ABSTRACT
This study investigated the influence of gas pressure on the submicrometer particle capture performance of an electrostatic precipitator (ESP). Current-voltage characteristics and particle capture performance of the ESP were studied in air and in simulated flue gas (SFG) under 1, 2, and 3 atm. Using negative corona and air as the feed gas, the penetration of most particles of 40–400 nm in diameter decreased from 8 × 10−4 − 2 × 10−2 to 2 × 10−4 − 1 × 10−2 as pressure increased from 1 atm to 3 atm at constant current; and increased from 3 × 10−5 − 1 × 10−3 to 2 × 10−4 − 1 × 10−2 as pressure was elevated when the voltage was held roughly constant. Similar type of disparity under different pressures was also observed for positive corona and for SFG. Experiments set up to capture fly ash in the ESP showed that with constant current, higher pressure resulted in a higher initial charge fraction of the particles from the furnace, which could facilitate the penetration of fly ash particles. A semiempirical model was developed based on the Deutsch–Anderson equation and experimental data under 1, 2, and 3 atm to calculate the particle penetrations under high pressure. The total charge number on a particle (n') is calculated by incorporating the effects of current (I) and pressure (P) on relative weights of the diffusion charging number (ndiff) and field charging number (nfield), that is, n' = B1(I,P)ndiff + B2(I,P)nfield, where B1(I,P) and B2(I,P) are both empirical coefficients dependent on current and pressure. Experimental penetrations under 1.5 and 2.5 atm validated this model over the particle diameter range in 100–400 nm.
Copyright © 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
For providing reliable and low-cost energy, advanced technologies, such as gasification (Minchener Citation2005), pressurized fluidized bed combustion (Jensen et al. Citation1995), and pressurized oxy-combustion (Gopan et al. Citation2014) are considered promising alternatives to conventional coal combustion. The application prospects of these systems are not determined only by the efficiency of energy generation, but also by the feasibility and performance of additional pollution control measures due to growing environmental concerns. Particle control technologies have been the focus of academic studies and industrial applications especially in developing countries, such as China (Che et al. Citation2009; Huang et al. Citation2014) and India (Pachauri et al. Citation2013; Mishra et al. Citation2015) over the past few years due to growing concerns regarding haze as a major hazard to their atmospheric environment.
Electrostatic precipitators (ESPs) are widely utilized in controlling particles from various emission sources, such as conventional coal-fired power plants (Yokoyama et al. Citation2000; Meij and te Winkel Citation2004) because of their high capture efficiency, low cost of operation and ability to work in extreme environments (high temperature and high pressure) (Brown and Walker Citation1971; Bush et al. Citation1979; Rinard et al. Citation1987; Villot et al. Citation2012). These qualities make them promising candidates for particle control equipment in gasification and pressurized combustion systems. It has been also reported that corona discharge, which is the main charging mechanism of ESPs, can be obtained at a pressure of up to 1.6 MPa (Bush et al. Citation1977, Citation1979) and a temperature of up to 927°C (1700°F) (Brown and Walker Citation1971). However, the capture efficiency of particle in an ESP operating under high-pressure condition is not well studied. In particular, no size-dependent capture efficiency data has been reported under varying pressure conditions.
Particles in the submicrometer range (particle sizes smaller than 1 μm) require special attention due to their more adverse effects on human health (Biswas and Wu Citation2005). Extensive studies have been done on the capture of submicrometer particles with ESPs under atmospheric pressure (Zhuang et al. Citation2000; Zhuang and Biswas Citation2001; Suriyawong et al. Citation2008; Bai et al. Citation2010). For example, fundamental studies have used bench-scale ESPs to study the capture efficiency of synthetic particles (Yoo et al. Citation1997; Zhuang et al. Citation2000; Huang and Chen Citation2002; Jing et al. Citation2013). Furthermore, research has been carried out on ESPs with particles and exhaust gases from real combustion systems (McCain et al. Citation1975; Ylätalo and Hautanen Citation1998; Li et al. Citation2009). Nonetheless, to the best of our knowledge, the influence of pressure on the submicrometer particle capture efficiency for ESPs has not been investigated yet.
Due to its simplicity and relative accuracy, the Deutsch–Anderson (D-A) equation is extensively employed in estimating the capture efficiency of ESPs and facilitating their design (Chen et al. Citation2014). However, because of certain simplifying assumptions, such as rapid mixing in the transverse direction and particles attaining the saturation charge immediately after entering the ESP, the D-A equation is limited in providing accurate particle capture efficiency estimates for certain cases. For example, it was reported that capture efficiencies predicted by the D-A equation for fine particles with diameters <2 nm are much lower than the measured efficiencies (Riehle and Löffler Citation1993). In order to improve the accuracy, many modifications to the D-A equation have been proposed (Robinson Citation1967a; Cooperman Citation1984; Park and Chun Citation2002; Lin et al. Citation2012). Up to now, there has apparently not been any study on whether the D-A equation can be applied under elevated pressure conditions (e.g., in a pressurized ESP).
In this study, the fundamental aspects of a pressurized ESP are explored by examining the current-voltage (I-V) characteristics, and studying the performance of the pressurized ESP by measuring its capture efficiency of submicrometer particles including homogeneous NaCl particles and fly ash particles at the outlet of a pressurized drop-tube furnace. To better predict the capture efficiencies of the submicrometer particles in the ESP under high pressures, a complete calculation procedure based on the original D-A equation and experimental data fitting was established and validated.
2. Experimental section
2.1. Experimental system
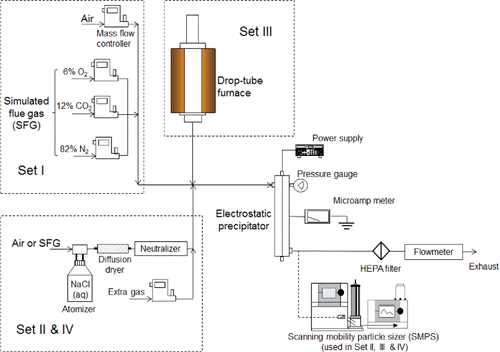
The experimental system is illustrated in . The ESP used in this study was a bench-scale DC corona-based wire-cylinder ESP, which mainly consisted of an outer PVC case, a central discharge electrode (stainless steel wire, 0.323 mm in diameter), and a cylindrical collecting electrode (stainless steel tube, 25.4 cm in length and 4.8 cm in diameter) enclosed by the outer case. The discharge electrode was sheathed using insulating materials at the top and bottom. A nozzle was installed at the outlet of the ESP to reduce the pressure from high level in the ESP to ambient pressure so that the particle size distribution measuring instrument can be normally used downstream the pressurized ESP. The nozzle used was actually a ball type valve, which has large-diameter, straight-through and open-position vent so that particle losses were minimized (Komhyr Citation1983; Williams et al. Citation2006). The ESP configuration is shown in Figure S1. A Bertan power supply (Model 230-20R, Spellman, Hauppauge, NY, USA) provided the high voltage for the ESP. The experimental system is shown in . Two types of systems generated test particles. NaCl particles were generated by an atomizer (Model 3076, TSI Inc., Shoreview, MN, USA). A Kr-85 radioactive source (Model 3077, TSI Inc., Shoreview, MN, USA) neutralized the charges on the NaCl particles from the atomizer, and a silica gel diffusion dryer removed water vapor from the particles. It should be noted that the particles downstream the neutralizer consist primarily of particles carrying zero charge or very low number of charges, which can be regarded negligible compared with charge number appeared in this study. Fly ash particles were generated in a pressurized drop-tube furnace. The configuration and operation parameters of the furnace have been described in previous papers (Suriyawong et al. Citation2006; Wang et al. Citation2013a,b, Citation2015). PRB (Powder River Basin) coal was fed at a rate of 1.5 g/h. The drop-tube furnace was operated at up to 3 atm in this study. Additional air, N2, O2, and CO2 flows were added to the system to adjust the gas composition and the total flow rate through the ESP. The flow rates through the ESP associated with the atomizer and the furnace were kept at 15 LPM (flow velocity: 0.14 m/s) and 25 LPM (flow velocity: 0.23 m/s), respectively. The current through the ESP was measured with a microamp meter (Model 17424, Simpson Electric, Lac du Flambeau, WI, USA). A scanning mobility particle sizer (SMPS, Model 3080, TSI Inc., Shoreview, MN, USA) measured the particle size distribution.
2.2. Experimental design
In this study, four sets of data were obtained. First, the operation of the pressurized ESP, that is, its current-voltage characteristics (Set I), were established. Next, the ESP's particle capture efficiency using NaCl particles (Set II) was determined. Then, the capture of fly ash particles from a drop-tube furnace in the pressurized ESP (Set III) was examined. The furnace was operated at the same gas pressure as the ESP for each run. Finally, using the experimental data (negative ESP with air feed) from Set II, a semiempirical calculation procedure of capture efficiency was developed and validated by comparing experimental capture efficiency data for NaCl particles under 1.5 and 2.5 atm (Set IV). summarizes the experimental objectives and parameters.
Table 1. Summary of performed studies.
3. Results and discussion
3.1. Current-voltage characteristics of the pressurized ESP
The current-voltage (I-V) characteristics provide information about the corona working range, while at the same time, the current indicates the ion concentration in the ESP. shows the I-V characteristics of the studied ESP under three pressures (1, 2, and 3 atm) for air and simulated flue gas (SFG for short) composed of 6% O2, 12% CO2, and 82% N2 (by mass) (similar to that used by Zevenhoven and Kilpinen Citation2001). For both positive and negative polarities, as the pressure increases, the corona onset voltage (at which point the corona began to appear) increases. In addition, at the same voltage, the ESP current is lower at higher pressure. To form the corona discharge, a certain amount of ions and electrons is needed. Most of the ions and electrons are generated by impaction between gas molecules and high-energy electrons. To initiate ionization, an electron must gain sufficient kinetic energy to knock a secondary electron out of a gas molecule. At higher pressure, the mean free path of the gas inside the ESP decreases. Thus, there is a higher probability that the electron will hit a gas molecule before being accelerated to achieve the critical kinetic energy necessary to ionize the gas. The ionization process is thus hindered (Robinson Citation1971).
Figure 2. The influences of gas pressure on (a) I-V characteristics and (b) corona onset voltage with different gas compositions (air and SFG). (a) I-V curves of the ESP under three pressure conditions (1, 2, and 3 atm) with air and SFG as the feed gas, respectively; (b) the relationship between the corona onset voltage and gas pressure (experimental results for air and SFG, estimation for air case based on Equation (Equation2[2] ), and fitting curve for SFG case; “pos”: short for “positive”; “neg”: short for “negative”).
![Figure 2. The influences of gas pressure on (a) I-V characteristics and (b) corona onset voltage with different gas compositions (air and SFG). (a) I-V curves of the ESP under three pressure conditions (1, 2, and 3 atm) with air and SFG as the feed gas, respectively; (b) the relationship between the corona onset voltage and gas pressure (experimental results for air and SFG, estimation for air case based on Equation (Equation2[2] ), and fitting curve for SFG case; “pos”: short for “positive”; “neg”: short for “negative”).](/cms/asset/2e0e8662-48ac-489e-b904-f25225b197fc/uast_a_1216071_f0002_b.gif)
also shows that for the positive ESP polarity, the onset voltages of the ESP fed with SFG were higher than the corresponding voltages for the air feed cases. A similar phenomenon was observed by Suriyawong et al. Citation(2008). This result can be attributed to the fact that N2 and CO2 have higher ionization potentials than O2 (15.6, 13.8, and 12.1 eV, respectively) (Condon and Odishaw Citation1967). Second, CO2 has a higher dielectric constant than O2 and N2 (1.6, 1.0, and 1.0, respectively), and thus a reduced effective electric field results (Lide Citation2000). Furthermore, because CO2 has negative electron affinity (Knapp et al. Citation1986), electron impact reactions with CO2 usually acts as electron traps by producing oxygen radicals (O), resulting in reduced electron ionization and suppression of electric discharge (Smyth and Stueckelberg Citation1930). SFG has more CO2, slightly more N2, and less O2 than air; thus, the ionization is less extensive compared to air or oxygen. However, for negative ESP polarity, the I-V characteristic curves for the corresponding air and SFG cases almost overlap with each other. It has been demonstrated that negative corona discharge need to be sustained by continuous collision between electrons and neutral gas molecules and generation of “secondary electron-positive ion” pairs (Chen and Davidson Citation2003). On the one hand, the short-wavelength photons emitted in the corona discharge are more energetic than positive ions (mean kinetic energy: 0.01–0.1 eV) in terms of knocking out electrons. On the other hand, the stainless steel discharge electrode has a lower work function (4.4 eV for stainless steel) than the ionization energy of any gas (12.1 eV for O2, 15.6 eV for N2, and 13.8 eV for CO2) used in this study. Therefore, the primary source of the secondary electrons in negative corona discharge is direct photoionization of the discharge electrode, indicating that the I-V characteristic of the negative corona in ESPs might depend rather on the material and the condition of the surface of the electrode than on the gas composition.
Because the corona onset voltage and field strength are parameters of critical importance to the ESP performance (Abdelsalam and Wiitanen Citation1993), the relationship between the onset field strength and the gas pressure was evaluated by using an equation (Robinson Citation1967b) derived from Peek's semiempirical equation (Peek Citation1920):[1a]
The relationship between the corona onset voltage and corona onset field strength at the wire surface () in a wire-cylindrical space is:
[1b]
By combining Equations (Equation1a[1a] ) and (Equation1b
[1b] ), the corona onset voltage as a function of gas pressure (
) is:
[2]
The constant Ag mainly depends on the gas type, and Bg depends on the ESP polarity. shows the estimated relationship between onset voltages and pressures as well as our experimental data. In the case of air, recommended values derived from the cumulative data of several investigators were used, namely 32.2 × 105 V/m for Ag and 8.46 × 104 V/m1/2 for Bg with negative polarity (Robinson Citation1967b). With these values, the estimated values match the experimental data well. Although constant values have been reported for different types of gases (Thornton Citation1939), data on gas mixtures, such as the SFG used in this study, which is a mixture of N2, O2, and CO2, are not reported in the literature. Thus, a weighted average method based on the mass percentage was used to estimate Ag for the SFG. Ag was estimated to be 36.0 × 105 V/m. Bg was still 8.46 × 104 V/m1/2 for negative polarity. By fitting the estimated relationship curve with the experiment data, Bg was determined to be 10.00 × 104 V/m1/2. With the aforementioned values, the calculated relationship matches the experimental data reasonably well. The recommended Bg value reported here can be used in estimation of the onset voltage for higher gas pressure cases and pressurized ESP design.
To better explain the experimental I-V curves and facilitate the calculation of particle penetration of the ESP, a mathematical derivation with a numerical fitting approach was used. Derived from the Poisson's equation for electric field distribution, the relationship between corona voltage and current for wire-cylinder ESPs is given (Yuan and Shen Citation2004) by[3]
Here, EC is given by Equation (Equation1b[1b] ), and φ is a function of current (I):
[4]
It can be implied from Equations (Equation3[3] ) and (Equation4
[4] ) that when the current is zero, the corona voltage is equal to the corona onset voltage, and the corona voltage increases monotonically with the current after corona inception. As shown in Supplementary Figure S2, the calculated corona currents based on Equations (Equation3
[3] ) and (Equation4
[4] ) are lower than, similar to, and higher than the experimental values with the same voltage under 1, 2, and 3 atm, respectively. Higher currents at 3 atm can be explained by the fact that the theoretical model above does not consider the inhibiting effect of high pressure on the ionization inside the ESP. The reason for lower calculated currents under 1 atm is that in Equation (Equation3
[3] ), the space charge considered is an ionic space charge, with no consideration given to the presence of charged particles in the interelectrode space (Yuan and Shen Citation2004). In reality, such particles do contribute to the total space charge. Ignoring the contribution of the charged particles could explain the lower predicted current.
Considering the deviations between the experimental I-V curves and the calculated values based on Equation (Equation3[3] ) under different pressures, the equation was modified by adding pressure factors in the form of
. Numerical iterations over wide ranges of a1 and a2 were computed by MATLAB until reaching the minimum summation of squared deviations (σ′ = (N − 1)σ2) between the experimental results and calculated values. As a result, the modified equations describing the I-V relationships for negative and positive polarity voltages are
[5]
[6]
For negative polarity, σ′ decreases by 82% from 143.7 kV2 after the modification, and for the positive voltage condition, σ′ is reduced by 64% from 46.1 to 16.8 kV2. graphically compares the experimental and computed data based on Equations (Equation5[5] ) and (Equation6
[6] ). Particularly, when the pressure is at 1 atm and the current is higher than 200 µA, the experimental values of voltages are slightly lower than the calculated voltages with the same current. Nonetheless, the other I-V curves under the three pressure conditions are well predicted by the calculations, which implies that the two semiempirical equations for I-V characteristics are valid for deriving a capture efficiency equation for pressurized ESPs.
3.2. Capture of NaCl particles using air as the feed gas
NaCl particles were used to evaluate the capture efficiencies of the ESP. The particles passed through a Kr-85 radioactive radiation source, which gave them an assumed Boltzmann charge distribution (Keefe et al. Citation1959). The size distributions of NaCl particles atomized under different gas pressures are shown in Supplementary Figure S3. Here the particle capture is quantified using particle penetration (p = 1 − η, where η is capture efficiency), which is calculated using the following equation:[7] where Noff and Non are particle number concentration downstream of the ESP when ESP is off and on, respectively.
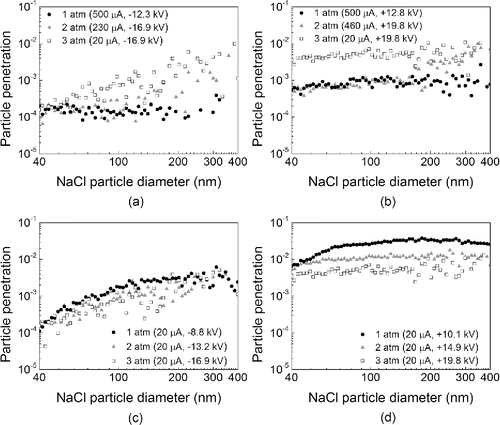
Voltage is one of the most important ESP operation parameters. Thus, ESP performances under different gas pressures were first compared for similar ESP voltages (±16.7 kV for the 2- and 3-atm cases; ±12.7 kV for the 1-atm case, because the maximum current output was reached). and b show the particle penetrations through the ESP under various pressures at positive and negative potentials. High collection efficiencies over 99% for most of the particles ranging 40–400 nm in diameter was observed under all the conditions. This outcome can be attributed to low flow velocity (Lin et al. Citation2010) and high onset voltage required for inception of corona discharge under high pressure. More detailed discussion can be found in supplemental materials.
Figure 4. NaCl particle penetrations through the ESP using air as feed gas under different gas pressures: (a) negative ESP, voltage controlled; (b) positive ESP, voltage controlled; (c) negative ESP, current controlled; (d) positive ESP, current controlled.
and b also show that the ESP behaviors under positive and negative polarities were similar: as the particle diameter increased, the particle penetration increased due to the lower electrical mobility of larger particles (Jing et al. Citation2013). There are similar shapes of the size dependent efficiency curve found in other studies. The experimental results in the study of Huang and Chen Citation(2002) indicate that ultrafine aerosol penetration through the single-stage ESP increases rapidly as aerosol size increases from 20 to 300 nm under 26.4 kV and 80 µA. Suriyawong et al. Citation(2008) found that particle penetration through a cylindrical-wire ESP also increases as particle diameter increases from 40 to 200 nm for different gas compositions. Interestingly, the penetration for small particles around 40–50 nm seem so low that the partial charging effects on the particle penetration might be insignificant. It has been reported (Huang and Chen Citation2002) that the partial charging effects on small particles tend not to be obvious when the applied voltage is high. This is probably due to the following reasons: (1) the particle space charge density is significantly high (Park and Kim Citation1998) due to high applied voltage, which could enhance the particle charging, and thus the collection efficiency. (2) High voltage may intensify the ionic wind (Liang and Lin Citation1994), which could subsequently compensate for efficiency loss due to partial charging.
It can also be observed for both polarities in and b that the penetrations of most particles ranging from 40 to 400 nm in diameter increased approximately from 3 × 10−5 − 1 × 10−3 to 2 × 10−4 − 1 × 10−2 with higher pressure. The reason for this result is that for similar ESP voltages, the ion concentrations in the ESP decreased with higher gas pressure, which is indicated by the ESP current measurements; The lower ion concentration thus resulted in weaker particle charging (Hinds Citation1999), which eventually led to higher particle penetration.
To further investigate the influence of factors other than the ion concentration on the particle capture, ESP performances under different gas pressures were compared under the same current (20 µA) to maintain the same ion concentration in the ESP. and d show the particle penetrations through the ESP at three pressures (1, 2, and 3 atm): Similar to the results in and b, for both positive and negative polarities, penetration increased with particle diameter. However, in terms of the effect of gas pressure, the penetrations of most particles ranging from 40 to 400 nm in diameter decreased from 8 × 10−4 − 2 × 10−2 to 2 × 10−4 − 1 × 10−2 as pressure increased from 1 to 3 atm for negative corona; and decreased from 1 × 10−4 − 5 × 10−2 to 6 × 10−5 − 2 × 10−2 as pressure increased from 1 to 3 atm for positive corona. In this set of experiments, particle capture was mainly determined by the transport of the charged particles in the ESP, and this conclusion can be attributed to the combined effect of three factors: (1) according to ideal gas law, it can be concluded that higher pressure results in lower volumetric flow rate, and therefore, lower particle axial velocity and higher collection efficiency; (2) it is generally known that higher radial terminal electrostatic velocity vTE, which is equal to neEB, where B is particle mechanical mobility, is conducive to particle collection. Given that voltage and electrical field strength (E) are higher under higher pressure, higher pressure is thus favorable to vTE and thus collection efficiency by increasing field strength; (3) the particle mechanical mobility B can be represented by CC/3μπdp and thus is positively proportional to the Cunningham correction factor (CC), which is positively correlated to the mean free path (λ). Higher pressure leads to lower gas density and lower mean free path, so higher pressure could suppress radial terminal electrostatic velocity and collection efficiency by diminishing particle mechanical mobility. Factors (1) and (2) enhanced particle capture with increasing gas pressure, while factor (3) weakened particle capture by increasing gas pressure. Nonetheless, it can be inferred that higher voltage can generally enhance performance of pressurized ESP with constant current.
3.3. Capture of NaCl particles using SFG as the feed gas
The composition of the coal combustion exhaust is very different from ambient air; thus, the ESP under different pressures was studied with SFG to understand its performance under exhaust conditions. As illustrated in , the trends for particle penetration with respect to gas pressures were similar to those of air. A particular observation where the case of SFG is different from the case using air was that for positive ESP polarity, the particle penetration did not change much as the particle diameter increased. For example, the widest range of the penetration of particles ranging from 40 to 400 nm in diameter among the five positive corona cases was from 7 × 10−3 to 3 × 10−2 at operating condition of 1 atm, 20 µA, and +10.1 kV, which spanned even less than one order of magnitude. More specifically, the penetrations for ultrafine particles with diameter less than 100 nm, which is in the size range dominated by diffusion charging, were much greater (1–2 orders of magnitude) than those in the case where air was used. The reason for this phenomenon is possibly that the diffusion charging was slower because of a lower thermal ion mobility. SFG may have higher concentrations of CO2+ and CO+ ions, and fewer O2+ ions when compared to ambient air (Stueckelberg Citation1929; Smyth and Stueckelberg Citation1930). The higher average ion mass would result in lower thermal ion mobility.
3.4. Capture of fly ash particles from coal combustion in a pressurized drop-tube furnace
Fly ash particles from real combustion have different physical properties from those of NaCl particles with Boltzmann charge distributions; thus, their capture behavior in the ESP could differ from that observed in the aforementioned experiments. The effect of pressure on the capture of fly ash particles (size distributions shown in Figure S4) at negative ESP polarity was studied. When the negative ESP is operated at the same flow rate as the experiment Set II, namely 15 LPM, the capture efficiencies were above 99.9% for both cases (as shown in Figure S5); thus, generally the capture efficiencies were higher for fly ash particles than NaCl particles with Boltzmann charge distributions. The initial charge fractions (when the particles entered the ESP) of fly ash particles were higher than that of NaCl particles (as shown in Figure S6), which tended to impact the capture of particles in the ESP. The high charge fraction was a result of diffusion charging and direct thermionization and photoionization of the particles (Burtscher et al. Citation1986; Burtscher Citation1992) during combustion.
As shown in , higher penetrations of fly ash particles were obtained by increasing the flow rate to 25 LPM. The penetration increased with particle size, which was also observed in the NaCl experiments. However, the penetrations of most particles ranging from 40 to 400 nm in diameter increased from 8 × 10−5–9 × 10−3 to 1 × 10−3–2 × 10−2 as pressure increased from 1 to 3 atm for negative corona, which is opposite to the trend obtained in the NaCl experiment as shown in . Although the ESP operating currents in the fly ash experiments were the same as in the NaCl experiments, the voltages were slightly different due to the differences in gas compositions, thereby affecting the I-V characteristics. This fact provides evidence that the charging status of particles fed into the ESP plays an important role in ESP performance. In the NaCl particle tests, all the particles had a Boltzmann charge distribution; however, in the fly ash particle tests, the charge fraction varied with pressure. From the results of the charge status test (Figure S6), it is very probable that there was a higher percentage of multiple positively charged particles from combustion under higher gas pressure, as a result of more residence time for diffusion charging (Hinds Citation1999) and direct thermionization and photoionization of particles. Those multiple positively charged particles caused an overall higher penetration in the negative ESP.
Figure 6. Particle penetrations through the ESP for fly ash particles of various sizes under different gas pressures.
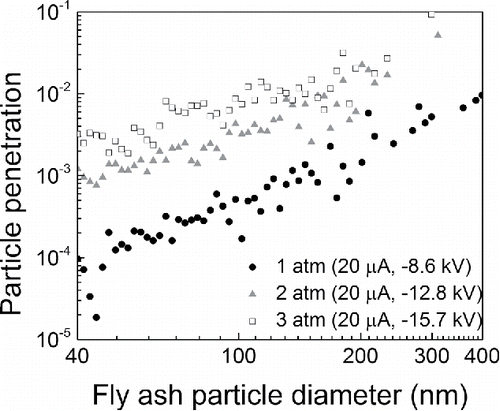
In addition, the dielectric constants of fly ash particles and NaCl particles are also different, which may influence diffusion charging in the ESP. However, because the difference is small (Table S1), the influence of the dielectric constant is expected to be insignificant.
3.5. Development of a modified D-A equation considering the effects of pressure
The D-A Equation (Equation8[8] ) (Hinds Citation1999) is usually used to calculate the capture efficiency of an ESP, which is given as:
[8]
This original D-A equation is based on three major assumptions: (1) particles stick if they come into contact with the tube wall and are not reentrained. (2) The feed gas in the ESP is regarded as an ideal gas. (3) The particles are uniformly distributed across every cross section and reach an equilibrium charge distribution as soon as they enter the ESP.
In order to explore the effect of gas pressure on the capture efficiency of an ESP, parameters, and variables in Equation (Equation8[8] ) were explicated termwise as follows.
According to ideal gas law, the pressure change can alter gas volume, so the volumetric flow rate in a pressurized ESP is given by[9] indicating that higher pressure can lead to lower volumetric flow rate and thus larger residence time of the aerosol flow in the ESP.
Assuming that Stokes' law applies, the terminal electrostatic migration velocity of particles derived from the balance between the electric force and drag force can be represented by[10] where E is the average electric field strength that can be approximated as the field strength at the middle layer of wire-cylindrical space (i.e., at
):
[11]
E0, the field strength at , is calculated from the corona voltage:
[12] where V0 is given by Equations (Equation5
[4] ) and (Equation6
[5] ).
CC considering noncontinuum effects is given by (Hinds Citation1999)[13] where λ can be related to gas pressure through the following equation (Hinds Citation1999) and ideal gas law:
[14]
Based on two charging mechanisms, the number of elementary charges can be divided into two components:[15]
Here, ndiff represents the number of elemental units of particle charges for diffusion charging, which is calculated by using Fuch's equation (Fuchs Citation1947):[16]
The number of elemental units of particle charges for field charging (nfield) is calculated by using the Pauthenier and Moreau-Hanot Citation(1932) equation:[17]
where Ni is a function of current and field strength:[18]
In the whole series of aforementioned equations, the only two independent variables are corona current and gas pressure in the ESP since the dimensions of the ESP are known and other regular physical quantities are assumed constant. Thus, a complete procedure for calculating the capture efficiency of particles with diameter (dp) by an ESP operated under particular pressure (P) and current (I) can be elaborated as follows (also shown in Figure S7):
plugging P into Equation (Equation9
[9] ) to obtain the volumetric flow rate (Q), and then calculating residence time (t = V/Q),
plugging P into Equation (Equation2
[1b] ) to obtain the onset voltage (VC, which can also be obtained simply by experimental measurement), calculating the onset field strength (EC) by using Equation (Equation1b
[1b] ), and then plugging VC, EC, I, and P into Equation (Equation5
[5] ) (or Equation (Equation6
[6] ) if polarity is positive) to estimate the applied voltage (V0), which can be subsequently put in Equations (Equation12
[12] ) and (Equation11
[10] ) to get the average applied field strength (E),
plugging P into Equation (Equation14
[14] ) and using Equation (Equation13
[13] ) to obtain the Cunningham correction factor (CC),
with values of I, E, and t, using Equations (Equation15–18) to calculate the number of elemental charges on a particle (n),
plugging n, E, and CC into Equation (Equation10
[9] ) to obtain the terminal migration velocity of the particles (vTE), and
plugging Q and vTE into Equation (Equation8
[8] ) to finally obtain the capture efficiency (η).
In order to see if this calculation procedure is applicable to high pressure conditions, the measured particle penetrations (using negative ESP with air feed) from experiment Set II were compared with the corresponding calculated penetrations based on Equations Equation(8)–(18). Figure S8 shows that the calculated particle penetrations at pressures higher than 1 atm are much lower than the corresponding experimental values, which indicates that the calculation model shown in Figure S7 might be a reasonable tool for calculating capture efficiency under atmospheric pressure conditions, but it is not feasible for pressurized cases.
It has been reported that the relative dominance of the diffusion charging effect compared with field charging might be dependent on applied pressure (Phelps Citation1990; Romay et al. Citation1991) and current (Pfafflin and Ziegler Citation1986) of the ESPs; moreover, Equations (Equation16[16] ) and (Equation17
[17] ) are oversimplified due to some restrictive assumptions. Therefore, to obtain better simulation of particle penetrations at high pressure, the weighted influence of each of the charging mechanisms in Equation (Equation15
[15] ) was modified by multiplying an empirical coefficient, which is a function of pressure and current, by each of the charge number terms:
[19] where b1 and b2 are constants to be numerically determined, and I0 ( = 20 µA) and P0 (= 1 atm) are set as reference values since the simulated results for the experimental condition of 20 µA and 1 atm are so close to the experimental penetrations, as shown in Figure S8, that they are not considered to be further modified.
Using MATLAB, numerical iterations of b1 and b2 over wide ranges were conducted. Eventually, the minimum summation of squared deviations (σ′) reached a minimum value of 0.0022 (decreased by 46% compared with Figure S8) when b1 and b2 were 1.77 and 2.84, respectively. Thus, the modified equation of total charge number on a particle is expressed as:[20]
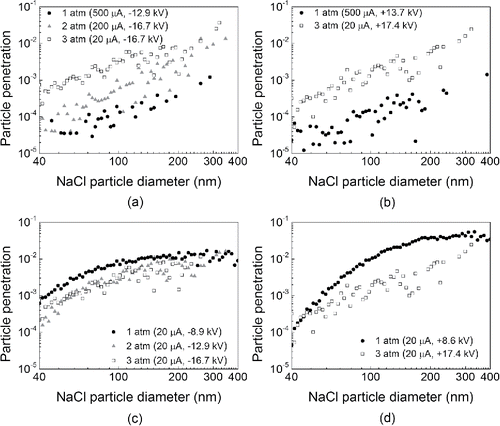
A modified calculating procedure of capture efficiency in pressurized ESPs using Equation (Equation20[19] ) as the expression for the number of elementary charges on a particle is illustrated in . Thus, a comparison between the measured particle penetrations (using negative ESP with air feed) from experiment Set II with the corresponding calculated penetrations using Equation (Equation20
[19] ) is shown in . The calculated curves fit the experimental penetrations reasonably well, especially in the diameter range of 70–400 nm for all five conditions, although the agreements are unfavorable for particles lower than 100 nm for the cases with high currents, that is, 200 and 500 µA, which is primarily due to limitations in computational cost and algorithm. The trend of particle penetration with increasing particle size is upward as demonstrated by the experimental results, which is similar to the trend of penetration curves produced by the modified calculating procedure—all of which serves to illustrate the legitimacy of this model. This trend makes physical sense because larger particles tend to have lower electrical mobility (Jing et al. Citation2013) so that they are much less likely to be captured in the limited residence time inside the ESP. However, the effect of field charging would probably facilitate collection of particles larger than 200 nm by increasing particle charge number, which can be supported by the trend shown in Figure S9. In fact, it can be observed in that the experimental penetration dotted curves become flattened at around 200–300 nm, and the three simulated curves under pressures over 1 atm proceed through penetration peaks and even toward lower penetration in the range of 200–400 nm. These facts allow us to reasonably speculate much clearer decreasing penetration curves (increasing efficiency) as particle size increases beyond visible scale (to over 400 nm) in our study, which is due to field charging.
Figure 7. Calculating procedure of capture efficiency in pressurized ESPs using Equation (Equation20[19] ) as the modified expression for the number of elementary charges of the particles.
![Figure 7. Calculating procedure of capture efficiency in pressurized ESPs using Equation (Equation20[19] ) as the modified expression for the number of elementary charges of the particles.](/cms/asset/f6393c98-80f1-4e17-bb2f-12b1aedb8107/uast_a_1216071_f0007_oc.gif)
Figure 8. Particle penetrations for various particle sizes measured under 1, 2, and 3 atm and the corresponding calculated values using Equation (Equation20[19] ) (summation of squared deviations [σ'] is 0.0022, which excludes the case of “1 atm and 20 µA”).
![Figure 8. Particle penetrations for various particle sizes measured under 1, 2, and 3 atm and the corresponding calculated values using Equation (Equation20[19] ) (summation of squared deviations [σ'] is 0.0022, which excludes the case of “1 atm and 20 µA”).](/cms/asset/f4705c80-5a9b-4853-b8a1-de780f418124/uast_a_1216071_f0008_b.gif)
The experimentally speculated significance of partial charging effect on this study can be verified by the modified D-A equation and Figure S9. As reported by Lin et al. Citation(2012), a partial charging effect factor (α= MIN(1, n)) can be used in a modified D-A equation. α is equal to 1 for particle charge number greater than or equal to 1.0, and is equal to n when n < 1.0, or particles are partially charged. Based on calculation using the Equation (Equation20[19] ), the value of n is no less than 2.3 for particles of all concerned sizes. This fact indicates that the value of α is constantly equal to 1 and partial charging effect is negligible. In addition, as implied by Figure S9, the total charge number increases rapidly as particle size rises, although the portion of particles carrying few charges downstream the neutralizer also increases with larger particle size. For instance, it can be seen in Figure S9 that a particle in diameter of 392 nm carries around 40 charges under 1 atm, 20 µA, and −8.9 kV, and it is known that particles carrying −3, −2, −1, 0, +1, +2, or +3 account for 94% (TSI 2009) of the total particles in the same size. These two facts mean that most of the particles in diameter of 390 nm carry negligible number of charges after passing the neutralizer compared with the ultimate charge number in the ESP. Therefore, charging effect by the radioactive source should have little impact on the performance of the ESP.
In order to test the robustness of this modified model under other pressure conditions, experimental particle penetrations of NaCl particles under 1.5 and 2.5 atm were compared with the corresponding calculated values based on the model. It can be observed in that the calculated particle penetration for particles of each particular size was lower when pressure rose with constant current, which is the same trend as presented by the experiments at 1.5 and 2.5 atm as well as the previous experiments at 1, 2, and 3 atm. Moreover, the calculated size distribution of particle penetrations is close to the corresponding experimental dotted line especially for particles larger than 100 nm, for both the 1.5- and 2.5-atm cases. This outcome demonstrates that the model using Equation (Equation20[19] ) is better able to predict the capture efficiency of particles with diameters greater than 100 nm.
Figure 9. Particle penetrations for various particle sizes measured under 1.5 and 2.5 atm and the corresponding calculated values using Equation (Equation20[19] ).
![Figure 9. Particle penetrations for various particle sizes measured under 1.5 and 2.5 atm and the corresponding calculated values using Equation (Equation20[19] ).](/cms/asset/c45c99a1-05fd-4b76-8a28-a6a1c512eb75/uast_a_1216071_f0009_b.gif)
4. Conclusions
This study examined the effect of gas pressure on the performance of an ESP. The corona onset voltage increased with the gas pressure, and under the same voltage, the ESP current was lower with higher gas pressure, because the higher gas density hindered the ionization. For positive ESP, under the same voltage, the ESP current was lower for the SFG than for the air. This phenomenon is attributed to the fact that the increase in CO2 concentration caused a higher ionization potential, a reduced effective electric field, and more electron traps. However, for negative voltages, the I-V characteristic curves for the cases of these two gases were similar, because the primary source of the ions for the negative corona is direct photoionization of the discharge electrode, which is independent of feed gas type.
The effect of gas pressure on the submicrometer particle capture in the ESP was studied with NaCl particles in both air and SFG. For both gas compositions, it can be concluded that: (1) at similar operating voltages, particle penetration was lower for a lower gas pressure, because the ion concentration was higher with lower gas pressure and (2) with the same current, the particle penetration was lower for a higher gas pressure, which was an outcome of the combined effects of lower particle axial velocity, lower particle radial velocity, and higher electrical field strength.
The capture of fly ash particles from a drop-tube furnace was also studied at the same three pressures. The charge status of the fly ash particles played an important role in this process. With higher pressure in the combustor, more fly ash particles might be multiple charged and gain opposite polarity to the ESP when they entered the ESP. As a result, for the same current, the particle capture efficiency became lower with higher pressure.
A semiempirical model was established based on the theoretical D-A equation and experimental data under 1, 2, and 3 atm to calculate the particle penetrations under high pressure. Instead of simply adding up charge numbers governed by the two charging mechanisms, the total number of charges on a particle (n′ = B1(I,P)ndiff + B2(I,P)nfield) was calculated by taking into account the effects of current and pressure on the relative weights of diffusion charging and field charging. Experimental penetrations under 1.5 and 2.5 atm validated this calculation model over the particle diameter range of 100–400 nm, which can facilitate the design of pressurized ESPs for controlling submicrometer particle emissions. It is necessary to note that the implementation of this model could be limited by particular configuration and scale of ESPs and oversimplified flow and charging conditions. More detailed studies on the fundamental mechanisms of how high pressure affects turbulence of the flow in ESPs and on charging efficiency of ultrafine particles are needed for a better understanding of capture efficiency in pressurized ESPs.
Nomenclature
| AC | = | collecting area of the ESP |
| Ag, Bg | = | constants of the gas of concern in the corona onset field strength estimation equation |
| B1, B2 | = | coefficients to adjust relative weights of charge numbers induced by diffusion charging and field charging (functions of current and pressure) |
| CC | = | Cunningham correction factor |
| = | rms average thermal speed of ions | |
| dm | = | collision diameter of molecules, approximately 3.7 × 10−10m for air |
| dp | = | particle diameter |
| E | = | average electric field strength in the wire-to-shell space |
| E0 | = | field strength at the surface of the wire ( |
| EC | = | field strength needed to start a corona discharge |
| e | = | elementary charge, 1.6 × 10−19 (C) |
| h | = | effective length of the wire |
| I | = | current |
| I0 | = | reference current, 20 (µA) |
| K0 | = | permittivity of free space, 8.85 × 10−12 (F/m) |
| KE | = | constant of proportionality, 9.0 × 109 (N·m2/C2) |
| k | = | Boltzmann constant, 1.38 × 10−23 (J/K) |
| m | = | mass of one ion |
| N | = | number of statistical values of concern |
| NA | = | Avogadro's number, 6.02 × 1023 |
| Ni | = | ion concentration |
| Noff | = | particle number concentration downstream of the ESP when ESP is off |
| Non | = | particle number concentration downstream of the ESP when ESP is on |
| n | = | number of elemental charges on a particle |
| nm | = | number of molecules per cubic meter |
| n′ | = | modified number of elemental charges on a particle |
| P | = | gas pressure |
| P0 | = | atmospheric pressure, 1.01325 × 105 (Pa) |
| p | = | particle penetration |
| Q | = | volumetric flow rate |
| Q0 | = | volumetric flow rate under atmospheric pressure and the reference temperature, T0 |
| R | = | idea gas law constant, 8.314 (J/mol·K) |
| r | = | horizontal distance from the center line of ESP |
| r0 | = | radius of the discharge wire |
| r1 | = | radius of the collection tube |
| T | = | temperature |
| T0 | = | reference temperature, 298.15 (K). |
| t | = | residence time of particles in the ESP ( |
| V | = | volume of the ESP |
| V0 | = | corona voltage at the surface of the central wire ( |
| VC | = | corona onset voltage |
| vTE | = | terminal migration velocity of a particle |
| Zi | = | mobility of ions, approximately 0.00015 (m2/V·s) |
Greek letters
| α | = | partial charging effect factor |
| ϵ | = | relative permittivity of particles |
| η | = | capture efficiency of the ESP |
| λ | = | mean free path |
| µ | = | dynamic viscosity, 1.8 × 10−5 (Pa·s) for air |
| δ′ | = | relative gas pressure compared to atmosphere pressure ( |
| σ′ | = | summation of squared deviations |
| σ | = | variance |
| φ | = | dimensionless current |
UAST_1216071_Supplementary_File.zip
Download Zip (797 KB)Acknowledgments
The authors thank Carla Roberts, Jim Ballard, Miguel Vazquez Pufleau, and Jiaxi Fang for their revisions and support.
Funding
This work was partially supported by the Consortium for Clean Coal Utilization (CCCU) at Washington University in St. Louis.
References
- Abdelsalam, M., and Wiitanen, D. (1993). Calculation of Corona Onset Voltage for Duct-type Precipitators. IEEE Trans. Ind. Appl., 29:274–280.
- Bai, M., Wang, S., Chen, Z., Leng, H., and Mao, S. (2010). The Effects of Submicrometer Dust Charging and Coagulation on ESP Efficiency by Using Alternating Electric Field. IEEE Trans. Plasma Sci., 38:127–132.
- Biswas, P., and Wu, C.-Y. (2005). Nanoparticles and the Environment. J. Air Waste Manag. Assoc., 55:708–746.
- Brown, R. F., and Walker, A. B. (1971). Feasibility Demonstration of Electrostatic Precipitation at 1700 °F. J. Air Pollut. Control Assoc., 21:617–620.
- Burtscher, H. (1992). Measurement and Characteristics of Combustion Aerosols with Special Consideration of Photoelectric Charging and Charging by Flame Ions. J. Aerosol Sci., 23:549–595.
- Burtscher, H., Reis, A., and Schmidtott, A. (1986). Particle Charge in Combustion Aerosols. J. Aerosol Sci., 17:47–51.
- Bush, J., Feldman, P., and Robinson, M. (1977). Development of a High-temperature/High-pressure Electrostatic Precipitator. U. S. Environmental Protection Agency, Office of Research and Development, Industrial Environmental Research Laboratory, Research Triangle Park, North Carolina, EPA-60017-77-132:36–48.
- Bush, J. R., Feldman, P. L., and Robinson, M. (1979). High Temperature, High Pressure Electrostatic Precipitation. J. Air Pollut. Control Assoc., 29:365–371.
- Chen, J., and Davidson, J. H. (2003). Model of The Negative DC Corona Plasma: Comparison to The Positive DC Corona Plasma. Plasma Chem. Plasma Process., 23:83–102.
- Chen, T. M., Tsai, C. J., Yan, S. Y., and Li, S. N. (2014). An Efficient Wet Electrostatic Precipitator for Removing Nanoparticles Submicron and Micron Sized Particles. Sep. Purif. Technol., 136:27–35.
- Che, H., Zhang, X., Li, Y., Zhou, Z., Qu, J. J., and Hao, X. (2009). Haze Trends over the Capital Cities of 31 Provinces in China, 1981–2005. Theor. Appl. Climatol., 97:235–242.
- Condon, E. U., and Odishaw, H. (1967). Handbook of Physics. McGraw-Hill, New York.
- Cooperman, G. (1984). A Unified Efficiency Theory for Electrostatic Precipitators. Atmos. Environ. (1967), 18:277–285.
- Fuchs, N. (1947). The Charges on the Particles of Aerocolloids. Izv. Akad. Nauk. SSSR, Ser. Geogr. Geofiz, 11:341–348.
- Gopan, A., Kumfer, B. M., Phillips, J., Thimsen, D., Smith, R., and Axelbaum, R. L. (2014). Process Design and Performance Analysis of a Staged, Pressurized Oxy-Combustion (SPOC) Power Plant for Carbon Capture. Appl. Energy, 125:179–188.
- Hinds, W. C. (1999). Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. John Wiley & Sons, New York.
- Huang, S.-H., and Chen, C.-C. (2002). Ultrafine Aerosol Penetration through Electrostatic Precipitators. Environ. Sci. Technol., 36:4625–4632.
- Huang, R.-J., Zhang, Y., Bozzetti, C., Ho, K.-F., Cao, J.-J., Han, Y., Daellenbach, K. R., Slowik, J. G., Platt, S. M., Canonaco, F., Zotter, P., Wolf, R., Pieber, S. M., Bruns, E. A., Crippa, M., Ciarelli, G., Piazzalunga, A., Schwikowski, M., Abbaszade, G., Schnelle-Kreis, J., Zimmermann, R., An, Z., Szidat, S., Baltensperger, U., El Haddad, I., and Prevot, A. S. H. (2014). High Secondary Aerosol Contribution to Particulate Pollution during Haze Events in China. Nature, 514:218–222.
- Jensen, A., Johnsson, J. E., Andries, J., Laughlin, K., Read, G., Mayer, M., Baumann, H., and Bonn, B. (1995). Formation and Reduction of NOx in Pressurized Fluidized Bed Combustion of Coal. Fuel, 74:1555–1569.
- Jing, H., He, S., Ou, Q., Hsiao, T.-C., and Chen, D.-R. (2013). Development of a Compact Electrostatic Nanoparticle Sampler for Offline Aerosol Characterization. Mapan, 28:217–226.
- Keefe, D., Nolan, P. J., and Rich, T. A. (1959). Charge Equilibrium in Aerosols According to the Boltzmann Law. Proc. Roy. Irish Acad. (Sect.A), 60:27–45.
- Knapp, M., Echt, O., Kreisle, D., Märk, T. D., and Recknagel, E. (1986). Formation of Long-lived CO2−, N2O−, and Their Dimer Anions, by Electron Attachment to Van Der Waals Clusters. Chem. Phys. Lett., 126:225–231.
- Komhyr, W. D. (1983). An Aerosol and Gas Sampling Apparatus for Remote Observatory Use. J. Geophys. Res., 88:3913–3918.
- Liang, W. J., and Lin, T. H. (1994). The Characteristics of Ionic Wind and Its Effect on Electrostatic Precipitators. Aerosol Sci. and Technol., 20:330–344.
- Lide, D. (2000). CRC Handbook of Chemistry and Physics 1999–2000: A Ready-Reference Book of Chemical and Physical Data, 81. CRC Press, Boca Raton, Florida.
- Lin, G.-Y., Chen, T.-M., and Tsai, C.-J. (2012). A Modified Deutsch-Anderson Equation for Predicting the Nanoparticle Collection Efficiency of Electrostatic Precipitators. Aerosol Air Qual. Res., 12:697–706.
- Lin, G. Y., Tsai, C. J., Chen, S. C., Chen, T. M., and Li, S. N. (2010). An Efficient Single-Stage Wet Electrostatic Precipitator for Fine and Nanosized Particle Control. Aerosol Sci. Technol., 44:38–45.
- Li, Y., Suriyawong, A., Daukoru, M., Zhuang, Y., and Biswas, P. (2009). Measurement and Capture of Fine and Ultrafine Particles from a Pilot-scale Pulverized Coal Combustor with an Electrostatic Precipitator. J. Air Waste Manag. Assoc., 59:553.
- McCain, J. D., Gooch, J. P., and Smith, W. B. (1975). Results of Field Measurements of Industrial Particulate Sources and Electrostatic Precipitator Performance. J. Air Pollut. Control Assoc., 25:117–121.
- Meij, R., and te Winkel, H. (2004). The Emissions and Environmental Impact of PM10 and Trace Elements from a Modern Coal-fired Power Plant Equipped with ESP and Wet FGD. Fuel Process. Technol., 85:641–656.
- Minchener, A. J. (2005). Coal Gasification for Advanced Power Generation. Fuel, 84:2222–2235.
- Mishra, D., Goyal, P., and Upadhyay, A. (2015). Artificial Intelligence Based Approach to Forecast PM2.5 During Haze Episodes: A Case Study of Delhi, India. Atmos. Environ., 102:239–248.
- Pachauri, T., Singla, V., Satsangi, A., Lakhani, A., and Kumari, K. M. (2013). Characterization of Major Pollution Events (Dust, Haze, and Two Festival Events) at Agra, India. Environ. Sci. Pollut. Res., 20:5737–5752.
- Park, J.-H., and Chun, C.-H. (2002). An Improved Modelling for Prediction of Grade Efficiency of Electrostatic Precipitators with Negative Corona. J. Aerosol Sci., 33:673–694.
- Park, S. J., and Kim, S. S. (1998). Effects of Particle Space Charge and Turbulent Diffusion on Performance of Plate–Plate Electrostatic Precipitators. J Electrostat., 45:121–137.
- Pauthenier, M., and Moreau-Hanot, M. (1932). Charging of Spherical Particles in an Ionizing Field. J. Phys. Radium, 3:590–613.
- Peek, F. W. (1920). Dielectric Phenomena in High Voltage Engineering. McGraw-Hill Book Company, New York.
- Pfafflin, J. R., and Ziegler, E. N. (1986). Advances in Environmental Science and Engineering. CRC Press, Boca Raton, Florida.
- Phelps, A. V. (1990). The Diffusion of Charged-particles in Collisional Plasmas - Free and Ambipolar Diffusion at Low and Moderate Pressures. J. Res. Natl Inst. Stand. Technol., 95:407–431.
- Riehle, C., and Löffler, F. (1993). Reflections on Similarity Laws Concerning Particle Transport in Electrical Precipitators. Powder Technol., 77:201–208.
- Rinard, G., Rugg, D. E., and Yamamoto, T. (1987). High-temperature High-pressure Electrostatic Precipitator Electrical Characterization and Collection Efficiency. IEEE Trans. Ind. Appl., 23:114–119.
- Robinson, M. (1967a). A Modified Deutsch Efficiency Equation for Electrostatic Precipitation. Atmos. Environ. (1967), 1:193–204.
- Robinson, M. (1967b). The Corona Threshold for Coaxial Cylinders in Air at High Pressures. IEEE Trans. Power App. Syst., 2:185–189.
- Robinson, M. (1971). Electrostatic Precipitation, in Air Pollution Control (Part 1), W. Strauss, ed., John Wiley & Sons, New York, pp. 227–335.
- Romay, F. J., Pui, D. Y. H., and Adachi, M. (1991). Unipolar Diffusion Charging of Aerosol-Particles at Low-Pressure. Aerosol Sci. Technol., 15:60–68.
- Smyth, H. D., and Stueckelberg, E. C. G. (1930). The Ionization of Carbon Dioxide by Electron Impact. Phys. Rev., 36:0472–0477.
- Stueckelberg, E. C. G. (1929). Simultaneous Ionization and Dissociation of Oxygen and Intensities of the Ultra-violet O2+ Bands. Phys. Rev., 34:65–67.
- Suriyawong, A., Gamble, M., Lee, M.-H., Axelbaum, R., and Biswas, P. (2006). Submicrometer Particle Formation and Mercury Speciation under O2-CO2 Coal Combustion. Energy Fuels, 20:2357–2363.
- Suriyawong, A., Hogan, C. J., Jiang, J. and Biswas, P. (2008). Charged Fraction and Electrostatic Collection of Ultrafine and Submicrometer Particles Formed during O2-CO2 Coal Combustion. Fuel, 87:673–682.
- Thornton, W. (1939). LXVIII. The Electric Strength of Gases, Measured by Corona Discharge. Philos. Mag., 28:666–678.
- TSI. (2009). Model 3080 SMPS Operation and Service Manual. Rev. J. TSI Inc., St Paul, MN.
- Villot, A., Gonthier, Y., Gonze, E., Bernis, A., Ravel, S., Grateau, M., and Guillaudeau, J. (2012). Separation of Particles from Syngas at High-Temperatures with an Electrostatic Precipitator. Sep. Purif. Technol., 92:181–190.
- Wang, X., Cotter, E., Iyer, K. N., Fang, J., Williams, B. J., and Biswas, P. (2015). Relationship Between Pyrolysis Products and Organic Aerosols Formed During Coal Combustion. Proc. Combust. Inst., 35:2347–2354.
- Wang, X., Daukoru, S. M., Torkamani, S., Wang, W.-N., and Biswas, P. (2013a). Role of Exhaust Gas Recycle on Submicrometer Particle Formation during Oxy-Coal Combustion. Proc. Combust. Inst., 34:3479–3487.
- Wang, X., Williams, B. J., Wang, X., Tang, Y., Huang, Y., Kong, L., Yang, X., and Biswas, P. (2013b). Characterization of Organic Aerosol Produced during Pulverized Coal Combustion in a Drop Tube Furnace. Atmos. Chem. Phys., 13:10919–10932.
- Williams, B. J., Goldstein, A. H., Kreisberg, N. M., and Hering, S. V. (2006). An In-Situ Instrument for Speciated Organic Composition of Atmospheric Aerosols: Thermal Desorption Aerosol GC/MS-FID (TAG). Aerosol Sci. Technol., 40:627–638.
- Ylätalo, S. I., and Hautanen, J. (1998). Electrostatic Precipitator Penetration Function for Pulverized Coal Combustion. Aerosol Sci. Technol., 29:17–30.
- Yokoyama, T., Asakura, K., Matsuda, H., Ito, S., and Noda, N. (2000). Mercury Emissions from a Coal-Fired Power Plant in Japan. Sci. Total Environ., 259:97–103.
- Yoo, K. H., Lee, J. S., and Oh, M. D. (1997). Charging and Collection of Submicron Particles in Two-stage Parallel-plate Electrostatic Precipitators. Aerosol Sci. Technol., 27:308–323.
- Yuan, C.-S. J., and Shen, T. T. (2004). Electrostatistic Precipitation, in Air Pollution Control Engineering, L. K. Wang, N. C. Pereira and Y.-T. Hung, eds, Humana Press, Totowa, New Jersey, pp. 153–196.
- Zevenhoven, R., and Kilpinen, P. (2001). Control of Pollutants in Flue Gases and Fuel Gases. Helsinki University of Technology, Espoo, Finland.
- Zhuang, Y., and Biswas, P. (2001). Submicrometer Particle Formation and Control in a Bench-Scale Pulverized Coal Combustor. Energy Fuels, 15:510–516.
- Zhuang, Y., Kim, Y. J., Lee, T. G., and Biswas, P. (2000). Experimental and Theoretical Studies of Ultra-fine Particle Behavior in Electrostatic Precipitators. J. Electrostat., 48:245–260.

![Figure 3. Experimental and calculated I-V curves by Equations (Equation5[5] ) and (Equation6[6] ) under different pressures.](/cms/asset/8fc32138-9ed8-4332-a78b-2254a1de0f57/uast_a_1216071_f0003_b.gif)