 2.5%, 2.0%, and 2.27% shielding of organisms by the aggregate for the unfiltered, 100-μm, 41-μm, and 20-μm filtrates, respectively. Since the organisms and particulates were water born, the aggregate geometries were variable and amorphous, such that the shielding was not able to incorporate a fixed geometry, instead giving a statistical average over aggregates within given size ranges.
The data obtained by Kesavan et al. Citation(2014) shows (see that when clusters of spores up to 4.4 microns in diameter are exposed to narrow band UV-C, the fraction of the spores inside the clusters that are killed reaches and slightly exceeds 99%. This is despite the fact that more than half of the spores inside the clusters (more than 75% for the case of spores exposed on a surface) are completely shielded from direct exposure by spores on the surface and in the intervening distance between the inside spore and the cluster outer shell of spores. The work reported here investigates the hypothesis that the decay of spores inside the cluster can be quantitatively predicted by accounting for the attenuation of the UV-C as it traverses the intervening spores between the cluster surface and the spores inside. The research described herein develops a new way of modeling the UV-C dose–response of spores in clusters, based on knowledge of the UV-C dose–response of single spores. The model is very simplified in that it assumes that light travels through the spore without reflecting or refracting at any of the interfaces, although it can be attenuated by absorption. The experimental data for BG presented in Kesavan et al. Citation(2014) is used to demonstrate that it is possible to predict the dose–response to narrow band UV-C of spores contained within clusters. This is demonstrated by accounting for the UV-C fluence reaching spores within clusters after attenuation by intervening material in the cluster and using the single spore dose–response to narrow band UV-C.
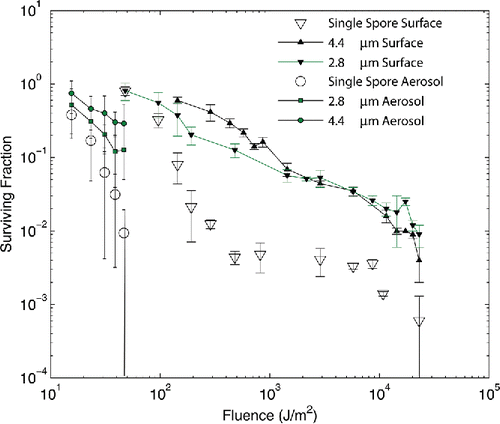
Figure 1. Measured survival rates from Kesavan et al. Citation(2014). Surviving fraction is plotted versus UV-C fluence. Single spore measurements on surfaces are shown as open triangles and when aerosolized as open circles. Cluster data are plotted as filled symbols: filled squares for aerosolized 2.8 μm clusters and filled circles for aerosolized 4.4 μm clusters, filled downward pointing triangles plot data for 2.8 μm clusters on surfaces and filled upward pointing triangles plot data for 4.4 μm clusters on surfaces. Error bars shown are 1 standard deviation.
Methods
Experimental data
The modeling work described here was made possible by the reporting of the first quantitative measurement of UV-C inactivation of Bacillus spore clusters of several definite monodispersed sizes, dry and contaminant-free, when aerosolized and surface-bound (Kesavan et al. Citation2014). Further, the UV-C inactivation of the single spores that comprised the spores was also measured under the same preparation and exposure conditions. Details of the experimental techniques can be found in Kesavan et al. Citation(2014). Here only the essentials will be sketched, with emphasis on aspects of particular relevance to the modeling done in this research. Spores of B. atrophaeus subspecies globigii (BG) were prepared using methods validated in previous studies (Kesavan and Doherty Citation1999; Kesavan et al. Citation2008; King et al. Citation2011). An ink jet aerosol generator (IJAG) (manufactured by U.S. Army Edgewood Chemical Biological Center) was used to generate single spores and clusters for the fixed-particle studies on surfaces. It is described in Kesavan et al. Citation(2008). A Collison nebulizer (BGI Inc., Waltham, MA, USA) was used to generate single spores for the aerosol studies and a SonoTek aerosol generator (Sono-Tek Corporation, Milton, NY, USA) was used to generate clusters for the aerosol testing. The single spore and cluster sizes were determined with an aerodynamic particle sizer (APS, TSI Inc., Shoreview, MN, USA) to be 1.2 (1.5) μm, 2.8 (1.3) µm, and 4.4 (1.3) µm, respectively, reported as mass median diameter (geometric standard deviation). The number of spores per cluster particle was determined microscopically to be:[1]
Single spores and clusters were deposited on polycarbonate membrane filters for the UV surface exposure, with microscopic confirmation of isolated particle deposition. The test filters were exposed to UV-C lamp irradiation for lengths of time, which provided the desired irradiation doses. After irradiation, filters were removed from the exposure chamber and the surviving fraction determined by comparing to controls (no irradiation) as described in Kesavan et al. Citation(2014). Aerosol experiments were conducted in a 0.14 m3 chamber as shown in Kesavan et al. (Citation2014, figure 1). Spores and clusters were injected into the chamber aerodynamically by feeding the outlet tubes of the Collison nebulizer or SonoTek aerosol generator, as described above, through a port in the wall of the chamber and the chamber air was mixed by a small fan. After selected time periods of UV-C irradiation, samples were drawn from the chamber and cultured to determine the surviving fraction, by comparing to controls (no irradiation).
The fluence of the aerosol test chamber was measured by dividing the chamber into a 5 × 5 × 3 matrix and by measuring the flux at each of those locations as shown in Kesavan et al. Citation(2014, figure 1), with the measurement plane of the meter pointing straight upward. From these measurements the average flux of the chamber was calculated by averaging over the measured values and the fluence was determined by multiplying the average of the flux readings by the exposure time. Previous sampling of aerosolized BG spores in the chamber found that the spores were well mixed by the circulating fan and differences in spore concentrations in different locations within the chamber were negligible (King et al. Citation2011). Kesavan et al. (2014) assumed each particle spent identical amounts of time in each section of the chamber volume.
Experimental dose–response for single spores and clusters
The measured survival rates for single spore and cluster data on surfaces and aerosolized are shown in , plotting surviving fraction of spores as a function of UV-C fluence in J/m2 as reported in Kesavan et al. Citation(2014). Exposures were limited for the aerosolized case to relatively low exposure times to maintain sufficient signal to noise, resulting in lower fluence being attainable. Aerosolized spores and clusters appear to be about an order of magnitude more sensitive than similar particles on surfaces and single spores are up to an order of magnitude more sensitive than clusters in similar exposure conditions. The surviving fraction for 2.8 μm clusters counterintuitively is identical to that for single spores at the lowest fluence (48 J/m2) and exceeds the surviving fraction of the 4.4 μm spores at large fluence. Also, the standard deviations for the surface 2.8 μm data are significantly larger than those for the 4.4 μm cluster data, particularly for the three lowest fluence, highest surviving fraction data points.
Modeling dose–response of single spores
Two kinetic models for dose–response were applied to the single spore data: the multi-hit model and a series-event model (Severin et al. Citation1983). The multi-hit model has the form (Kowalski et al. Citation2000)[2] where k1 is the inactivation constant of the susceptible subpopulation (m2/J), n1 is the threshold event level at which inactivation occurs in the susceptible population, f is the fraction of the total population that is susceptible, k2 is the inactivation constant of the resistant subpopulation (m2/J), n2 is the threshold event level at which inactivation occurs in the resistant subpopulation, F is the UV dose or fluence applied (J/m2), N(F) is the number of spores that survive after dose F, N0 is the initial number of spores, and S(F) is the surviving fraction. The dose
, the integral of the incident flux I over time t. If I is constant, then F = I•t. Experiments on B. subtilis spores indicate that the effective dose F is independent of the rate of delivery of photons so that the dose is simply equal to the integrated flux (Rice and Ewell Citation2001). If no resistant population is evident, f = 1.
The series-event model has the form (Pennel et al. Citation2008; Lim and Blatchley Citation2012)[3] where here the constants are as defined for Equation Equation(2)
[2] and “i” is the index of summation and ranges from 0 to n1 – 1 or n2 – 1 as indicated. Equation Equation(3)
[3] differs from the form used by Pennel et al. Citation(2008) and Lim and Blatchley Citation(2012) in that the second term, for the resistant population, contains a series-event term instead of a simple exponential.
Modeling dose–response of spore clusters
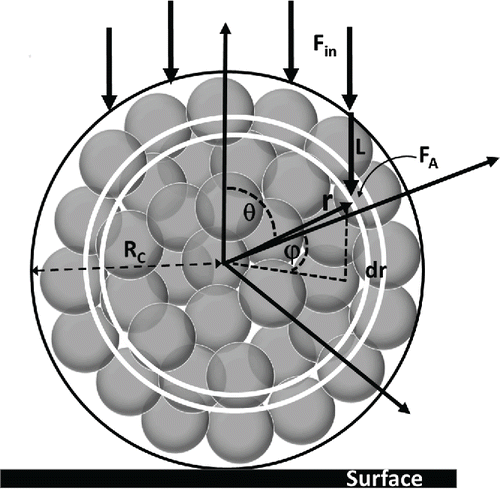
The exposures in Kesavan et al. Citation(2014) were done on dry spores and clusters in air, such that the clusters had rigid and fixed geometries. Using this geometry, if SSS(F) is the surviving fraction of single spores after exposure to fluence F, then the surviving fraction of spores located at a specific location within a cluster, Sc, should be[4] where r, ϕ, θ are spherical coordinates representing the radial distance from the spore center, the equatorial, and polar angles, respectively; FA(r, ϕ, θ) is the attenuated fluence reaching the spore location within the cluster designated by r, ϕ, θ; τ is the attenuation coefficient of the intervening spore material in the cluster along the path length L from the surface of the cluster to the spore; ε is the fraction of the distance occupied by spore material; and Fin is the fluence incident on the spore cluster surface. The geometry is illustrated in . Spore clusters are assumed to be spherical, which simplifies the analysis but still allows assessment of the utility of the approach. The very simplified model applied here uses the straight line distance from a spore location within the cluster to the cluster surface, along the line of incidence of the incoming irradiance, as a crude measure of the potential relative attenuation lengths for radiation reaching the spore location. A more detailed calculation of the light intensity within the cluster accounting for refraction and attenuation within the cluster, including detailed accounting for internal air-spore boundaries, would be necessary to improve estimates of irradiance on spores within the clusters (see for example, Chowdhury et al. Citation1992; Velesco and Schweiger Citation1999). The simple approach used here is intended to assess the possible benefits of the general approach and stimulate more detailed calculations. In particular, the intention is to investigate the plausibility of two key underlying hypotheses. First, that UV dose–response of single bacterial spores, uniformly prepared and exposed to narrow band UV irradiation, depends only on the actinometric (integrated over all incident angles), time integrated fluence on the organism. Second, that the first hypothesis holds for single bacterial spores bound within clean, dry clusters. (See section Discussion for some of the drawbacks of the simplified approach used here.) The clusters produced in Kesavan et al. Citation(2014) were dry clusters comprised only of spores. If the cluster contained other material, such as residual slurry material, then τε could be replaced by an average over the materials within the cluster, viz. τε = τ1ε1 + τ2ε2 + τ3ε3 … etc. The empirically determined number of spores per cluster given in Equation Equation(1)
[1] allows the estimate ε = .62, for the clusters produced in Kesavan et al. Citation(2014). If Rc is the radius of the cluster, then
−rcosθ under these assumptions.
Figure 2. Geometry of spore cluster exposure. Spores are indicated as shaded circles. A dark circle indicates the outer boundary of the cluster, which has radius RC. A polar coordinate system is used where r is the radial distance from the spore center, θ is the polar angle from the upward pointing axis, and ϕ is the azimuthal angle. The incoming UV-C flux is denoted by Fin. The model evaluates the attenuated fluence FA at a location in the spore given by (r, ϕ, θ) and considers the length L from the surface of the cluster to the point of evaluation as an approximation of the effective attenuation length. The two white circles illustrate the integration interval dr. Integration intervals for dϕ and dθ are not shown. The surface on which the cluster rests is shown by the black rectangle.
Given the single spore dose–response obtained from the data expressed by either Equation Equation(2)[2] or Equation Equation(3)
[3] and using Equation Equation(4)
[4] the fraction of spores surviving within a cluster is obtained by integrating over the cluster volume
[5] where ρ is the density (number of spores per unit volume) of spores within the cluster and Rc is the radius of the cluster. Equation Equation(5)
[5] can be fit to the data using nonlinear regression, performing the triple integral numerically, to obtain the value of τ that best fits the cluster dose–response data. Nonlinear regression was performed using Matlab routine nlinfit, which is part of the statistics toolbox and called in the form [beta,r,J,COVB,mse] = nlinfit(X,y,fun,beta0) (MathWorks Citation2015). In the call, X is the fluence, y is the surviving fraction measured, fun is the function nonlinearly regressed on the X, y data, and beta0 is the vector of initial values of the fit parameters. To examine possible differences from using either multi-hit or series-event models for the single spore dose–response, regressions on the cluster dose–response were performed using both Equations Equation(2) and (3) as the regression function denoted fun in the call to nlinfit, with the values of k1, k2, n1, and n2 determined by the regressions on the single spore dose–response. The fit parameters obtained by regression on the cluster does–response are contained in the functional form of τ in Equation Equation(5)
[5] , which is of the form
, where τ0, χ, and k3 are the fitted parameters. This expression for τ is discussed and derived below in the Results section. Explicit forms of the regression functions are given in the online supplementary information (SI). Note that Equation Equation(5)
[5] does not require a mechanistic representation of single spore dose–response, SSS(F), such as the commonly used multi-hit or series-event fits. A spherical cluster admits several symmetries that simplify the numerical integration. For spore clusters exposed on a surface, assuming plane incident UV radiation and ignoring refraction, symmetry about the polar axis removes the need to integrate over the equatorial angle ϕ at each value of r and θ, and simply multiply by 2π. For aerosolized spore clusters, assuming the spore clusters rotate randomly when suspended averages the attenuated fluence received over the polar and equatorial angles, for given r, and the averaged attenuated fluence applies for all spores within the cluster at that radius. Finally, although spores are shown as spheres in , Equation Equation(5)
[5] treats spores within the cluster as a continuous distribution of spores with density
, using the experimentally determined Equation Equation(1)
[1] . This value agrees with the measured volume of BG spores (Carrera et al. Citation2007) 0.273 μm3 and the packing fraction measured by Kesavan et al. Citation(2014) of 0.62.
Results
Single spore dose–response
The data obtained in Kesavan et al. Citation(2014) for single spores exposed on a surface covered a range of fluence from 50 J/m2 to over 2 × 104 J/m2 ( and shows evidence of shoulder behavior in both the susceptible and resistant subpopulations. Most previous work extended only to fluence of order 102 J/m2 and consequently has not shown shoulder behavior in resistant subpopulations (Pennel et al. Citation2008; King et al. Citation2011; Lim and Blatchely Citation2012). Previous applications of Equations Equation(2) and (3) have thus used a simple exponential for the resistant population dose–response. The analysis presented here models both susceptible and resistant populations (for the surface exposures) with the same functional form as indicated in Equations Equation(2) and (3). The data from Kesavan et al. Citation(2014) for aerosolized single spores did not extend to high-enough fluence to resolve the resistant subpopulation and so that data is modeled here as a single, susceptible population.
For single spores on surfaces, the data were fit with nonlinear regression using both the multi-hit Equation Equation(2)[2] and the series-event Equation Equation(3)
[3] . The multi-hit model was evaluated for the case of integer-constrained n values as well as unconstrained n values. The results are shown in . Since the survival rates at high fluence for the resistant subpopulation are several orders of magnitude below the rates for the susceptible population at lower fluence, regression using both populations together and minimizing residual errors has limited sensitivity to resistant population parameters. Therefore, the regressions were performed iteratively for both Equations Equation(2) and (3). First, the data at low fluence, below 400 J/m2, were regressed to determine initial estimates of k1 and n1. Regressions were iterated over values of n1 to determine the minimum mean square error for the series-event model and the integer-constrained multi-hit model. The initial values of k1 and n1 were then used as fixed parameters for a two-population fit of the data above 400 J/m2, which determines f (and hence 1 – f by constraint), k2, and n2, again iterating over n2 values to minimize the mean square error. Then the values of k2 and n2 were used as fixed parameters for regression of the low fluence data for revised estimates of k1, n1, and f. The process was repeated until the values for parameters and mean square error changed by less than one part in 104.
Table 1. Fits of series-event and multi-hit models of UV dose–response behavior of Bacillus atrophaeous single spores in air on surfaces and aerosolized using data from Kesavan et al. Citation(2014).
For aerosolized single spores, nonlinear regressions were done on the data for a single population (f = 1), iterated over n for the integer-constrained cases (series-event and multi-hit) and with unconstrained n for the unconstrained multi-hit case. The aerosolized results are also shown in .
Plots of the data and the fitted equations are shown in for both the aerosolized and surface data. The lines for the series-event and multi-hit fits are not distinguishable. For prediction of cluster dose–response, the particular form of the single spore dose–response is unimportant so long as the fit is a good predictor of the behavior.
Figure 3. Fits of survival fractions to experimental data from Kesavan et al. Citation(2014). Surviving fraction of spores after exposure to UV-C is plotted as a function of the exposure fluence.
Dose–response for spores in clusters
The attenuation experienced by fluence before it reaches spores in the cluster depends on the type of exposure (aerosolized or on a surface) as discussed above. Aerosolized spore clusters are assumed to rotate randomly, taking on all values of ϕ and θ equally. Aerosolized exposure can be called brasa exposure (as in cooking a la brasa or on a rotiserrie) in which the attenuation length L is averaged over both angles and is a function of the radial coordinate only. Spore clusters on a surface are exposed as shown in . Exposure of clusters on a surface can be called parilla exposure (as in cooking a la parilla or on a grill). In this exposure, configuration L is constant for all ϕ for a given r and θ. The geometric dependence of attenuation lengths on position within the clusters for both brasa and parilla cases is discussed in the SI.
The attenuation of the fluence, exp(-τεL), depends on the attenuation coefficient of the spore material, τ, which is obtained by regression of Equation Equation(5)[5] on the survival data for clusters. Fitting all of the surface data for 4.4 μm clusters using the single spore parameters given in , and assuming τ is constant, yields the value τ = 1.20 μm−1, but the predicted values fall below the surviving fraction data for fluence greater than about 103 J/m2. This behavior is illustrated in a plot in the SI, Figure S3. Fitting datasets with starting points at increasing fluence levels, i.e., deleting the first point, then the second point, etc., yields increasing estimates of τ with fluence. This behavior is illustrated in a plot in the SI, Figure S4. Since incident fluence generates a variety of photoproducts some of which could increase the attenuation, a mechanistic, dimensional interpretation of the increase of absorption suggests attenuation could be increased with fluence proportional to the number of such photoproducts formed, via an expression of the form
[6] where τ0 is the background, constant, extinction coefficient, σ is the cross section for formation of the additional photoproducts with units of area, L is the path length, μ is the number density of potential sites with units number per area, F is the fluence, k3 is the rate constant associated with the transitions, and τ1 is the extinction coefficient of the additional photoproducts, with units μm−1. The constants τ0 and χ
τ1σμ and k3 can then be determined by nonlinear regression, iteratively as described earlier.
The results are given in and plotted in . The baseline (zero fluence) regression constant τ0ε was 0.32 μm−1 and 0.68 μm−1 for 2.8 μm and 4.4 μm clusters, respectively. The average value is then 0.50 μm−1. With ε = 0.62, this corresponds to baseline attenuation of 0.52 μm−1 and 1.1 μm−1 for the 2.8 μm and 4.4 μm clusters, respectively, and an average 0.81 μm−1 for BG spores.
Figure 4. Fits of Equation Equation(5)[5] to cluster survival data on surfaces. Open circles plot the experimental data for 4.4 μm clusters on surfaces and open squares plot the data for 2.8 μm clusters. Surviving fraction is plotted versus UV-C fluence in a log10–log10 plot. Dashed and dotted lines plot the modeled prediction of the surviving fraction for the 4.4 μm and 2.8 μm cases, respectively. Note that the 4.4 μm surviving fractions are plotted on the left vertical axis and the 2.8 μm surviving fractions use the right vertical axis. The right axis is displaced upward one order of magnitude with respect to the left axis for clarity.
![Figure 4. Fits of Equation Equation(5)[5] to cluster survival data on surfaces. Open circles plot the experimental data for 4.4 μm clusters on surfaces and open squares plot the data for 2.8 μm clusters. Surviving fraction is plotted versus UV-C fluence in a log10–log10 plot. Dashed and dotted lines plot the modeled prediction of the surviving fraction for the 4.4 μm and 2.8 μm cases, respectively. Note that the 4.4 μm surviving fractions are plotted on the left vertical axis and the 2.8 μm surviving fractions use the right vertical axis. The right axis is displaced upward one order of magnitude with respect to the left axis for clarity.](/cms/asset/e8a7eff0-fae4-476f-8581-3524e1badb7a/uast_a_1137556_f0004_b.gif)
Table 2. Fits UV of dose–response behavior of Bacillus atrophaeous spore clusters in air on surfaces and aerosolized using data from Kesavan et al. Citation(2014).
Dose–response for aerosolized spore clusters
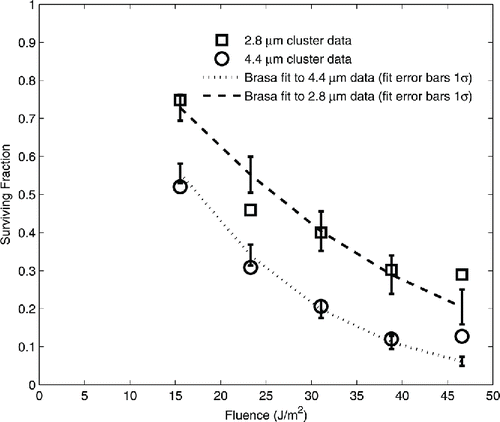
The data for aerosolized single spores and aerosolized clusters were all below 50 J/m2, and consequently did not extend high enough to show evidence of the resistant subpopulation. Equation Equation(5)[5] was regressed on this data using the single spore fit results given in . The series-event and multi-hit single spore expressions gave identical results. The fit of the brasa exposures of 2.8 μm and 4.4 μm clusters are given in and plotted in . The regression yielded τε values of 0.32 μm−1 and 0.525 μm−1 for the 2.8 μm and 4.4 μm clusters, respectively, for an average of 0.42 μm−1. With ε = 0.62, this corresponds to an average baseline attenuation of 0.68 μm−1 for the spore material.
Figure 5. Data and fits of the model to the aerosolized cluster survival data. Surviving fraction is plotted as a function of UV-C fluence. 2.8 μm cluster data are plotted as open squares. 4.4 μm data are plotted as open circles. The lines indicate the regression fit of the model to the data. Error bars show 1 standard deviation of the regression.
Discussion
Single spore results
Kesavan et al. Citation(2014) discussed differences in the narrow band 254 nm UV-C decay constants derived from this data for aerosolized and surface exposures and results of other narrow band 254 nm UV-C experiments that may be due to experimental and biological factors such as differences in culturability versus death of spores, particle placement (air or surface), particle sizes, percent of vegetative cells and spores, experimental design, test species studied, inert versus nutritive substrates, etc. The discussion here focuses on modeling and physical aspects to compare the aerosolized to the surface results from Kesavan et al. Citation(2014) and the results from Kesavan et al. Citation(2014) to other experiments. Throughout this section, decay constants, D values, and attenuation coefficients are for narrow band 254 nm UV-C irradiation unless explicitly indicated otherwise.
The primary reference measurement of BG decay was obtained by derivation from the D96 = ln(1 – 0.96)/k value, 198.26 J/m2, yielding a decay constant of k = 0.0162 m2/J (EPA Citation2006). The interpolated D96 value from the Kesavan et al. Citation(2014) data is 176.5 J/m2, corresponding to k = 0.018. It is shown in the SI that the surface derived values for single spore decay constants derived in this work agree reasonably well with the previous measured value and are internally consistent.
The aerosolized exposures yielded a multi-hit derived value for k = 0.118 m2/J with n = 2.75. As shown in the SI, this corresponds to a D96 derived value of (0.62)(0.118) = 0.07 m2/J. Detailed consideration of the dosimetry may account for the differences between the surface and aerosolized k values. As described above in methods/experimental data section, Kesavan et al. Citation(2014) derived the UV-C fluence for the aerosolized exposures by measuring the UV-C flux on the front, back, and mid planes of the chamber, and averaging these values. This approach doubly weights the readings furthest from the UV-C bulb (the front and back planes) and gives a conservative estimate of average flux. King et al. Citation(2011) evaluated the average flux in the same aerosol chamber by applying a contour analysis and using the average contour-based flux as an estimate of average flux and found an average flux of 0.54 W/m2, which is about 1.8 times the value obtained by averaging over the front, back, and mid plane UV-C meter readings, 0.31 W/m2 for that experiment. This implies the corresponding D96 derived value should be about 0.0732/1.8 = 0.0407 m2/J, which is a factor 2.5 higher than the EPA result. Applying a three-dimensional analysis of the UV-C field produced by the bulb as described in Kowalksi and Bahnfleth Citation(2000) and averaging that flux over the total box volume could further increase the average flux, reducing the derived decay constant further, but that is beyond the scope of this article.
Experimental data is available for B. subtilis exposed as an aerosol and on surfaces. Kowalski Citation(2011) provides three measurement results for spores in air and three on surfaces. Averages of the aerosolized and surface k values are 0.0262 and 0.0228 m2/J, respectively, within the overall standard deviation of 0.006 m2/J of each other, despite being taken under differing humidity. A more recent measurement (Lim and Blatchley Citation2012) yielded an average value of 0.0276 m2/J for spores on surfaces under humidities of 20%, 60%, and 100%. Low humidity apparently accounts for a small increase, about 20%, in the k value. Hence, for B. subtilis, the decay constants appear to be essentially the same for aerosolized and surface bound spores of B. subitilis. This also suggests that the high values from Kesavan et al. Citation(2014) for BG may be due to dosimetry offsets. Measurements with precision dosimetry or lagrangian actinometry (Blatchley et al. Citation2006) might resolve this uncertainty.
Cluster results
As shown in and , the modeling approach described here is capable of producing reasonable fits to aerosol and surface bound cluster UV-C dose–response data based on knowledge of single spore dose–response. The derived values for the UV-C attenuation are plotted in . The estimates for the low fluence attenuation of the spores, τ0, range from a low of 0.51 μm−1 for the 2.8 μm aerosolized clusters to a high of 1.1 μm−1 for the 4.4 μm cluster surface exposures. The values for the 2.8 μm clusters (aerosolized and surface) differ by less than a percent from the average of the two and the values for the 4.4 mm clusters differ by about ±13% from their average. Artificially adjusting the aerosolized fluence values upward by a factor of 4.5 reconciles the aerosolized decay constants with the surface and literature value of 0.016 J//m2, but this adjustment does not affect the shoulder parameters, or the resulting attenuation constants. Further, the fact that the baseline attenuation values derived from both 2.8 μm exposures are quite close suggests that the cause is associated with the cluster size, not the fluence. Imagery of BG (and other spore clusters) indicate the cluster shapes can be somewhat irregular (Holler et al. Citation1998; Duncan and Ho Citation2008), particularly at smaller diameters (compare figure 6 of Duncan and Ho Citation2008 with figure 2 of Holler et al. Citation1998). The 8 μm diameter cluster from Holler et al. Citation(1998) was produced by the same mechanism (IJAG) as the clusters used in Kesavan et al. Citation(2014) and appears nearly spherical. Measurements of fall speeds of clusters of spores of varying size and shape have indicated that fall speed, for a given overall size, is insensitive to particle shape, i.e., spherical, prolate spheroid, flat, etc. (Ferrandino and Aylor Citation1984). Hence, the APS measurements of BG cluster size in Kesavan et al. Citation(2014) did not necessarily indicate spherical shape of the clusters. In fact, the APS measurements of the single spores in Kesavan et al. Citation(2014) gave mass median diameter of 1.2 μm whereas microscopic imaging indicates BG spores are elongated, 1.2 μm × 0.65 μm (Carrera et al. 2006). Prolate spheroid or otherwise elongated or flattened cluster shape could account for reduced attenuation lengths and could change the derived attenuation constant. Reducing the 2.8 μm cluster diameter numerically to an effective diameter of 1.7 μm results in agreement of the 2.8 μm cluster derived values between aerosolized and surface exposures and with the value derived from the aerosolized 4.4 μm clusters. Imagery of the clusters could resolve this issue and if necessary the approach described here could be extended to other shapes such as prolate spheroids.
Figure 6. Derived UV-C attenuation coefficients for BG spores as a function of fluence. The UV-C attenuation coefficients, τ, derived from regression of the data are plotted as a function of fluence. The extent in fluence for the aerosolized results is exaggerated for visibility. The solid and dashed lines extending across the full range of fluence plot the variation of attenuation with fluence derived from the cluster exposures on surfaces. The short thick lines indicate the attenuation coefficients derived from the aerosolized cluster exposures to UV-C. The aerosolized exposures attained a maximum of 50 J/m2 and the attenuation coefficient plots for the aerosolized case would not be visible on the scale of the plot so the length of the short thick lines has been exaggerated for improved visibility.
The baseline (low fluence) UV-C attenuation values, from τ0ε in , for aerosolized and surface bound clusters range from a low τ0 of 0.51 μm−1 to a high of 1.1 μm−1 with an average of 0.74 μm−1. This is compared to theoretical estimates, which found a reduction of 38% through dry spores (τ0 ˜ 0.76 μm−1) (Coohill Citation1986) and a calculated complex index of refraction for dry Bacillus spores of 0.048 at 266 nm, equivalent to τ0 ˜ 1.1 μm−1 (Hill et al. Citation2013). Measurements of the index of refraction of B. subtilis spores in water and glycerol found an imaginary part of the complex index of refraction at 255 nm of 0.0138 (water) and 0.00767 (glycerol) giving τ0 values 0.33 μm−1 and 0.19 μm−1, respectively (Tuminello et al. Citation1997).
As discussed above, possible shape variations in the 2.8 μm clusters, coupled with the anomalously high values for surviving fraction at large fluence, compared to the values for the 4.4 μm clusters, may account for the low initial attenuation value and the large increase in attenuation at high fluence, compared to the 4.4 μm clusters. The large increase in the regression-derived attenuation for the 2.8 μm clusters, from 0.6 μm−1 to 1.6 μm−1, evident in is responsible for the abrupt change in slope of the 2.8 μm cluster fit in , which occurs around log10(Fluence) = 2.5. This is readily seen from a plot of attenuation and fit data on the same fluence scale, which is provided in the SI. The total attenuation derived from the 4.4 μm clusters is 1.1 μm−1 at zero fluence and increases to 1.33 μm−1 at high fluence. No measurements have been reported of UV-C attenuation of bacterial spores or DNA as a function of fluence; however, data has been reported (Roleda et al. Citation2006) for Saccorhiza dermatodea zoospores suspended in sea water indicating a decrease in ln(UV-B transmittance) of about 30% over a 5 h exposure of UV-A and UV-B, totaling about 104 J/m2 UV-B and 105 J/m2 UV-A, in general accord with the change reported in this work of about 20% in UV-C transmittance over 2×104 J/m2. A variety of photochemical and subsequent reactions on DNA, lipids, and proteins, and other cellular structures, have been reported to result from exposure of spores to UV-A, UV-B, and UV-C radiation (Pattison and Davies Citation2006; Santos et al. Citation2013), which modify the chemical composition of spore material. While it is plausible these changes could result in changes in overall UV-C attenuation, the phenomenon has not been confirmed experimentally or predicted theoretically for bacterial spores.
The difficulty of cross-comparison of experimental results for UV-C absorption and attenuation from different researchers using differing methods of growth, preparation, and exposure is worth noting. For example, Tuminello et al. Citation(1997) do not report the growth media used for the B. subtilis or whether or how many times it was washed. Later measurements (Kunnil et al. Citation2005) on dry B. Globigii spores indicated that a single washing reduced fluorescence quantum efficiencies by factors of 2 to 4, depending on wavelength, compared to the same measurements on the unwashed spores. Tuminello et al. Citation(1997) report measurements of index of refraction for B. subtilis (as cited above) for spores not previously exposed to UV (A, B, and C) as well as spores that have been previously exposed, i.e., “used spores” from a previous measurement, and some differences between the two results are evident. However, the total fluence of the exposure was not reported and, while reported as a function of wavelength, the same samples appear to have been exposed to multiple wavelengths, both of which make interpretation in terms of a possible fluence dependence of attenuation impossible.
The calculation presented here used a rough proxy for potential attenuation length: a straight line distance from the cluster surface to the spore location. A more realistic, detailed calculation of light intensities within the cluster would produce more definitive results and confirm the utility of the general approach. For a homogenous sphere with real refractive index around 1.5 at 255 nm (see, e.g., Tuminello et al. Citation1997), Mie theory treatment with geometric optics indicates refraction at the outer edge of the cluster and could yield an angle of refraction about 40°, with the potential to shield the outer lower segments of the cluster (Chowdhury et al. Citation1992). However, regressions done in this work with various size segments of the cluster shielded and subjected to reduced fluence still required increasing attenuation with fluence. Further, inspection of the cluster dose–response in suggests the fraction shielded would be small, on the order of 5% of the spores or less. In fact, however, the clusters are aggregates of spores not homogeneous and internal propagation would involve interaction with multiple spores. Multipole analysis of UV radiation in interstellar grains finds that the interiors of aggregates of spherical particles subjected to narrow band UV radiation at six wavelengths between 100 nm and 200 nm are well illuminated without significant shadowed regions (Cecchi-Pestellini et al. Citation2005).
Conclusions
An approach was demonstrated for modeling the UV-C dose–response of clusters of BG spores exposed on surfaces and when aerosolized based on characterization of the single spore dose–response and explicit accounting for attenuation of the UV-C fluence by the spores in the cluster. The approach provides quantitative predictions of the dose–response of the clusters that are in reasonable accord with the data. The baseline, low fluence (less than about 500 J/m2), attenuation of fluence derived from the model is in reasonable accord with reported theoretical and measured values. Clusters were assumed to be spherical in this analysis but the results and reported experimental observations suggest that small clusters (less than about 4 μm) may deviate from this assumption. Future efforts to characterize the dose–response of spore clusters should include geometric characterization of the clusters. The analysis presented here suggests that UV-C attenuation by BG spores increases as fluence increases, but this phenomenon remains to be confirmed experimentally or theoretically. It is likely that the dose–response of single BG spores on surfaces and when aerosolized are described by the same decay characteristics. Dose–response experiments, particularly aerosolized exposures, should apply rigorous actinometric dosimetry to insure reliable relation to other experimental results.
Supplementary_Information.docx
Download MS Word (6.8 MB)Acknowledgments
The work reported here was only made possible by the experimental results of Jana Kesavan, Deborah Schepers, Jerold Bottiger, and Jason Edmonds and would have been impossible without the direct, contemporaneous, and animated interaction with the entire experimental team. The author also acknowledges the reviewer's insightful and useful comments, which greatly improved the final result.
References
- Blatchley, E. R., Meeusen, A., Aronson, A. I., and Brewster, L. (2005). Inactivation of Bacillus Spores by Ultraviolet or Gamma Radiation. J. Environ. Eng., 131(9):1245–1252.
- Blatchely, E. R., Shen, C., Nuanovic, Z., Lin, L.-S., Robinson, J. P., Ragheb, K., Gregori, G., Bergstrom, D. E., Fang, S., Guan, Y., Jennings, K., and Gunaratna, N. (2006). Dyed Microspheres for Quantification of UV Dose Distributions: Photochemical Reactor Characterization by Lagrangian Actinometry. J. Environ. Eng., 132(11):1390–1403.
- Bohrerova, Z., and Linden, K. G. (2006). Ultraviolet and Chlorine Disinfection of Mycobacterium in Wastewater: Effect of Aggregation. Water Environ. Res., 78(6):565–571.
- Carrera, M., Zandomeni, R. O., Fitzgibbon, J., and Sagripanti, J.-L. (2006). Difference Between the Spore Sizes of Bacillus anthracis and Other Bacillus Species. J. Appl. Microbiol., 102:303–312.
- Checchi-Pestellini, C., Saija, R., Antonia, M., Giusto, A., Borghese, F., Denti, P., and Aiello, S. (2005). Ultraviolet Radiation Inside Interstellar Grain Aggregates. I. The Density of Radiation. Astrophys. J., 624:223–231.
- Chowdhury, D. Q., Barber, P. W., and Hill, S. C. (1992). Energy-Density Distribution Inside Large Nonabsorbing Spheres by Using Mie Theory and Geometrical Optics. Appl. Opt., 31(18):3518–3523.
- Coohill, T. P. (1986). Virus Cell Interactions as Probes for Vacuum Ultraviolet Radiation Damage and Repair. Photochem. Photbiol., 44:359–363.
- Duncan, S., and Ho, J. (2008). Estimation of Viable Spores in Bacillus atrophaeous (BG) Particles of 1 to 9 μm Size Range. Clean: Soil, Air, Water, 36(7):584–592.
- EPA. (2006). Biological Inactivation Efficiency by HVAC In-Duct Ultraviolet Light Systems: Steril-Aire, Inc. Model SE 1 VO with GTS 24 VO Emitter. Report NR EPA 600/R-06/052. U.S. Environmental Protection Agency, Washington, DC.
- Ferrandino, F. J., and Aylor, D. E. (1984). Settling Speed of Clusters of Spores. Phytopathology, 74(8):969–972.
- Handler, F. A., and Edmonds, J. M. (2015). Quantitative Analysis of Effects of UV Exposure and Spore Cluster Size on Deposition and Inhalation Hazards of Bacillus Spores. Aerosol Sci. Technol., 49(11):1121–1130.
- Hill, S. C., Pan, Y. L., Williamson, C., Santarpia, J. L., and Hill, H. (2013). Fluorescence of Bioaerosols: Mathematical Model Including Primary Fluorescing and Absorbing Molecules in Bacteria. Opt. Exp., 21:22285–22313.
- Hofmann, W. (2011). Modelling Inhaled Particle Deposition in the Human Lung—A Review. J. Aerosol Sci., 42:693–724.
- Hofmann, W., and Koblinger, L. (1992). Monte Carlo Modeling of Aerosol Deposition in Human Lungs. Part III: Comparison with Experimental Data. J. Aerosol Sci., 23(1):51–63.
- Holler, S., Pan, Y., Chang, R. K., Bottiger, J. R., Hill, S. C., and Hillis, D. B. (1998). Two-Dimensional Angular Optical Scattering for the Characterization of Airborne Microparticles. Opt. Lett., 23(18):1489–1491.
- Kesavan, J., Bottiger, J. R., and McFarland, A..R. (2008). Bioaerosol Concentrator Performance: Comparative Tests with Viable and with Solid and Liquid Nonviable Particles. J. Appl. Microbiol., 104:285–295.
- Kesavan, J., Schepers, D., Bottiger, J., and Edmonds, J. (2014). UV-C Decontamination of Aerosolized and Surface Bound Single Spores and Bioclusters. Aerosol Sci. Technol., 48:450–457.
- Kesavanathan, J., and Doherty, R. (1999). Test Procedure for Removing Polystyrene Latex Microspheres from Membrane Filters. ECBC Technical Report, ECBC-TN-001. U.S. Army Edgewood Chemical Biological Center, Aberdeen, MD.
- King, B., Kesavan, J., and Sagripanti, J.-L. (2011). Germicidal UV Sensitivity of Bacteria in Aerosols and on Contaminated Surfaces. Aerosol Sci. Technol., 45:645–653.
- Kowalski, W. (2011). Ultraviolet Germicidal Irradiation Handbook. Springer-Verlag, Berlin.
- Kowalski, W. J., and Bahnfleth, W. P. (2000). Effective UVGI System Design Through Improved Modeling. ASHRAE Trans., 106:721–730.
- Kowalski, W. J., Bahnfleth, W. P., Witham, D. L., Severin, B. F., and Whittam, T. S. (2000). Mathematical Modeling of Ultraviolet Germicidal Irradiation for Air Disinfection. Quant. Microbiol., 2:249–270.
- Kunnil, S., Sarasanandarjah, S., Chacko, E., and Reinisch, L. (2005). Fluorescence Quantum Efficiency of Dry Bacillus globigii Spores. Opt. Exp., 13(22):8969–8979.
- Lighthart, B., Shaffer, B. T., Balkumar, M., and Ganio, L. (1991). Trajectory of Aerosol Droplets from a Sprayed Bacterial Suspension. Appl. Environ. Microbiol., 57:1006–1012.
- Lim, S., and Blatchley, E. R. (2012). UV Dose-Response Behavior of Air-Exposed Microorganisms. J. Environ. Eng. 138(7):780–785.
- MathWorks. (2015). nlinfit: Documentation. MathWorks, Natick, MA. http://www.mathworks.com/help/stats/nlinfit.html.
- Matsumoto, G. (2003). Bioterrorism: Anthrax Powder: State of the Art? Science, 302:1492–1497.
- Meselson, M., Guillemin, J., Hugh-Jones, M. (1994). The Sverdlovsk Anthrax Outbreak of 1979. Science, 266:1202–1208.
- Moeller, R., Setlow, P., Reitz, G., and Nicholson, W. L. (2009). Roles of Small, Acid-Soluble Spore Proteins and Core Water Content in Survival of Bacillus subtilis Spores Exposed to Environmental Solar UV Radiation. Appl. Environ. Microbiol., 75(16):5202–5208.
- Munakata, N., Makita, K., Bolsee, D., Gillotay, D., and Horneck, G. (2000). Spore Dosimetry of Solar UV Radiation: Applications to Monitoring of Daily Irradiance and Personal Exposure. Adv. Space Res., 26(12):1995–2003.
- Nicholson, W. L., and Galeano, B. (2003). UV Resistance of Bacillus anthracis Spores Revisited: Validation of Bacillus subtilis Spores as UV Surrogates for Spores of B. anthracis Sterne. Appl. Environ. Microbiol., 69:1327–1330.
- Pattison, D. I., and Davies, M..J. (2006). Actions of Ultraviolet Light on Cellular Structures, in Cancer: Cell Structures, Carcinogens, and Genomic Instability, Leon P. Bignold, ed., Birkhäuser, Basel, Switzerland, pp. 131–157.
- Pennell, K. G., Aronson, A. I., and Blatchley, E..R. (2008). Phenotypic Persistence and External Shielding Ultraviolet Radiation Inactivation Kinetic Model. J. Appl. Microbiol., 104:1192–1202.
- Rice, J. K., and Ewell, M. (2001). Examination of Peak Power Dependence in the Inactivation of Bacterial Spores. Appl. Environ. Microbiol., 67(12):5830–5832.
- Riesenman, P. J., and Nicholson, W. L. (2000). Role of the Spore Coat Layers in Bacillus subtilis Spore Resistance to Hydrogen Peroxide, Artificial UV-C, UV-B, and Solar UV Radiation. Appl. Environ. Microbiol., 66(2):620–626.
- Roleda, M. Y., Clayton, M. N., and Wiencke, C. (2006). Screening Capacity of UV-Absorbing Compounds in Spores of Arctic Laminariales. J. Exp. Marine Biol. Ecol., 338:123–133.
- Santos, A. L., Moreirinha, C., Lopes, D., Esteves, A. C., Henriques, I., Almeida, A., Domingues, M. R. M., Delgadillo, I., Correia, A., and Cunha, A. (2013). Effects of UV Radiation on the Lipids and Proteins of Bacteria Studied by Mid-Infrared Spectroscopy. Environ. Sci. Technol., 47:6306–6315.
- Setlow, P. (2006). Spores of Bacillus subtilis: Their Resistance to and Killing by Radiation, Heat and Chemicals. J. Appl. Microbiol., 101:514–525.
- Severin, B. F., Suidan, M. T., and Engelbrecht, R. S. (1983). Kinetic Modeling of UV Disinfection of Water. Water Res., 17(11):1669–1678.
- Sinha, R. P., and Hader, D.-P. (2002). UV-Induced DNA Damage and Repair: A Review. Photochem. Photobiol. Sci., 1:225–236.
- Sykes, I. R., Parker, S. F., Henn, D., and Chowdhury, B. (2011). SCIPUFF Version 2.7: Technical Documentation. Sage Management, Princeton, NJ.
- Tuminello, P. S., Arakawa, E. T., Khare, B. N., Wrobel, J. M., Querry, M. R., and Milham, M. E. (1997). Optical Properties of Bacillus subtilis Spores from 0.2 to 2.5 μm. Appl. Opt., 36(13):2818–2824.
- Velesco, N., and Schweiger, G. (1999). Geometrical Optics Calculation of Inelastic Scattering on Large Particles. Appl. Opt., 38(6):1046–1052.
- Weber, S. (2005). Light-Driven Enzymatic Catalysis of DNA Repair: A Review of Recent Biophysical Studies on Photolyase. Biochim. Biophys. Acta. 1707:1–23.
