ABSTRACT
Mineral dust is the second largest emission by mass into the atmosphere. Aerosol particles affect the radiative forcing budget by directly scattering and absorbing light, acting as cloud condensation and ice nuclei, and by providing surfaces for heterogeneous chemistry. Factors that affect how the particles scatter and absorb light include their composition, shape, size, and concentration. In this study, we characterize the most common components of mineral dust, quartz, and aluminosilicate clay minerals. In addition, we apply our results from calcite, feldspars, quartz, and aluminosilicate clay minerals to model the optical properties of Arizona test dust (ATD). We use cavity ring-down spectroscopy to measure the extinction cross sections of size-selected particles, electron microscopy to characterize the size selection, and Mie theory as well as the discrete dipole approximation as models. For quartz, the extinction cross sections can be well modeled assuming the particles are spheroids or spheres. For clay minerals, even spheroids fail to model the extinction cross sections, potentially due to orientation effects and lift forces in our flow system. In addition, aluminosilicate clay minerals experience weak size selectivity in the differential mobility analyzer. For ATD, the extinction cross sections are best modeled by treating each component of the mixture separately in terms of shape and size distribution. Through the application to ATD, our study outlines the procedure that can be used to model the optical properties of complex airborne dust mixtures.
Copyright © 2016 American Association for Aerosol Research
EDITOR:
Introduction
Mineral dust is the second largest emission of particulate matter by mass into the atmosphere with 1000–3000 Tg emitted annually (Dentener et al. Citation1996; Ginoux et al. Citation2001; Forster et al. Citation2007; Ginoux et al. Citation2012). The major source of this dust is the dust belt that stretches from the Sahara through central Asia (Prospero et al. Citation2002). From modeling and satellite data, 25% of the total dust is estimated to originate from anthropogenic sources (Ginoux et al. Citation2012). The majority of this dust will gravitationally settle near the source region, but particles under 7.3 μm, which includes a large fraction of submicron particles, can undergo long-range transport (Maring et al. Citation2003; Zhao et al. Citation2003). Gravitational settling of the particles during transport is also dependent on particle shape (Yang et al. Citation2013). Quartz composes up to 50% by weight (wt.%) of mineral dust, aluminosilicate clay minerals compose approximately 60 wt.% of Saharan dust and 85 wt.% of Asian dust, and carbonates compose up to 11 wt.% of Asian dust (Liu Citation1985; McNaughton et al. Citation2009).
Arizona test dust (ATD) has been used as a proxy of mineral dust for experimental studies of ice nucleation, heterogeneous chemistry, and optical properties (Marcolli et al. Citation2007; Curtis et al. Citation2008; Ndour et al. Citation2008; Kanji and Abbatt Citation2010; Broadley et al. Citation2012; Glen and Brooks Citation2013). Note that while ATD has been used extensively, NX-illite, an illite-rich mixture of minerals described in more detail in the Experimental Methods, has recently been proposed as a more representative proxy for long-range transported dust (Broadley et al. Citation2012). ATD consists of quartz (17.1 wt.%), feldspar (33.2 wt.%), carbonate (5.6 wt.%), illite (7.5 wt.%), kaolinite (2 wt.%), smectites (10.2 wt.%), and other aluminosilicate clays (24.4 wt.%; Broadley et al. Citation2012). These components have a range of refractive indices from 1.522 to 1.601 (Sokolik and Toon Citation1999; Egan and Hilgeman Citation1979). In addition, each particle type has a different shape, with quartz, carbonates, and feldspars having aspect ratios less than 2 while the aluminosilicate clay mineral components (kaolinite, illite, and smectites) have large aspect ratios (Nadeu Citation1985; Inoue and Kitagawa Citation1994; Veghte and Freedman Citation2014).
7w?>Particle interactions with solar and terrestrial radiation are termed the aerosol direct effect. The direct radiative effect from aerosol particles has been calculated to be −0.5 ± 0.4 W m−2 with mineral dust aerosol particles contributing −0.1 ± 0.2 W m−2 (Forster et al. Citation2007). The unknowns of composition, emission amounts, size distribution, and shape of mineral dust contribute to uncertainties in radiative balance (Johnson et al. Citation2009; Kok Citation2011; Johnson et al. Citation2012). Models tend to use externally mixed particles (Kalashnikova et al. Citation2005), but particle radiative properties depend on the internal structure of the particle as well (Bohren and Huffman Citation2004).
Particle shape has been shown to be important for modeling the optical properties of mineral dust aerosol particles (Gasteiger et al. Citation2011; Kahnert et al. Citation2012). Particles such as aluminosilicate clay minerals consist of alternating layers of tetrahedrally coordinated silicon and octahedrally coordinated aluminum and have large aspect ratios (Martin et al. Citation1991). The reported aspect ratios of aluminosilicate clay minerals range from 3 to 500 (Nadeau Citation1985; Inoue and Kitagawa Citation1994; Veghte and Freedman Citation2014). In contrast, quartz (SiO2) has an aspect ratio near unity at 1.7–1.9 (Siegesmund et al Citation2002). While particles are often modeled using spheres, researchers have started to incorporate spheroids with a large range of aspect ratios that more accurately model the scattering properties of mineral dust (Kahnert Citation2004; Nousiainen et al. Citation2006; Merikallio et al. Citation2011; Haapanala et al. Citation2012; Koepke et al. Citation2015).
The scattering and extinction cross sections of high aspect ratio mineral dust aerosol have been investigated previously using polar nephelometers and long pass infrared instruments. For aluminosilicate clay minerals, Mie theory does not model the phase functions well and either calculated phase functions based on experimental phase functions and size distribution data (Curtis et al. Citation2008) or models that account for the high aspect ratios of these particles are needed (Hudson et al. Citation2008; Meland et al. Citation2012). For these studies, spheroids showed a great improvement in modeling the experimental phase function compared to spheres modeled with Mie theory (Volten et al. Citation2001; Hudson et al. Citation2007, Citation2008; Meland et al. Citation2012). Even though better agreement with theory is observed for these models, they underestimate the scattering of the particles. Quartz has also been modeled as spheres and spheroids, and elongated shapes were found to provide a better model for the optical properties of the particles (Curtis et al. Citation2007, Citation2008; Hudson et al. Citation2008). Mie theory has not been able to model the phase functions of Arizona Test Dust; only empirical models have been able to accurately describe the scattering properties (Curtis et al. Citation2008; Glen and Brooks Citation2013).
Cavity ring-down aerosol extinction spectroscopy (CRD-AES) has been previously used to study mixed organic/salt particles (Riziq et al. Citation2007; Freedman et al. Citation2009; Lang-Yona et al. Citation2009; Mellon et al. Citation2011) and mineral dust aerosol (Attwood and Greenslade Citation2011; Veghte and Freedman Citation2012; Veghte et al. Citation2015). CRD-AES has a high sensitivity due to having a long effective path length and independence from laser output intensity (Pettersson et al. Citation2004; Riziq et al. Citation2007; Li et al. Citation2011). Because of the high sensitivity of CRD-AES, it can be used for size-selected samples at low concentrations. This high sensitivity has been recently demonstrated by the use of CRD-AES for single particle extinction cross section measurements (Walker et al. Citation2013). Other techniques to measure the scattering properties of aerosol particles include nephelometers and mid-IR extinction spectrometers, although they have a high detection limit and require the use of bulk samples (Heintzenberg and Charlson Citation1999; Mogili et al. Citation2007, Citation2008).
In this article, we have characterized the extinction cross sections of aluminosilicate clay minerals (kaolinite, montmorillonite, and NX-illite), quartz, and Arizona Test Dust using CRD-AES and electron microscopy. Electron microscopy has been used to obtain the aspect ratios and size distributions of the particles (Veghte and Freedman Citation2014). The extinction cross sections obtained for spheroids using the discrete dipole approximation (DDA) are compared to calculations for spheres using Mie theory to understand the effect of shape on the particle optical properties. Calculations are performed for both monodisperse and polydisperse size distributions of particles.
Experimental methods
Generation of aerosol particles
Particles were aerosolized using the dry generation method that we have previously described (Veghte and Freedman Citation2012; Veghte et al. Citation2015). Mineral dust analogs studied included: silicon dioxide (quartz; >99%, Sigma Aldrich), kaolinite, Warren County, Georgia, USA (KGa-2; Clay Mineral Society, West Lafayette, IN, USA); montmorillonite, Gonzales County, TX, USA (STx-1b; Clay Mineral Society, West Lafayette, IN, USA), NX-illite (Arginotec, NX Nanopowder, B+M Notenkämper, Munich, Germany), and Arizona test dust (0–3 μm fraction, Powder Technology Inc., Burnsville, MN, USA). NX-Illite is an illite-rich mixture of minerals consisting of quartz (6.6 wt.%), feldspar (9.8 wt.%), carbonate (2.1 wt.%), illite (60.5 wt.%), mixed illite-smectite (13.8 wt.%), and kaolinite (7.2 wt.%; Broadley et al., Citation2012). Each species was aerosolized by directing a flow of nitrogen at 1.5 lpm over the powder while constantly stirring. These aerosolized particles were then directed through an Erlenmeyer flask to maintain a steady concentration of particles (Sullivan et al. Citation2009). Excess particles were pumped away and the flow was diluted to 1.5 lpm. A diagram of the setup is included in the online supplemental information (SI).
Size selection of aerosol particles
Aerosolized particles were size selected using an electrostatic classifier (TSI 3080, Shoreview, MN, USA) and a differential mobility analyzer (DMA; TSI 3081) for sizes ranging from 250 nm to 900 nm. The DMA size selects particles based on their electrostatic mobility. The recommended ratio of the aerosol flow to sheath flow is 1:10, but we have previously shown better size selection for larger, irregularly shaped particles with a smaller difference between the two flows (Veghte and Freedman, Citation2012). As a result, each particle type was passed through the DMA at 1.5 lpm, adjusting the sheath flow rate from 2.7 to 8.8 lpm, depending on the size selected. These size-selected particles are then passed through the CRD-AES to determine extinction coefficients before being counted by a condensation particle counter (CPC; TSI 3775). Size distributions of the particles were determined using a scanning mobility particle sizer (SMPS; TSI).
Extinction cross sections of aerosol particles
A cavity ring-down aerosol extinction spectrometer was used to determine the extinction cross sections of the size-selected particles at 643 nm as described previously (Veghte and Freedman Citation2012; Veghte et al. Citation2015). The characteristic ring-down time for our instrument is 165 μs, corresponding to an effective path length longer than 48 km. The extinction coefficient, bext, can be calculated from the ring-down time of each pulse using[1] where τ0 is the decay constant without aerosol particles and τ is the ring-down time with aerosol particles in the cavity. The constants in Equation (Equation1
[1] ) are RL, the ratio of the total cavity length to that occupied by aerosol particles, and c, the speed of light. The extinction cross section can be calculated according to
[2] where N is the number of particles per cm3.
Scanning electron microscopy (SEM)
The size selection of the aerosol particles from the DMA was characterized by SEM. We have shown previously the utility of using electron microscopy to describe the size selection of irregular particles to model aerosol extinction cross sections. SEM was used for quartz, aluminosilicate clay minerals, and ATD because it can be used to obtain the aspect ratios for both top-down and side-on orientations of the particles (Veghte and Freedman Citation2014). We have, in addition, determined the aspect ratios of two feldspar samples, labradorite (Sonora, Mexico, Ward's Scientific) and orthoclase (Lago, Maggiore, Baveno, Italy, Ward's Scientific), which were ground using a mortar and pestle. After size selection with the DMA, the aerosol flow was directed into a cascade impactor (PIXE International Corp. Tallahassee, FL, USA) that was backed with a pump at 1.0 lpm. Particles were collected on silicon wafer chips (Virginia Semiconductor Inc., Fredericksburg, VA, USA) for SEM analysis. For the quartz particles, the silicon substrate was coated with a thin layer of Formvar 15/95 (Electron Microscopy Science, Hatfield, PA, USA) to prevent damage to the substrate from the particle impaction. For all species, the silicon was coated with a low molecular weight polymer (3M double-sided sticky tape) dissolved in chloroform (J. T. Baker, HPLC grade) to increase the collection efficiency. The extinction coefficients of the particles were monitored by CRD-AES to obtain enough particles for analysis. A FEI NanoSEM 630 FESEM operated at 3 keV was used to image the particles at each size selected, and 419 to 643 particles were analyzed per sample.
From the SEM images, the area equivalent diameter of each particle was calculated using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The side-on aspect ratios, which account for the height of the particles, were used from our previous study (Veghte and Freedman Citation2014) and the volume equivalent diameters were calculated assuming that the particles were spheroids. Mean values used for the top-down/side-on aspect ratios were: 1.38/1.64 for quartz, 1.44/4.80 for NX-illite, 1.39/8.89 for KGa-2, and 1.38/7.49 for STx-1b (Veghte and Freedman Citation2014). ATD particles up to 2 μm in diameter and feldspar particles up to 4 μm in diameter were analyzed.
Modeling optical properties of aerosol particles
Quartz is a birefringent material with an ordinary and extraordinary refractive index of approximately 1.55. All the clay minerals are biaxial, causing a trirefringence in the particles. Because of the complex behavior of the refractive indices of the clay minerals, the literature refractive indices are reported as an average over a bulk sample with no orientation effects (Egan and Hilgeman Citation1979). For illite, the range of refractive indices reported is 1.54–1.60 with an average value of 1.57. Kaolinite has a refractive index range of 1.55–1.57, which averages to 1.56. Montmorillonite has the lowest refractive index of these clay minerals with a range of 1.49–1.55, which averages to 1.52 (Egan and Hilgeman Citation1979; Sokolik and Toon Citation1999). None of the minerals absorb significantly at 643 nm.
For the ATD samples, three different types of calculations and three different methods for accounting for the size distribution at each mobility diameter were performed for a total of nine models. The models are summarized in . In the first type of calculation (models 1–3), all components of ATD were modeled using Mie theory. In the second type of calculation (models 4–6), all components were modeled as spheroids using the DDA with an aspect ratio of 1.84. The aspect ratio of 1.84 was used because it is the median aspect ratio from the SEM analysis. In the third type of calculation (models 7–9), each component was either modeled as a sphere using Mie theory or spheroid using the DDA, depending on which shape has been shown to best model the extinction cross section. For calcite and quartz, spheres have been shown previously to model the extinction cross sections well (Veghte and Freedman Citation2012). Because feldspars are non-absorbing minerals at 643 nm and have a small aspect ratio similar to calcite and quartz, Mie theory was used to calculate the optical extinction (see the supporting information). For the clay mineral species of illite, kaolinite, and montmorillonite, spheroids with an aspect ratio that is the same as the actual aspect ratios of the species were used (Veghte and Freedman Citation2014). The aspect ratios used for models 7–9 are summarized in . The size distribution at each mobility diameter was accounted for in three different ways, by assuming the particles were monodisperse (models 1, 4, 7), by using the size distribution measured for ATD (models 2, 5, 8), or by using the size distributions of the individual components (models 3, 6, 9). All of these size distributions were obtained from electron microscopy. For each of these models, the refractive indices of the individual components were used (). Calculation of the extinction cross section of a polydisperse distribution at a given mobility diameter will be described in the following section. The resultant extinction cross sections for any type of size distribution used are weighted by the composition of ATD to calculate the modeled extinction cross section at a given mobility diameter.
Table 1. Summary of the nine models used to calculate the optical properties of Arizona test dust.
Table 2. Description of the refractive indices and aspect ratios for the components of Arizona test dust.
Mie calculations of extinction cross section
Extinction cross sections were determined for spherical and non-spherical particles. To model spherical particles, Mie theory was performed using a MATLAB version of BHCOAT (Mätzler Citation2002; Bohren and Huffman Citation2004; Riziq et al. Citation2007; Freedman et al. Citation2009). To calculate the extinction efficiency, Qext, at each size, the average literature value for the refractive index, m, is used and the size parameter xae =πdae/λ was calculated from the volume equivalent diameter. The extinction cross section, σext, for each volume equivalent diameter was determined from σext =πQext (dae/2)2. Because the size distributions of the particles are polydisperse, a weighted average of the extinction cross sections for a distribution of polydisperse spheres was used to calculate the extinction cross section according to[3] for each mobility diameter from 250 to 900 nm, where ntot is the total number of particles, nbin,i is the number of particles in each particle size bin, and σext,i is the extinction cross section of the particles in that bin. Error was estimated by dividing each of the SEM images into three subgroups. For each of these three subgroups, the extinction cross section for polydisperse spheres was found and the standard deviation between these three measurements was used as an overestimate of the error (Veghte and Freedman Citation2012).
Discrete dipole approximation calculations of extinction cross section
The discrete dipole approximation (DDA) was used to calculate the scattering properties of spheroidal particles. In the DDA, a particle is defined as a finite number of dipoles in an array. DDA calculations were performed using DDSCAT 7.2 developed for FORTRAN-90 (Draine and Flatau Citation1994, Citation2010). For calculations of the scattering properties of roughened particles, an average of 196 scattering angles is required to obtain convergence of each extinction cross section (Hajihashemi and Jian Citation2013; Veghte et al. Citation2015). Because spheroidal particles have multiple symmetry axes compared to roughened particles, fewer particle orientations are needed. As a result, only 24 scattering angles were used for the spheroidal particles due to the symmetry of the particles. This number corresponds to the same number of particle orientations as used for rough, asymmetric particles in our previous study (Veghte et al. Citation2015).
To determine the volume equivalent radius, aeff, at which the DDA will be accurate given the number of dipoles, Nd, we have used the equation[4] where λ is the wavelength, and m is the refractive index. At larger volume equivalent diameters, the dipole spacing is too large, introducing additional error into the calculations. The dipole spacing was chosen so that there were 40 dipoles spanning the radius of a volume-equivalent sphere, which, according to Equation (4), leads to accurate scattering and absorption cross sections for spheroidal particles up to a 4 μm volume equivalent diameter.
Mie theory calculations were used to fit the extinction cross sections obtained from the DDA calculations of spheroids for quartz and the aluminosilicate clay minerals. The retrievals of effective refractive index, m, were performed using the results from the DDA calculations for various size ranges and fitting to Mie theory using the minimum in the cumulative fractional difference (CFDR) to determine the best fit (Freedman et al. Citation2009; Hasenkopf et al. Citation2010; Veghte et al. Citation2015). CFDR is defined according to[5] where P is the number of particle sizes selected and bext,measured are the extinction coefficients measured with cavity ring-down spectroscopy. This method has the same minimum value as using the χ2 function (Riziq et al. Citation2007). For each mineral type, best fit parameters were determined for the diameter ranges: 30–210 nm, 230–410 nm, 430–610 nm, 630–810 nm, 830–1010 nm, 30–490 nm, 510–1010 nm, and 30–1010 nm.
Results and discussion
The optical properties of mineral dust components (quartz and aluminosilicate clay minerals) and a heterogeneous mixture of mineral dust (ATD) have been studied using CRD-AES and computational methods. A comparison of the bulk size distributions measured using SEM and SMPS was performed. To characterize the size selection of the mineral dust particles, electron microscopy was used to analyze the volume equivalent diameters at various mobility diameters selected using the DMA, as described above. The size distributions obtained from the SEM measurements were used to calculate the theoretical optical extinction cross sections at each mobility diameter.
Size distributions of quartz
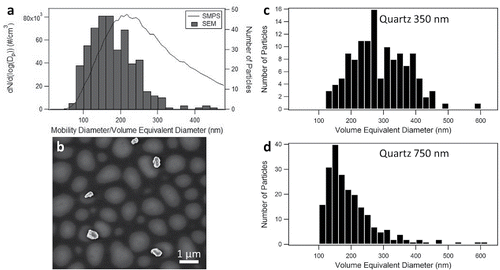
The size distribution from SMPS, which is plotted as a function of mobility diameter, was compared to the size distribution obtained by SEM images (), which is displayed as a function of volume equivalent diameter. The peak in the SMPS spectrum occurs at a mobility diameter of 217 nm, whereas the peak in the size distributions of the bulk powder obtained from SEM occurs at a volume equivalent diameter of 160 nm. In addition, we observe that particles with a mobility diameter above 500 nm tend to be aggregates. When the aggregates are impacted on the SEM substrate, some break apart to form particles that are between 100 and 200 nm in size (; Figure S3). The breakup of these aggregates skews the SEM size distribution towards smaller particle sizes. In addition, the polydispersity of particles size selected at a given mobility diameter, such as we have previously observed for particles with a similar shape to quartz, likely results in the broadening of the size distribution observed with SMPS (Veghte and Freedman Citation2012). show a comparison of size distributions plotted as a function of volume equivalent diameter for particles size selected at mobility diameters of 350 nm and 750 nm. For a mobility diameter of 350 nm, the mean particle size is 269 nm. For a mobility diameter of 750 nm, a distribution of smaller particles is observed that is similar to the size distribution for the bulk sample in the SEM measurements. In contrast, when the size distributions at these mobility diameters were compared for calcite, both electron microscopy size distributions had a maximum near the mobility diameter selected, but with a higher polydispersity than for nearly spherical ammonium sulfate particles (Veghte and Freedman Citation2012).
Figure 1. Quartz aerosol particles: (a) comparison of bulk sample by SEM (bars) and SMPS (line), (b) SEM image of quartz particles at 450 nm, and size distribution from SEM of size-selected particles at mobility diameters of (c) 350 nm and (d) 750 nm. Note that the particles in (b) have crisp edges; the lighter, out-of-focus areas are due to the coating on the substrate.
Size distributions of aluminosilicate clay minerals
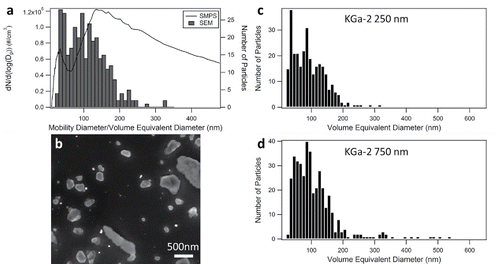
A large difference was observed between the size distribution of kaolinite obtained with SMPS and volume equivalent diameters of the bulk sample measured with SEM. Specifically, SEM analysis of bulk samples of kaolinite (KGa-2) yield the largest average particle size of the aluminosilicate clay mineral species studied with a maximum at a volume equivalent diameter of approximately 80 nm (). In comparison, the size distribution from the SMPS system has a peak at a mobility diameter of 136 nm with a broad distribution. A representative SEM micrograph of KGa-2 particles is shown in . Little difference was observed in the size distributions of volume equivalent diameters collected at mobility diameters of 250 and 750 nm with the peak of the distribution centered at a volume equivalent diameter of approximately 80 nm (). The major difference in the size selection of the particles at mobility diameters of 250 and 750 nm is that particles above a volume equivalent diameter of 300 nm are transmitted at a mobility diameter of 750 nm. These results indicate that kaolinite is not well size selected using the DMA.
Figure 2. Kaolinite (KGa-2) aerosol particles (a) comparison of bulk sample by SEM (bars) and SMPS (line), (b) SEM image of KGa-2 particles, and size distribution from SEM of size-selected particles at (c) 250 nm and (d) 750 nm.
The other aluminosilicate clay minerals (montmorillonite and NX-illite) had similar results to kaolinite. Montmorillonite (STx-1b) had a SMPS maximum at 275 nm while the SEM measurements had a maximum at 30 nm. Similarly, NX-illite had a SMPS maximum at 269 nm and a SEM volume equivalent diameter maximum at 30 nm. The size-selected volume equivalent diameters from SEM analysis at small and large diameters were similar to the bulk distributions and only a few larger particles were size selected at the larger diameters. A full discussion of the results for STx-1b and NX-illite, and a comparison of the size selection for each particle type, can be found in the SI.
Particle size selection through a DMA is contingent on factors that include aerosol flow rate, sheath flow rate, applied potential, particle size, and particle shape. Spherical and near-spherical particles (such as ammonium sulfate) are size selected well at all sizes using a DMA. When particles deviate in shape from spheres, but retain a similar aspect ratio (e.g., calcite or quartz), the peak in the size distribution of area or volume equivalent diameters is close to the mobility diameter, but with a much larger polydispersity (Veghte and Freedman Citation2012). When the particles deviate further in shape, such as high aspect ratio aluminosilicate clay minerals, little size selection occurs. Previous studies have shown a difference between the ice nucleation activity of size-selected particles using a DMA, with larger mobility diameters acting as better ice nuclei (Welti et al. Citation2009; Lüönd et al. Citation2010). We have shown here and previously that the size distribution selected using a DMA is dependent on the shape of the particles. Furthermore, the distribution of platelet-shaped particles is similar at different mobility diameters. Any difference in ice nucleation activity with mobility diameter, especially for aluminosilicate clay minerals, may be due to the few larger particles transmitted at larger mobility diameters.
ATD particle morphology
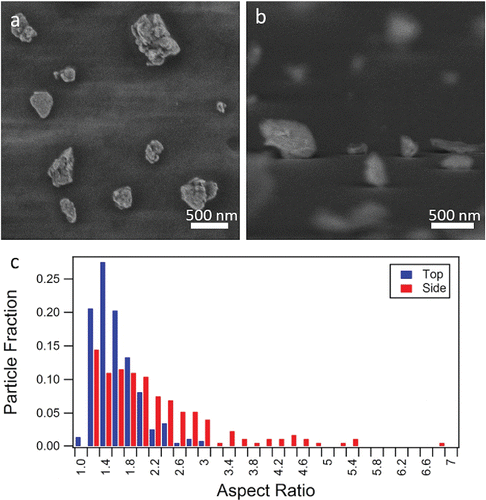
SEM was used to analyze the shape of the ATD particles. shows irregularly shaped particles, of similar roughness to particle types that we have studied previously (Veghte and Freedman Citation2012; Veghte and Freedman Citation2014). From the side-on orientation (), different particle aspect ratios are observed, ranging from near unity to 7. shows the range of aspect ratios for the top-down and side-on orientations. From a top-down orientation, the particles have a fairly uniform aspect ratio with a median value of 1.41. From a side-on view, two different modes are observed: a mode with an aspect ratio of 1.2–1.6 and a mode with a relative maximum at 4.4. Particles with high aspect ratios are indicative of clay minerals (Veghte and Freedman Citation2014). The mean aspect ratio from a side-on view is 2.09, but for modeling purposes the median aspect ratio of 1.84 is used. Additional discussion and figures of ATD aspect ratios are included in the SI.
Figure 3. SEM images of ATD from the (a) top-down and (b) side-on orientations with (c) the distribution of aspect ratios determined for both orientations.
Additional information was needed to model the feldspar species. As in our previous work, we used SEM to measure the aspect ratios of two different feldspars (Veghte and Freedman Citation2014). Both feldspars have aspect ratios less than 2 (Figure S8). Labradorite has a top-down aspect ratio of 1.62 and side-on aspect ratio of 1.65 while orthoclase has a top-down aspect ratio of 1.58 and a side-on aspect ratio of 1.53. Species with similar aspect ratios that do not absorb light at 643 nm such as calcite have been modeled well using Mie theory (Veghte and Freedman Citation2012). We have therefore used Mie theory to model feldspars in calculations where the different components of ATD are treated individually ().
Extinction cross sections of quartz aerosol
Quartz minerals have a slightly elongated shape with aspect ratios of 1.38 and 1.64 (Veghte and Freedman Citation2014). Because the shape is elongated, the optical properties of these particles are different than for spherical particles. Previous experiments have shown that the scattering phase functions of these particles are better modeled by spheroids than spheres (Volten et al. Citation2001; Curtis et al. Citation2007; Curtis et al. Citation2008). Light extinction in the mid-IR region is also better modeled through incorporation of a spheroid model (Hudson et al Citation2008).
shows the results from CRD-AES measurements for quartz. As we have previously observed for calcite, the polydispersity of volume equivalent diameters for quartz at each mobility diameter is large, which suggests that it needs to be incorporated to accurately model the extinction cross sections of these particles (Veghte and Freedman Citation2012). For mobility diameters smaller than 450 nm, the extinction cross sections from CRD-AES (diamonds) are higher than predicted by Mie theory for monodisperse spheres (black line) and lower for mobility diameters greater than 450 nm. When the polydispersity of volume equivalent diameters for each mobility diameter was included in the calculation, Mie theory modeled the experimental results well for mobility diameters up to 550 nm. At larger sizes, the calculated extinction cross sections were lower than the experimental results because larger aggregated particles break apart when impacted on the SEM substrates, resulting in a larger number of smaller particles.
Figure 4. Extinction cross section vs. mobility diameter for quartz. Mie theory for monodisperse spheres (solid line) and DDA calculations for monodisperse spheroids (dashed line) are compared to the extinction cross sections obtained from CRD-AES (diamonds) and theoretical Mie calculations for spheres that incorporate the polydispersity determined from the SEM measurements.
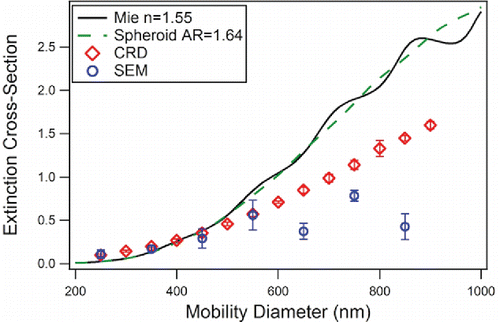
The quartz particles were also analyzed using the DDA for spheroids with aspect ratios of 1.64 (Veghte and Freedman Citation2014). The DDA calculations did not greatly differ from Mie theory. The major difference between the two sets of calculations was that the oscillations present in the Mie theory calculations for monodisperse spheres were dampened in the DDA calculations (). Even when the polydispersity of the size distribution was included in the calculations, no improvement was observed between modeling quartz particles as spheroids or spheres. The major difference between Mie theory and the DDA calculations was in the scattering phase functions of the particles (Figure S7).
Extinction cross sections of aluminosilicate clay mineral dust aerosol
The extinction cross sections of the aluminosilicate clay mineral particles were measured using CRD-AES. Previous research has shown that Mie theory does not model the optical properties of these compounds well, and that accounting for shape allows the models to better agree with the measured scattering properties (Volten et al. Citation2001; Hudson et al. Citation2007; Curtis et al. Citation2008; Hudson et al. Citation2008; Meland et al. Citation2012). Our measured extinction cross sections were much lower than predicted from calculations for monodisperse particles, especially for kaolinite ( and S6). We note that the kaolinite particles have a smoother surface morphology than NX-illite or montmorillonite. The rough features of NX-illite and montmorillonite increase the overall surface area of the particles, allowing for additional light scattering (Figure S6). Mie theory calculations for monodisperse particles are compared to the experimental results for NX-illite, montmorillonite, and kaolinite in Figure S6. When the polydispersity of volume equivalent diameters observed at each mobility diameter was incorporated into the Mie theory calculations, the model results for all aluminosilicate clay minerals were lower than the measured extinction cross sections.
Figure 5. Extinction cross section vs. mobility diameter for KGa-2. The CRD-AES data ([blue] circles) show the experimentally determined cross section at each mobility diameter. The solid lines represent the models using monodisperse particles for Mie theory, randomly oriented spheroids, and oriented spheroids. Red circles and triangles indicate models where polydispersity is taken into account for mobility diameters of 250 and 750 nm and is shown for both the particles treated as area equivalent spheroids and volume equivalent spheroids. The same plot with an expanded y-axis is shown in the inset.
![Figure 5. Extinction cross section vs. mobility diameter for KGa-2. The CRD-AES data ([blue] circles) show the experimentally determined cross section at each mobility diameter. The solid lines represent the models using monodisperse particles for Mie theory, randomly oriented spheroids, and oriented spheroids. Red circles and triangles indicate models where polydispersity is taken into account for mobility diameters of 250 and 750 nm and is shown for both the particles treated as area equivalent spheroids and volume equivalent spheroids. The same plot with an expanded y-axis is shown in the inset.](/cms/asset/d135ae7a-6fe1-4b85-92b4-3857c5bc5a08/uast_a_1225153_f0005_oc.gif)
We analyzed KGa-2 further to determine how particle shape and orientation affects the extinction cross sections of these particles. In addition to calculations of isotropically distributed spheroids, we have modeled the extinction cross sections of oriented particles. Elongated particles, such as disk-like aluminosilicate clay minerals, orient in the flow of the cavity with their long axis in the direction of the flow (Dahneke Citation1973; Gallily and Eisner Citation1979; Hinds Citation1996; Baron et al. Citation2001). The orientation is dependent on the flow rates and the sizes of the particles. Researchers have determined that systems that have a Reynolds number greater than 0.1 will have some alignment to the flow (Hinds Citation1996; and the Reynolds number inside the CRD-AES cavity is approximately 182. In , the experimental extinction cross sections are compared to the calculations for monodisperse spheres, spheroids, and spheroids oriented parallel to the flow. For calculations of monodisperse spheroids compared with monodisperse spheres, the isotropically oriented particles have lower extinction cross sections than the spherical particles, while particles oriented parallel to the flow have lower extinction cross sections below approximately 600 nm and higher extinction cross sections at diameters greater than 600 nm. None of the models correct the theory to agree with the experimental CRD-AES results. When the polydispersity of the particles was included, if only the area equivalent diameter was used then there is agreement at 250 nm, but the extinction cross sections decrease with diameter. When a volume equivalent diameter is used that includes the height of the particles from SEM analysis, the agreement is worse than any of the calculations that assume a monodisperse distribution of particles. The fact that the modeled extinction cross sections for polydisperse, isotropically oriented spheroids are lower than the extinction cross sections obtained experimentally could be due to an increase in the concentration of kaolinite in the center of the cavity due to lift forces. These lift forces are caused by a pressure and flow differential that causes particles to move away from the walls towards the center of the cavity (Segré and Silberberg Citation1961). This force will affect disk shaped particles oriented in the flow greater than spherical particles and cause a particle gradient within the cylinder (Kishore and Gu Citation2010). Since the laser is most intense in the center of the CRD-AES instrument cavity, an increase in particle concentration in the center of the cavity could increase the values of the extinction coefficients observed, but not affect the particle concentration measured with the CPC. In addition, spheroids have a smaller surface area for scattering than the actual kaolinite particles, which could reduce the calculated extinction cross sections. For the phase functions, the forward scattering is enhanced for the spheroids (Figures S7).
Extinction cross sections of ATD
Nine different models were used to simulate the extinction cross sections of ATD: three each using spheres (Mie theory), spheroids (DDA), and a combination of spheres and spheroid (). In the first set of calculations, each component in ATD was treated as a collection of monodisperse or polydisperse spheres and Mie theory was used to calculate the extinction cross sections. When the CRD-AES results were compared to any of the models that did not include the polydispersity at each mobility diameter, there was poor agreement. As a result, we only discuss results below that include the polydispersity at each mobility diameter. To include the polydispersity of the size selection, the extinction cross section for each of the mineral components was calculated separately at each mobility diameter and the weighted average extinction cross section at each mobility diameter was calculated (). When the size distribution of ATD is used for the calculations, the extinction cross section is underestimated at all diameters except 450 nm. The average percent difference from the CRD-AES results is 39%. When the size distributions of the individual components are used, the average percent difference drops to 28%. In this case, the theoretical results have a higher extinction cross section than the data at 300 nm, but are lower than the experimental data for the other mobility diameters.
Figure 6. Experimental extinction cross sections retrieved using CRD-AES (circles) compared to different models using either the ATD polydispersity (squares) or individual polydispersity (diamonds) for (a) Mie theory (models 1–3), (b) spheroids with an aspect ratio of 1.84 (models 4–6), and (c) using individual models for each component (models 7–9). (d) Summary of percent differences between models that incorporate the polydispersity at each mobility diameter and experiment.
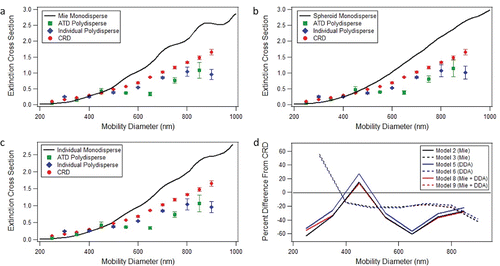
We next modeled the extinction cross sections using the discrete dipole approximation to calculate the extinction cross sections of spheroids with an aspect ratio of 1.84, which is the median side-on aspect ratio of the ATD particles. In the literature, several models have shown that spheroids with an aspect ratio of 1.8 model the optical properties of mineral dust better than spheres (Gasteiger et al. Citation2011; Merikallio et al. Citation2011). Similar to the Mie theory calculations, three different ways of accounting for the size distributions of the particles were used. The particles were assumed to be monodisperse spheroids, the particles were assumed to be polydisperse spheroids where the size distribution of each species was assumed to be the same as the size distribution of ATD, and the particles were assumed to be polydisperse spheroids where the individual size distributions of each species were used (). Incorporation of polydispersity into the model provides better agreement to the experimental data. Similar to the Mie theory calculations, the extinction cross sections of spheroids that use the ATD size distribution gave lower values than the CRD-AES results except at a mobility diameter of 450 nm. The average percent difference between the experimental results and the calculations was 35%. When the individual size distributions were used in the calculations, the average percent difference was 27%.
In the final approach to modeling the extinction cross sections of ATD, each component was treated individually, meaning that some species were treated as spheres (quartz, feldspar, carbonate) and others as spheroids (aluminosilicate clay minerals) with different aspect ratios (, ). When the size distribution of ATD is used to account for the polydispersity, then the average percent difference from the experimental results is 38%. Note that Alexander et al. (Citation2013) also found good agreement between T-matrix calculations and measured light scattering properties of ATD when the size distribution of ATD was used with each component of the mixture treated separately in terms of optical properties and shape. Incorporation of individual size distributions yields an average percent difference of 28%. A comparison of the percent differences for the different calculations is shown in . When the size distributions of the individual components are incorporated instead of the size distribution of ATD, the CRD-AES data are modeled better. The average percent difference between using individual vs. ATD size distributions was 10%.
Atmospheric implications: Modeling mineral dust aerosol components and ATD
Because models often use Mie theory to estimate the optical properties of mineral dust aerosol, we used Mie theory to retrieve the refractive index that best fit the model output for spheroids at different size ranges for quartz, NX-illite, KGa-2, and STx-1b. shows the literature refractive index used for the calculations of spheroids and the retrieved effective refractive index obtained from Mie theory for the size range 30 to 1010 nm. Because the quartz particles have a low aspect ratio (1.64), the retrieved effective refractive index was the same as the literature value at 1.55. For the aluminosilicate clay minerals, the retrieved effective refractive indices were 0.05 to 0.09 lower than the literature refractive indices. In the supporting information, we provide effective refractive indices for different size ranges. These effective refractive indices allow Mie theory to be used to accurately determine the extinction cross sections of high aspect ratio particles in different size regimes. Note, however, that these effective refractive indices are retrieved for use with Mie theory and as a result, average over the effects of particle shape. They may therefore be very sensitive to the size, shape, and orientational distributions of a sample as well as the exact composition of the sample.
Table 3. Literature and retrieved effective refractive indices for the particle size range 30–1010 nm.
Several papers have shown that Mie theory cannot account for the scattering properties of ATD, and instead use empirical models (Curtis et al. Citation2008; Glen and Brooks Citation2013). As a result, we have investigated how an empirical fitting factor could be used to improve the models for ATD. In particular, we have shown that using spheroids to model the extinction cross sections of high aspect ratio aluminosilicate clay minerals is not suitable. The factors by which the model results for aluminosilicate clay minerals need to be increased to agree with the CRD-AES data are 67 for a mobility diameter of 750 nm and 207 for a mobility diameter of 250 nm from our kaolinite results. Using these correction factors for the aluminosilicate clay minerals in the ATD sample, the average percent difference can be reduced from 28 to 24% using a factor of 67 or to 23% using 207. The error increased at the lowest diameter (300 nm), but using diameters larger than 300 nm leads to an error of 23% without the correction and 14% using a correction factor of 207. Based on these results, we expect that much of the error in modeling the extinction cross sections of ATD stems from the error in modeling the extinction cross sections of aluminosilicate clay minerals. Additional factors that would affect the modeling of the ATD mineral dust mixture include the possibility that the aerosolized portion of dust is different from the bulk in composition and size as well as having different compositions at different sizes of interest.
Conclusions
We have used cavity ring-down aerosol extinction spectroscopy to measure the extinction cross sections of quartz, aluminosilicate clay minerals, and ATD. For quartz and aluminosilicate clay minerals, SEM was used to determine the size distribution of bulk and size-selected particles for comparison with SMPS results. For the quartz particles, the size selection corresponded closely to that of the mobility diameter selected. For the aluminosilicate clay minerals, little size selection of the particles occurred using the DMA. The extinction cross sections of quartz and aluminosilicate clay minerals were modeled assuming that the particles were spheres, using Mie theory, and spheroids, using the DDA. Quartz was equally well modeled using spheres or spheroids with an aspect ratio of 1.64. For the aluminosilicate clay minerals, spheroids with high aspect ratios (4.8–8.89) showed considerable improvement over spheres, but due to the poor size-selection, possible orientation effects, and lift forces in the flow system, more elaborate methods are needed to model the extinction cross sections of these particles. ATD was best modeled using spheres or spheroids and incorporating the polydispersity of each individual component. Using an empirical factor to improve the models of the aluminosilicate clay minerals was necessary to better model the extinction cross section of ATD.
UAST_1225153_Supplemental_file.zip
Download Zip (3.5 MB)Acknowledgments
We would like to thank the shared instrumentation facilities at the University Park campus of the Pennsylvania State University for the use of SEM along with helpful advice. We also thank the Material Characterization Lab for use of the FEI NanoSEM 630 FESEM and the staff of Research Computing and Cyberinfrastructure (RCC), a unit of ITS at Penn State, for their significant help in meeting our computational needs.
Funding
This study was supported by funding from the Pennsylvania State University.
References
- Alexander, J. M., Laskina, O., Meland, B., Young, M. A., Grassian, V. H., and Kleiber, P. D. (2013). A Combined Laboratory and Modeling Study of the Infrared Extinction and Visible Light Scattering Properties of Mineral Dust Aerosol. J. Geophys. Res.: Atmos., 118:435–452.
- Attwood, A. R., and Greenslade, M. E. (2011). Optical Properties and Associated Hygroscopicity of Clay Aerosols. Aerosol Sci. Technol., 45:1350–1359.
- Baron, P. A., Sorensen, C. M., and Brockmann, J.E. (2001). Nonspherical Particle Measurements: Shape Factors, Fractals, and Fibers. In Aerosol Measurements: Principles, Techniques, and Applications, 2nd Edition, P. A. Baron and K. Willeke, eds. John Wiley, New York, pp. 705–749.
- Bohren, C. F., and Huffman, D. R. (2004). Absorption and Scattering of Light by Small Particles. Wiley-VCH, Weinheim, Germany.
- Broadley, S. L., Murray, B. J., Herbert, R. J., Atkinson, J. D., Dobbie, S., Malkin, T. L., Condliffe, E., and Neve, L. (2012). Immersion Mode Heterogeneous Ice Nucleation by an Illite Rich Powder Representative of Atmospheric Mineral Dust. Atmos. Chem. Phys., 12:287–307.
- Curtis, D. B., Aycibin, M., Young, M. A., Grassian, V. H., and Kleiber, P. D. (2007). Simultaneous Measurement of Light-Scattering Properties and Particle Size Distribution for Aerosols: Application to Ammonium Sulfate and Quartz Aerosol Particles. Atmos. Environ., 41:4748–4758.
- Curtis, D. B., Meland, B., Aycibin, M., Arnold, N. P., Grassian, V. H., Young, M. A., and Kleiber, P. D. (2008). A Laboratory Investigation of Light Scattering from Representative Components of Mineral Dust Aerosol at a Wavelength of 550 nm. J. Geophys. Res., 113:D08210.
- Dahneke, B. (1973). Slip Correction Factors for Nonspherical Bodies—I Introduction and Continuum Flow. J. Aerosol Sci., 4:139–145.
- Dentener, F., Carmichael, G. R., Zhang, Y., Lelieveld, J., and Crutzen, P. J. (1996). Role of Mineral Aerosol as a Reactive Surface in the Global Troposphere. J. Geophys. Res., 101(D17):22869–22889.
- Draine, B. T., and Flatau, P. J. (1994). Discrete Dipole Approximation for Scattering Calculations. J. Opt. Soc. Am. A, 11:1491–1499.
- Draine, B. T., and Flatau, P. J. (2010). User Guide for the Discrete Dipole Approximation Code DDSCAT 7.1. Available at http://arxiv.org/abs/1002.1505.
- Egan, W. G., and Hilgeman, T. W. (1979). Optical Properties of Inhomogeneous Materials. Academic Press, New York.
- Forster, P., Ramaswamy, V., Artaxo, P., Berntsen, T., Betts, R., Fahey, D. W., et al. (2007). Changes in Atmospheric Constituents and in Radiative Forcing, in Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. Averyt, M. Tignor, H. Miller, eds., Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- Freedman, M. A., Hasenkopf, C. A., Beaver, M. R., and Tolbert, M. A. (2009). Optical Properties of Internally Mixed Aerosols of Dicarboxylic Acids and Ammonium Sulfate. J. Phys. Chem. A, 113:13584–13592.
- Gallily, I., and Eisner, A. D. (1979). On the Orderly Nature of the Motion of Nonspherical Aerosol Particles. J. Colloid Interf. Sci., 68:320–337.
- Gasteiger, J., Weigner, M., Grob, S., Freudenthaler, V., Toledano, C., Tesche, M., and Kandler, K. (2011). Modelling Lidar-Relevant Optical Properties of Complex Mineral Dust Aerosols. Tellus, 63B:725–741.
- Ginoux, P., Chin, M., Tegen, I., Prospero, J.M., Holben, B., Dubovik, O., and Lin, S. (2001). Sources and Distributions of Dust Aerosol Simulated with the GOCART Model. J. Geophys. Res., 106(D17):20255–20273.
- Ginoux, P., Prospero, J. M., Gill, T. E., Hsu, N. C., and Zhao, M. (2012). Global-scale Attribution of Anthropogenic and Natural Dust Sources and Their Emission Rates Based upon MODIS Deep Blue Aerosol Products. Rev. Geophys., 50:RG3005.
- Glen, A. and Brooks, S. D. (2013). A New Method for Measuring Optical Scattering Properties of Atmospherically Relevant Dusts using the Cloud and Aerosol Spectrometer with Polarization (CASPOL). Atmos. Chem. Phys., 13:1345–1356.
- Haapanala, P., Raisanen, P., Kahnert, M., Nousiainen, T. (2012). Sensitivity of the Shortwave Radiative Effect of Dust on Particle Shape: Comparison of Spheres and Spheroids. J. Geophys. Res., 117:D08201.
- Hajihashemi, M. R., and Jian, H. (2013). Gaussian Random Ellipsoid Geometry-Based Morphometric Recovery of Irregular Particles Using Light Scattering Spectroscopy. J. Quant. Spectrosc. Radiat. Transfer, 118:86–95.
- Hasenkopf, C., Beaver, M., Trainer, M., Dewitt, H., Freedman, M. A., Toon, O. B., McKay, C., and Tolbert, M. (2010). Optical Properties of Titan and Early Earth Haze Laboratory Analogs in the Mid-Visible, Icarus, 207:903–913.
- Heintzenberg, J., and Charlson, R. (1999). Design and Applications of the Integrating Nephelometer: A Review. J. Atmos. Ocean Tech., 13:987–1000.
- Hinds, W. C. (1996). Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. Wiley, New York.
- Hudson, P. K., Gibson, E. R., Young, M. A., Kleiber, P. D., and Grassian, V. H. (2007). A Newly Designed and Constructed Instrument for Coupled Infrared Extinction and Size Distribution Measurements of Aerosols. Aerosol Sci. Technol., 41:701–710.
- Hudson, P. K., Gibson, E. R., Young, M. A., Kleiber, P. D., and Grassian, V. H. (2008). Coupled Infrared Extinction and Size Distribution Measurements for Several Clay Components of Mineral Dust Aerosol. J. Geophys. Res., 113:D01201.
- Inoue, A., and Kitagawa, R. (1994). Morphological Characteristics of Illitic Clay Minerals from a Hydrothermal System. Am. Mineral., 79:700–711.
- Johnson, B., Christopher, S., Haywood, J., Osborne, S., McFarlane, S., Hsu, C., Salustro, C., and Kahn, R. (2009). Measurements of Aerosol Properties from Aircraft, Satellite and Ground-Based Remote Sensing: A Case-Study from the Dust and Biomass-Burning Experiment (DABEX). Q. J. R. Meteorol. Soc., 135:922–934.
- Johnson, M., Meskhidze, N., and Kiliyanpilakkil, P. (2012). A Global Comparison of GEOS-Chem-Predicted and Remotely-Sensed Mineral Dust Aerosol Optical Depth and Extinction Profiles. J. Adv. Model. Earth Sys., 4:M07001.
- Kahnert, F. M. (2004). Reproducing the Optical Properties of Fine Desert Dust Aerosols using Ensembles of Simple Model Particles. J. Quant. Spectrosc. Radiat. Transfer, 85:231–249.
- Kahnert, M., Nousiainen, T., Thomas, M. A., and Tyynela, J. (2012). Light Scattering by Particles with Small-Scale Surface Roughness: Comparison of Four Classes of Model Geometries, J. Quant. Spectrosc. Radiat. Transfer, 113:2356–2367, doi:10.1016/j.jqsrt.2012.03.017.
- Kalashnikova, O., Kahn, R., Sokolik, I., and Li, W. (2005). Ability of Multiangle Remote Sensing Observations to Identify and Distinguish Mineral Dust Types: Optical Models and Retrievals of Optically Thick Plumes. J. Geophys. Res., 110:D18S14.
- Kanji, Z. A., and Abbatt, J. P. D. (2010). Ice Nucleation onto Arizona Test Dust at Cirrus Temperatures: Effect of Temperature and Aerosol Size on Onset Relative Humidity. J. Phys Chem. A, 935–941.
- Kishore, N., and Gu, S. (2010). Wall Effects on Flow and Drag Phenomena of Spheroid Particles at Moderate Reynolds Numbers. Ind. Eng. Chem. Res., 49:9486–9495.
- Koepke, P., Gasteiger, J., and Hess, M. (2015). Technical Note: Optical Properties of Desert Aerosol with Non-Spherical Mineral Particles: Data Incorporated to OPAC. Atmos. Chem. Phys., 15:5947–5956.
- Kok, J. F. (2011). A Scaling Theory for the Size Distribution of Emitted Dust Aerosols Suggests Climate Models Underestimate the Size of the Global Dust Cycle. Proc. Natl. Acad. Sci., 108(3):1016–1021.
- Lang-Yona, M., Rudich, Y., Segre, E., Dinar, E., and Abo-Riziq, A. (2009). Complex Refractive Indices of Aerosols Retrieved by Continuous Wave-Cavity Ring Down Aerosol Spectrometer. Anal. Chem., 81:1762–1769.
- Li, L., Chen, L., Chen, H., Yang, X., Tang, Y., and Zhang, R. (2011). Monitoring Optical Properties of Aerosols with Cavity Ring-Down Spectroscopy. J. Aerosol Sci., 42:277–284.
- Liu, T. (1985). Loess in China. China Ocean Press, Beijing.
- Lüönd, F., Stetzer, O., Welti, A., and Lohmann, U. (2010). Experimental Study on the Ice Nucleation Ability of Size-Selected Kaolinite Particles in the Immersion Mode. J. Geophys. Res., 115:D14201.
- Marcolli, C., Gedamke, S., Peter, T., and Zobrist, B. (2007). Efficiency of Immersion Mode Ice Nucleation on Surrogates of Mineral Dust. Atmos. Chem. Phys., 7:5081–5091.
- Maring, H., Savoie, D. L., Izaguirre, M. A., Custals, L., and Reid, J. S. (2003). Mineral Dust Aerosol Size Distribution Change During Atmospheric Transport. J. Geophys. Res., 108(D19):8592.
- Martin, R. T., Bailey, S. W., Eberl, D. D., Fanning, D. S., Guggenheim, S., Kodama, H., Pevear, D. R., Środoń, J., and Wicks, F. J. (1991). Report of the Clay Minerals Society Nomenclature Committee: Revised Classification of Clay Materials. Clay. Clay Miner., 39(3):333–335.
- McNaughton, S., Clarke, A., Kapustin, V., Shinozuka, Y., Howell, S., Anderson, B., Winstead, E., Dibb, F., Scheuer, E., Cohen, R., Wooldridge, P., Perring, A., Huey, G., Kim, S., Jimenez, S., Dunlea, E., DeCarlo, E., Wennberg, P., Crounse, J., Weinheimer, R., and Flocke, F. (2009). Observations of Heterogeneous Reactions Between Asian Pollution and Mineral Dust over Eastern North Pacific during INTEX-B. Chem. Phys., 2:8469–8539.
- Meland, B., Alexander, J. M., Wong, C. S., Grassian, V. H., Young, M. A., and Kleiber, P. D. (2012). Evidence for Particle Size-Shape Correlations in the Optical Properties of Silicate Clay Aerosol. J. Quant. Spectrosc. Ra., 113:549–558.
- Mellon, D., King, S. J., Kim, J., Reid, J. P., Orr-Ewing, A. J. J. (2011). Measurements of Extinction by Aerosol Particles in the Near-Infrared Using Continuous Wave Cavity Ring-Down Spectroscopy. Phys. Chem. A, 115:774–783.
- Merikallio, S., Lindqvist, H., Nousiainen, T., and Kahnert, M. (2011). Modelling Light Scattering by Mineral Dust using Spheroids: Assessment of Applicability. Atmos. Chem. Phys., 11:5347–5363.
- Mogili, P., Yang, K., Young, M., Kleiber, P., and Grassian, V. H. (2007). Environmental Aerosol Chamber Studies of Extinction Spectra of Mineral Dust Aerosol Components: Broadband IR-UV Extinction Spectra. J. Geophys. Res., 112:D21204.
- Mogili, P., Yang, K., Young, M., Kleiber, P., and Grassian, V. H. (2008). Extinction Spectra of Mineral Dust Aerosol Components in an Environmental Aerosol Chamber: IR Resonance Studies. Atmos. Environ., 42:1752–1761.
- Mätzler, C. (2002). IAP Research Report No. 2002–08 [Online], Universität Bern, Bern, Switzerland. Available at http://diogenes.iwt.unibremen.de/vt/laser/wriedt/Mie_Type_Codes/body_mie_type_codes.html, accessed May 2008.
- Nadeau, P. H. (1985). The Physical Dimensions of Fundamental Clay Particles. Clay Miner., 20:499–514.
- Ndour, M., D'Anna, B., George, C., Ka, O., Balkanski, Y., Kleffman, J., Stemmler, K., and Ammann, M. (2008). Photoenhanced Uptake of NO2 on Mineral Dust: Laboratory Experiments and Model Simulations. Geo. Phys. Res. Lett., 35:L05812.
- Nousiainen, T., Kahnert, M., and Veihelmann, B. (2006). Light Scattering Modeling of Small Feldspar Aerosol Particles using Polyhedral Prisms and Spheroids. J. Quant. Spectrosc. Radiat. Transfer, 101:471–487, doi:10.1016/j.jqsrt.2006.02.038.
- Pettersson, A., Lovejoy, E. R., Brock, C., Brown, S. S., and Ravishankara, A. R. (2004). Measurement of Aerosol Optical Extinction at 532 nm with Pulsed Cavity Ring Down Spectroscopy. J. Aerosol Sci., 35:995–1011.
- Prospero, J. M., Ginoux, P., Torres, O., Nicholson, S., and Gill, T. (2002). Environmental Characterization of Global Sources of Atmospheric Soil Dust Identified with the Nimbus 7 Total Ozone Mapping Spectrometer (TOMS) Absorbing Aerosol Product. Rev. Geophys., 40:1002.
- Riziq, A. A., Erlick, C., Dinar, E., and Rudich, Y. (2007). Optical Properties of Absorbing and Non-Absorbing Aerosols Retrieved by Cavity Ring Down (CRD) Spectroscopy. Atmos. Chem. Phys., 7(6):1523–1536, doi:10.5194/acp-7-1523-2007.
- Segré, G. and Silberberg, A. (1961). Radial Particle Displacements in Poiseuille Flow of Suspensions. Nature, 189:209–210.
- Siegesmund, S., Weiss, T., and Vollbrecht, A. (2002). Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies, Geological Society, London, Special Publications, 205:137–147.
- Sokolik, I. N., and Toon, O. B. (1999). Incorporation of Mineralogical Composition into Models of the Radiative Properties of Mineral Aerosol from UV to IR Wavelengths. J. Geophys. Res., 104(D8):9423–9444, doi:10.1029/1998JD200048
- Sullivan, R. C. M., Moore, J., Petters, M. D., Kreidenweis, S. M., Roberts, G. C., and Prather, K. A. (2009). Effect of Chemical Mixing State on the Hygroscopicity and Cloud Nucleation Properties of Calcium Mineral Dust Particles. Atmos. Chem. Phys., 9:3303–3316.
- Veghte, D. P., and Freedman, M. A. (2012). The Necessity of Microscopy to Characterize the Optical Properties of Size-selected, Nonspherical Aerosol Particles. Anal. Chem., 84:9101–9108.
- Veghte, D. P., and Freedman, M. A. (2014). Facile Method for Determining the Aspect Ratios of Mineral Dust Aerosol by Electron Microscopy. Aerosol Sci. Technol., 48(7):715–724.
- Veghte, D. P., Moore, J. E., Jensen, L, and Freedman, M. A. (2015). Influence of Shape on the Optical Properties of Hematite Aerosol. J. Geophys. Res. Atmos., 120:7025–7039.
- Volten, H., Munoz, O., Rol, E., De Haan, J. F., Vassen, W., Hovenier, J. W., Muinonen, K., and Nousiainen, T. (2001). Scatttering Matrices of Mineral Aerosol Particles at 441.6 nm and 632.8 nm. J. Geophys. Res., 106(D15):17375–17401.
- Walker, J. S., Carruthers, A. E., Orr-Ewing, A. J., and Reid, J. P. (2013). Measurements of Light Extinction by Single Aerosol Particles. J. Phys. Chem. Lett., 4:1748–1752.
- Welti, A., Lüönd, F., Stetzer, O., and Lohman, U. (2009). Influence of Particle Size on the Nucleating Ability of Mineral Dust. Atmos. Chem. Phys., 9:6705–6715.
- Yang, W., Marshak, A., Kostinski, A. B., and Varnai, T. (2013). Shape-Induced Gravitational Sorting of Saharan Dust During Transatlantic Voyage: Evidence from CALIOP Lidar Depolarization Measurements. Geophys. Res. Lett., 40:3281–3286.
- Zhao, T., Gong, S., Zhang, X., and McKendry, I. (2003). Modeled Size-Segregated Wet and Dry Deposition Budges of Soil Dust Aerosol during ACE-Asia 2001: Implications for Trans-Pacific Transport. Geophys. Res., 10:D23, doi: 10.1029/2002JD003363.
