ABSTRACT
A new experimental technique is reported to visualize agglomeration of submicron aerosol particles by laser-induced fluorescence (LIF). The basic idea is to produce or activate the fluorescent tracer material by a chemical reaction triggered by the agglomeration between two chemically different primary particles. Different types of chemical reactions are able to fulfil this task, among others acid–base reactions or molecule solvation. In this work, we demonstrate the feasibility of the fluorescent tracer activation by means of solvation. The fluorescence is activated almost instantaneously when the dry fluorescent material (Fluorescein or Rhodamine B) contained in the dye aerosol is dissolved by a water/glycerol mixture constituting the particles of the solvent aerosol. Estimations of the timescale for diffusional mixing suggest that the fluorescence is activated within 1 ms. Agglomerates can be detected as single particles or in bulk quantities depending on the available laser excitation energy and light sensitivity. In order to enhance agglomeration in the validation experiments, two aerosol streams were electrostatically charged with opposite polarity. Finally, potential variations and applications of the newly introduced technique are briefly discussed, mentioning the detection of humidity among others.
© 2017 American Association for Aerosol Research
EDITOR:
1. Introduction
Agglomeration of aerosols is seen as a promising technique for the production of advanced nanoparticle structures. Aerosol agglomeration may be used, for example, to deposit a solid or liquid coating material on the surface of aerosol particles (Pietsch Citation2003), to produce so-called Janus particles which offer special catalytic properties (Wei Seh et al. Citation2011), or might be used as surfactants (Ruhland et al. Citation2011). Also, thermally or electrostatically induced agglomeration may give a significant contribution to the precipitation of highly concentrated aerosols (Löffler and Gutsch Citation1993).
The authors are motivated to study the interaction between highly concentrated streams of charged aerosols. In this situation, electrostatic dispersion (Kasper Citation1981) causes clouds of the uniformly charged aerosol to expand or to deposit on the walls. At the same time, the mutual electrostatic attraction between clouds of oppositely charged aerosols produces aerosol mixing by means of an electrostatic instability, which tends to evolve into a transient mixing process with laminar or turbulent eddies. Eventually, agglomeration takes place due to micromixing, diffusion and electrostatic attraction between the individual particles. In the investigation and optimization of such aerosol agglomeration processes, a tool for visualization is useful, since it can be used to determine the production rate of agglomerates and aid in the evaluation of the mixing quality.
Bipolar agglomeration of oppositely charged aerosol particles is quite fast compared to diffusional mixing and thermal coagulation. The agglomerates appear localized to the areas where the different aerosol streams come into contact. Hence, it can be deduced that the spatial distribution of the agglomerates corresponds to the flow structures of the mixing process.
2. Possibilities for the activation of fluorescence
Fluorescence is widely used to visualize processes in living cells. In many of these applications, chemical or biochemical reactions are used to activate fluorescence (Bastiaens and Squire Citation1999). Examples of other applications are the spatial distribution of chemical reaction products (Stewart and Judeikis Citation1974) or the study of the internal structure of combustion flames by means of the OH-radical fluorescence (Campbell Citation1984).
From water quality analyses (Patel-Sorrentino, Mounier, and Benaim Citation2002) it is well known that the fluorescence yield and absorption/emission wavelengths of many fluorescent dyes depend on the pH value. For example, the non-ionic, hydrophobic, non-fluorescent indicator Fluorescein can also occur as an anionic, water-soluble, fluorescent Uranine (disodium fluorescein salt). As the acid–base reaction is reversible, the transition between these two states takes place over a wide range of pH-values. Hence, the pH-dependent variation of fluorescence intensity can be used to quantitatively determine and visualize chemical reaction rates (Lehwald, Thévenin, and Zähringer Citation2010).
Extending this concept toward the intended application in this work, i.e., the visualization of agglomerate concentration, implies that aerosol particles containing high concentrations of strong acids and bases would be needed to produce a good contrast between the fluorescent and the non-fluorescent state. Unfortunately, such aerosols would be toxic, corrosive, and difficult to handle.
An improved approach for fluorescence activation was found during the attempts to handle the acid–base reaction with aerosols. Some fluorescent dyes like Uranine or Rhodamine B show very little fluorescence in the solid state. This offers the possibility to prepare a solid aerosol by completely drying a fine spray of dissolved fluorescent dye, and a solvent aerosol containing water. When the two aerosols agglomerate, the dye dissolves in water again and the fluorescence intensity increases by several orders of magnitude. However, in order to prevent the evaporation of water and to stabilize the second aqueous aerosol, the vapor pressure of water has to be lowered by adding some hygroscopic substance. A 50% solution of glycerol in water is well suited for experiments in ambient air.
An alternative for producing a stable solvent aerosol is to use ionic liquids (IL). ILs offer the advantage to have a very low vapor pressure such that the diameter of aerosols produced from an IL is very stable as it does not decrease due to evaporation effects. In a screening experiment, it was found that Uranine dissolves very well in the IL 1-Ethyl-3-methyl-imidazolium-ethylsulfat and that it shows nice fluorescence very similar to aqueous solutions. Again, ILs can be applied for fluorescence activation either by means of solubilisation or by pH change. However, the use of ILs produces some problems as well, as they are prone to cause corrosion. IL aerosols might damage electronic devices and cause health problems when the aerosol is inhaled. Due to these reasons, no further experiments have been conducted with the ILs.
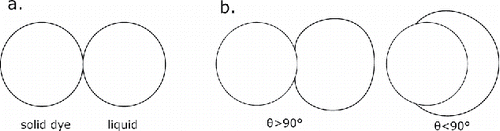
A general objection against the fluorescence activation method by mixing a solid and a solvent aerosol might be that the dissolution of the solid fluorescent dye by molecular diffusion within the solvent (water+glycerol) might be too slow to instantaneously visualize the rapidly evolving turbulent mixing flow structures. However, the distances involved in this diffusive transport are very small. For a quantitative estimate of the dissolution time we consider a worst case scenario where the agglomerate consists of two spherical particles barely touching each other (), and where the root mean square (RMS) displacement of the dye molecules necessary to activate fluorescence equals the radius of the glycerol/water droplet. As an additional assumption, there is only diffusion from the dye surface into the water droplet and not the other way around. The diffusion coefficient of the dye may be estimated from the diameter of the dye molecule together with the Stokes–Einstein equation of diffusion and the viscosity of the liquid. Applied to the present example this yields a diffusion coefficient of approximately 8 × 1011 m/s, assuming the Stokes–Einstein radius of the Uranine ion to be 0.45 nm (Montermini, Winlove, and Michel Citation2002) in a 50:50 weight % glycerol/water mixture (approximate viscosity of 6 mPa s). Further assuming the radius of an aerosol droplet to be 150 nm this gives a characteristic diffusion time of τdiff = 0.14 × 10−3 s. Depending on the contact angle θ between solid particle and solvent droplet, the real situation will be little to largely more favorable (). With Rhodamine B, the characteristic time is higher, due to the larger molecule. Additionally, the molecular diffusion causing the dissolution of the dried dye particle takes place in both directions between dye and water/glycerol particles. The diffusive transport might also be enhanced by Marangoni convection, given that Uranine is also a good detergent. It is furthermore noticed that fluorescence will become visible when just a small part of the dye has dissolved. However, complete data on the quantitative dependence of fluorescence intensity on dissolved concentration of the dye are not available.
3. Experimental setup for verification of the method
The visualization of aerosol agglomeration by Laser-Induced Fluorescence (LIF) was demonstrated for a case of electrostatically enhanced agglomeration between two streams of aerosol with opposite charge. The experimental setup and the settings which have been used are described below.
The experimental setup is shown in . The aerosols are produced by two Laskin nebulizers using multiple (5) orifices made from thin-walled stainless steel needles with 1 mm inner diameter. Each of the needles is brazed to a 10 mm diameter gas distributor pipe on one end (see ). The other end of the needle was modified by squeezing it to a thin slit in order to reduce the cross-section and therefore accelerate the gas flow.
The nebulizers are operated with a gas pressure of 4 bar approximately. Coarse droplets are removed by packings of coarse stainless steel turnings. The fluorescent dye aerosol was produced from a 14 g/L solution of Rhodamine B in water. The strong occurrence of foaming was reduced by applying silicone grease to the inner nebulizer walls above the liquid surface. The solvent aerosol was produced from a 50:50 glycerol–water mixture. The original aerosol droplet diameters (median diameter) are around 300 nm for both aerosols and number concentrations are around 107/cm−3. Measurements were done with an SMPS model from TSI (Long DMA 3081 and CPC 3010, Kr-85 neutralizer).
Both aerosols enter separately into charging chambers with dimensions of 50 mm x 50 mm x 50 mm. Both charging chambers have air suction holes through which ambient air is mixed into the aerosol with a ratio of 3:1. This air is needed to keep the electrode insulations in a dry state and also to dry the dye aerosol, while the solvent aerosol remains in a liquid state due to the strong hygroscopicity of glycerol. The suction holes also help to average out the flow rates of both aerosols, independent of the gas pressures used to run the Laskin nebulizers. Point discharge electrodes with a corona onset voltage around 4 kV are used to produce a positive charge on the dye aerosol and a negative charge on the solvent aerosol. As the charging of aerosols is intrinsically connected with a significant loss of particles (Marquard et al. Citation2004), the operation voltages were adjusted manually to reach optimum visual effects. Typically, the chargers were operated at voltages around 10 kV.
Since the aerosol is almost saturated with water when leaving the aerosol generator, the diluted and charged aerosol will have a higher relative humidity compared to the ambient air. This increase is negligible as the aerosol particles are very small.
The charged aerosols are introduced into the agglomeration duct with a cross-section of 100 mm × 50 mm and a length of 500 mm. The duct walls are made of transparent PMMA polymer (Plexiglas®). At the entrance of the duct the two flows with cross sections of 50 mm × 50 mm each are separated by a thin metal wall of 50 mm length. Both flows are equalized using metal screens (stainless steel, 1 mm mesh width, open surface fraction 39%). With an average flow velocity of around 0.44 m/s, the aerosol flows are in a laminar/transient flow region with a bulk velocity Reynolds number of around 1630. For calculation purposes, the hydraulic diameter depending on the duct dimensions was used.
For the visualization by fluorescence, different lasers and corresponding filters have to be used with the different dyes.
Uranine has its maximum absorption at around 485 nm and maximum fluorescence at around 520 nm in aqueous solutions. For illumination, a 473 nm, 100 mW CW blue laser with 0.7 mm laser beam diameter (LASOS single frequency diode-pumped solid-state (DPSS) Laser, BLK 7310 TS) was used together with a DT-green/yellow colour longpass filter for observation.
Rhodamine B has its maximum absorption at 555 nm and maximum fluorescence at 580 nm. Here, for illumination a 532 nm, 500 mW (max) CW green laser with 1.5 mm laser beam diameter (Dantec Dynamics, Code: 9080 × 0542, S/N: 106) was used. Fluorescence was observed through a monochromatic 532 nm Notch-filter.
4. Experimental results and discussion
Results of the experiments are only discussed forRhodamine B and the green laser, since there is no difference neither in the procedure nor the findings for the other laser/dye combination. At the beginning, lenses are used to widen the cross-section of the laser beam to approximately 20 mm × 1.5 mm, in order to cover a larger area and to make two-dimensional flow structures visible.
A video (, see the online supplementary information [SI]) was recorded with 24 frames/s. For the images, grayscale imaging, image complementation and contrast adjustment were applied to enhance the visibility of the fluorescence.
Figure 3. Top view on duct shows four different snapshots of the agglomeration zone at about 80 mm from the end of the flow separation wall with an expanded laser beam. Flow direction is from top to bottom. The two upper images show that there is only little fluorescence due to agglomeration when only one charger is active. The bottom images show that agglomeration is greatly enhanced when both chargers are active. The width of the laser beam in flow direction is 20 mm. The patterns on both sides originate from laser light scattered by the sidewalls of the duct and only designate the duct boundary (see the SI).
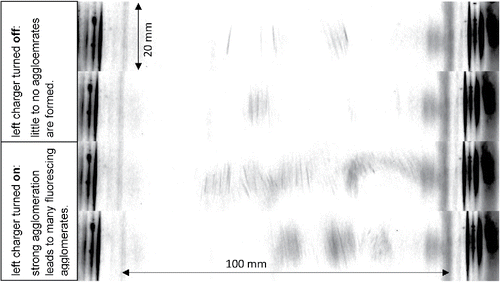
The video shows the appearance of agglomerates depending on whether both aerosols or only one aerosol (in our case the dye aerosol) is charged. When both aerosols remain uncharged, no visible amount of agglomerates can be detected. This is congruent with the fact that thermal coagulation of a monodisperse aerosol with concentration of 2 × 107/cm3 and a diameter of 300 nm yields an agglomeration rate of only around 2 × 105/(cm3 s). Furthermore, in a laminar flow this agglomeration rate will be found only in a very narrow boundary where both aerosols intermix by diffusion. For example, after a contact time of 0.1 s between the aerosols, the width of the diffusion zone is 10 µm only.
Charging of one of the aerosols enhances mixing the electrostatic dispersion of the charged aerosol causes additional pressure gradients in the flow. Further, the kinetics of agglomerate formation are slightly enhanced as well, given that charged and uncharged aerosol particles are mutually attracted via (rather weak) image forces (Hill and Nielsen Citation1974). Agglomeration is much stronger when both chargers are active.
The video images also reveal a clear internal structure of the fluorescent regions with streaks oriented in the flow direction. The length of the streaks (around 10–15 mm) matches quite well with the exposure time of each image (less than 1/24 s = 41 ms) multiplied with the average flow velocity of 0.44 m/s. This suggests that the streaks depict the trajectories of (large) single agglomerate particles or possibly of groups of agglomerate particles.
In a second series of experiments, we used higher light intensity from an unexpanded laser beam and post processing to improve the visibility of the turbulent mixing structures (). An unexpanded laser beam, which helps to increase fluorescence strength, is used to obtain images at different distances from the end of the flow separation wall. Post-processing is helpful to eliminate disturbances which are in part caused by scratches in the duct wall, and by traces of fluorescent dye on the duct walls. First, a picture subtraction to reduce background fluorescence from the duct walls is applied. Then, negative grayscale images with adapted contrast ranges are used to make the fluorescence caused by agglomeration better visible.
Figure 4. Agglomeration zones from top view of agglomeration duct at different distances from the flow separation wall. The black areas show where agglomeration occurs. An unexpanded Laser beam of 532 nm wavelength was used to illuminate the tracer Rhodamine B.
The images show that the occurrence of agglomerates increases with the distance from the end of the flow separation wall. They show that the quantity of agglomerates reaches a maximum after a distance of about 6 cm from the separation wall. Probably the agglomerate concentration decreases later due to deposition. As the video shows (, see the SI), agglomeration takes place mainly in the middle of the duct but has an overall shift to the right-hand side. This shift might be caused by different space charge densities of the two aerosols resulting from differing particle size distributions and hence different charging kinetics. This phenomenon will be investigated more in future work.
Regarding the flow regime, it is highly interesting to notice that the agglomeration zones appear randomly at different locations with different intensities ( and ). This strongly indicates the formation of complex 3D-structures, where fluorescence is only visible once large enough agglomerate concentrations appear in the laser plane. Considering that there is an underlying laminar/transient flow, one can assume that the observed mixing is mostly independent from the global pressure gradient driving the flow.
In supplementary measurements, the number concentration of the aerosols was measured, whereby samples were taken between duct outlet and fan. A total decrease of number concentration of around 80% for bipolar agglomeration was measured. The sampling points of the setup, however, do not allow for a distinction between the decrease due to agglomeration and the decrease due to precipitation losses on the duct walls caused by electrostatic dispersion, and within the charger unit. This will be remedied in future work.
The same experiments were also conducted with a solution of 10 g/L Uranine in water, producing similar results. Therefore, they are not presented in this article.
5. Conclusion and outlook
Experiments have shown that switchable fluorescence can be used to visualize the hetero-agglomeration of submicron aerosols. Fluorescence is activated by solvation when dry dye aerosol particles containing Rhodamine B or Uranine coagulate with liquid solvent (water/glycerol) aerosol particles. Due to the nature of solvation, the activation of fluorescence in an agglomerate occurs in one step after the first contact between a dye particle and a solvent particle has been made. Most likely, subsequent agglomeration steps cannot be detected separately from the first one, except by a gradual increase of the fluorescence intensity of the individual agglomerate.
An estimation of the characteristic diffusion time shows that fluorescence of the dye will occur within 10−3 s after the contact between the aerosol particles. The technique is therefore very well suited for the visualization of mixing in laminar and turbulent flows. In cases where the agglomeration step is fast compared to the mixing process, e.g., in the case of electrostatically enhanced bipolar agglomeration, the appearance of fluorescent agglomerates may be used to visualize the evolution of the boundary between two chemically different aerosol streams.
In the demonstration experiment, agglomeration was enhanced by opposite electrostatic charge on two aerosol streams mixing in a duct at laminar/transient flow conditions. LIF was viewed both with a widened and a non-widened laser beam, respectively. The streaky structure of the images indicates that possibly single submicron agglomerates can be viewed, supposed that the laser intensity and the camera resolution are sufficient.
Different applications may be developed from the technique described here.
Quite generally, the technique is specific for the detection of hetero-agglomerates formed by agglomeration between chemically different aerosol particles.
The intensity of fluorescent light emanating from a certain volume of aerosol is (with certain limitations) proportional to the mass or volume of actively fluorescent material, and hence proportional to the mass concentration of hetero-agglo. The mass concentration of hetero-agglomerates can then be monitored from fluorescence intensity measurements. Detection systems for this task will require only a relatively moderate resolution in time and space.
With highly sensitive measurements providing a high resolution in time and space, the spatial distribution of hetero-agglomerates can be viewed, and even the birth of individual hetero-agglomerates might be observed. Provided the fluorescence light emission from single agglomerates can be resolved, the further progress of the agglomeration process with the formation of larger agglomerates may be observed as well. These types of measurements are well suited for the verification of theoretical or numerical predictions on hetero-agglomeration, as they can provide a direct insight into the agglomeration process and its spatio-temporal kinetics.
For relatively small Reynolds numbers and an overall laminar/transient flow, the agglomeration step of electrostatically enhanced hetero-agglomeration is fast enough to depict flow structures in electro-hydrodynamically driven mixing processes.
In addition, this method might be developed further to visualize areas of different relative humidity or to highlight condensation zones. For example, in aerosol particles containing dry fluorescent dye, the fluorescence might be activated when these particles act as condensation nuclei for the condensation of (supersaturated) humidity. Probably, an admixture of hygroscopic chemicals to the dry aerosol particles might be used to define a specific value of relative humidity at which the activation of fluorescence occurs (e.g., salts). This would also work when liquid aerosol dries out and fluorescence disappears.
UAST_1339863_Supplemental_File.zip
Download Zip (13.2 MB)References
- Bastiaens, P. I., and Squire, A. (1999). Fluorescence Lifetime Imaging Microscopy: Spatial Resolution of Biochemical Processes in the Cell. Trends Cell Biol., 9:48–52.
- Campbell, D. H. (1984). Collisional Effects on Laser-Induced Fluorescence Measurements of Hydroxide Concentrations in a Combustion Environment. 2: Effects for v′ = 1 Excitation. Appl. Opt., 23:1319–1327.
- Hill, J. C., and Nielsen, K. A. (1974). Effect of Electrical Forces on Target Efficiencies for Spheres; Report ERI-Project 947; Engineering Research Institue; Iowa State University; Iowa State University; Ames
- Kasper, G. (1981). Electrostatic Dispersion of Homopolar Charged Aerosols. J. Colloid Interface Sci., 81:32–40.
- Lehwald, A., Thévenin, D., and Zähringer, K. (2010). Quantifing Macro-Mixing and Micro-Mixing in a Static Mixer Using Two-Tracer Laser-Induced Fluorescence. Exp. Fluids, 48:823–836.
- Löffler, F., and Gutsch, A. (1993). Electrically Induced Aerosol Agglomeration: Formulation of the Collision Frequency and Simulating Agglomerate Growth. J. Aerosol Sci., 24(Suppl.1):505–506.
- Marquard, A., Breadin, A., Meyer, J., and Kasper, G. (2004). Efficiency-Loss-Relations of Unipolar Nanoaerosol Chargers“. in Proceedings of the Fifth International Conference on Applied Electrostatics, Shanghai, China.
- Montermini, D., Winlove, C., and Michel, C. (2002). Effects of Perfusion Rate on Permeability of Frog and Rat Mesenteric Microvessels to Sodium Fluorescein. J. Physiol., 15:959–975.
- Patel-Sorrentino, N., Mounier, S., and Benaim, J. (2002). Excitation–Emission Fluorescence Matrix to Study pH Influence on Organic Matter Fluorescence in the Amazon Basin Rivers. Water Res., 36:2571–2581.
- Pietsch, W. (2003). An Interdisciplinary Approach to Size Enlargement by Agglomeration. Powder Technol., 130:8–13.
- Ruhland, T. M., Gröschel, A. H., Walter, A., and Müller, A. E. (2011). Janus Cylinder at Liquid - Liquid Interfaces. Langmuir, 27:9807–9814.
- Stewart, T. B., and Judeikis, H. S. (1974). Measurements of Spatial Reactant and Product Concentrations in a Flow Reactor Using Laser‐Induced Fluorescence. AIP- Rev. Sci. Instrum., 45:1542–1545.
- Wei Seh, Z., Liu, S., Zhang, S.-Y., Barathi, M., Ramanarayan, H., Low, M., Wei Shah, K., Zhang, Y.-W., and Han, M.-Y. (2011). Anisotropic Growth of Titania onto Various Gold Nanostructures: Synthesis, Theoretical Understanding, and Optimization for Catalysis. Angew. Chem. Int. Ed., 50:10140–10143.