?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nano-embedded microparticles represent promising carrier systems to tackle the challenges of nanoparticle delivery into the lungs by inhalation. While spray drying is widely used for the incorporation of nanoparticles into microparticles, the template-assisted technique is a novel method to prepare aspherical, cylindrical microparticles composed of nanoparticles. In this work, both techniques were applied to produce both spherical and cylindrical nano-embedded microparticles. For both geometries particles consisting of gelatin nanoparticles, mannitol and leucine were prepared in three different sizes each. Cylindrical microparticles could be prepared with defined dimensions and narrow size distributions, allowing to target a wide range of aerodynamic diameters. The size of spherical microparticles was influenced by the spraying feed concentration, yielding only small differences in geometric and aerodynamic diameters and broad particle size distributions. Regarding the redispersibility of the nano-embedded microparticles, spherical particles showed better disintegration behavior and higher nanoparticle release in comparison to cylindrical particles upon contact with water. The template-assisted technique yielded higher nanoparticle content in contrast to spray drying. In summary, cylindrical particles represent a promising drug delivery system with high potential for later application. However, further improvements in the preparation method are required to enable higher yields and a possible later scale-up.
Copyright © 2018 American Association for Aerosol Research
EDITOR:
1. Introduction
Nanoparticles for pulmonary drug delivery can provide advantages such as penetration of mucus and biofilm, reduced clearance from the lungs and controlled drug release (Hadinoto and Cheow Citation2014). The delivery of nanoparticles to the lungs is however challenging, as deposition efficiency upon pulmonary application is low due to their typically inappropriate size. Particles with a size of 0.1–1 µm are too small for deposition by impaction and sedimentation and at the same time show slow diffusion. Hence, they are to the largest part exhaled before being able to deposit (Carvalho, Peters, and Williams Citation2011). The preparation of nano-embedded microparticles, also called Trojan particles, is a prominent approach to tackle this issue (Tsapis et al. Citation2002; Ungaro et al. Citation2012). Embedded into microparticles of appropriate dimensions, nanoparticles can be transported into the lungs with efficient lung deposition. By subsequent disintegration of the microcarriers, the incorporated nanoparticles can be released (Bohr, Ruge, and Beck-Broichsitter Citation2014; Ruge et al. Citation2016; Torge et al. Citation2017). Spray drying is widely used for the preparation of nano-embedded microparticles, while the preparation of aspherical microparticles composed of nanoparticles by template-assisted technique represents a novel method (Kohler et al. Citation2011; Mohwald et al. Citation2017; Tscheka et al. Citation2015). This technique foresees the filling of nanoparticles into pores of track-etched membranes and their subsequent interconnection. By dissolving the membranes, cylindrical particles are released. Custom-tailored microparticles composed of nanoparticles can be produced with exact shape by using templates of definite dimensions. Cylindrical particles are promising drug delivery systems for the inhalative application, as particles with elongated shape are supposed to show high lung deposition, as known from fiber materials such as asbestos and mineral wool. These materials cause lung diseases such as fibrosis, lung cancer and mesothelioma (Lippmann Citation1990; Pott Citation1987). The toxicity of such fibers can be related to their good aerodynamic properties in combination with a long retention time. Due to the alignment of fibers in the airflow, they can be transported into the deep lungs, where they persist for long time. Clearance of these persistent materials is low and hence toxic effects are induced (Champion and Mitragotri Citation2006; Lippmann Citation1990; Pott Citation1987). The good aerodynamic properties of cylindrical particles however can inspire for the development of pulmonary drug delivery systems. As long as biocompatibility and biodegradability is provided, toxicological effects due to long retention time can be avoided. Of special interest in this context are nano-embedded microparticles, as the cylindrical microparticles serve only as intermediate carriers for the nanoparticles into the lungs. Upon deposition, they are supposed to disintegrate so that no accumulation occurs in the lungs.
Cylindrical microparticles composed of nanoparticles have been prepared so far from silica nanoparticles interconnected by agarose or by layer-by-layer technique using charged polymers (Kohler et al. Citation2011; Mohwald et al. Citation2017; Tscheka et al. Citation2015). Due to insolubility in water, these interconnection materials would not allow fast disintegration of microparticles in the lungs.
In this work, the preparation of cylindrical microparticles was adapted to biodegradable and biocompatible nanoparticles. Gelatin nanoparticles were used, being a promising carrier system for the delivery of hydrophilic macromolecules (Elzoghby Citation2013; Khan and Schneider Citation2013). Water soluble excipients were utilized for interconnection of nanoparticles to enable the disintegration of microparticles. For comparison with conventional nano-embedded microparticles, spherical microparticles composed of the same materials were prepared by spray drying. A comparison of the two preparation techniques, template-assisted technique and spray drying, will be conducted regarding the tunability of the particle size, feasibility of scale-up and expenditure of time. The resulting microparticles will be furthermore compared in terms of the size distribution, aerodynamic properties, nanoparticle content and disintegration behavior.
2. Materials and methods
2.1. Materials
Gelatin from bovine skin (Type B), glutaraldehyde solution grade II (25% in H2O), d-mannitol, l-leucine, and rhodamine B for fluorescence labeling were obtained from Sigma–Aldrich Co. (Steinheim, Germany). Pluronic® F68 (Poloxamer 188) was purchased from PanReac AppliChem (Darmstadt, Germany) and FITC–dextran 70 (Mw 70 kDa) from TdB Consultancy (Uppsala, Sweden). Acetone and ethyl acetate (both analytical reagent grade) were supplied by Fisher Scientific (Loughborough, UK) and dichloromethane (HPLC grade) by VWR International (Leuven, Belgium).
2.2. Nanoparticle preparation and characterization
Gelatin nanoparticles (GNP) with fluorescent labeling were prepared as previously described by nanoprecipitation (Khan and Schneider Citation2013). In short, 20 mg gelatin type B and 1 mg FITC-dextran (Mw 70 kDa) were dissolved in 1 mL of water under gentle heating (40 °C). The gelatin solution was added dropwise to a stabilizer solution (450 mg Poloxamer 188 in 15 mL acetone + 1 mL water). About 0.5 mL glutaraldehyde solution (2%, w/v) was added slowly and crosslinking was performed for 6 h under stirring. GNP were purified by three times centrifugation and redispersion in water. The nanosuspension’s concentration was determined by weighing the pellet after lyophilization (Alpha 2-4 LSC, Christ, Osterode am Harz, Germany). Size and zeta potential of GNP were analyzed by photon correlation spectroscopy (PCS) and laser Doppler velocimetry, respectively. Samples were diluted 1:100 (v/v) with water and measured with a Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK). pH of the undiluted nanosuspension was determined by a Seven compact pH meter (Mettler Toledo, Columbus, USA).
2.3. Preparation of cylindrical microparticles by template-assisted technique
Polycarbonate membranes (Nuclepore Track-Etched Membranes 25 mm; Whatman, Maidstone, UK) with the desired template pore size diameter (1, 3, or 5 µm) were placed above blocking membranes (0.05 µm pore diameter) and fixed in a Swinnex 25 mm PP filter holder (EMD Millipore Corporation; Billerica, USA). The GNP nanosuspension was adjusted to a concentration of 1.5 mg mL−1 and mannitol and rhodamine B were added in concentrations of 50 and 0.05 mg mL−1, respectively. The filling liquid was infiltrated into the template membrane using a syringe pump (Legato 210; KD Scientific, Holliston, USA). The filled membranes were wiped with a humid tissue and dried overnight (30 °C). The filling, cleaning and drying cycle was repeated with fresh blocking membranes till no further filling was possible. Five filling cycles were required for 1 and 3 µm pore size membranes, for 5 µm pore diameters 7–9 filling steps were necessary. Finally, the nanoparticles inside the pores were interconnected. Each membrane was placed onto 250 µL interconnection solution (200 mg mL−1 mannitol, 10 mg mL−1 leucine, and 0.05 mg mL−1 rhodamine B) dropped onto a glass slide. After drying at 30 °C, the interconnection step was repeated with the other membrane side. Membranes were completely dried and wiped with a humid tissue. After storing the membranes for several hours at 30 °C, they were dissolved in dichloromethane. Released rods were separated from the dissolved membrane material by three centrifugation steps (6,500×g; 10 min) and subsequent redispersion in dichloromethane. To remove free nanoparticles, the dispersion was subsequently centrifuged three more times at lower forces (2,000×g; 10 min) and the pellet was redispersed in ethyl acetate. For NGI analysis, the microparticle dispersion was completely dried at ambient temperature and pressure in an Eppendorf tube and stored till further use in a desiccator.
Depending on the used pore size diameter, the microparticle samples were named CYL 1 µm, CYL 3 µm, and CYL 5 µm. For geometric size analysis, three different batches were prepared for each type of cylindrical microparticles.
2.4. Preparation of spherical microparticles by spray drying
An aliquot of the nanosuspension containing 50 mg GNP was used for each spray drying run together with 900 mg mannitol, 50 mg leucine, and 2.5 mg rhodamine B. The primary suspension was diluted by water to achieve a final concentration of 1%, 10%, or 20% solids, respectively. Spray drying was performed with a B-290 spray dryer (Büchi, Flawil, Switzerland) at an inlet temperature of 100 °C and a resulting outlet temperature of 57–60 °C. The feed pump rate was 5.5 mL min−1, the atomizing air flow rate approx. 742 L h−1 and the aspirator was used at 100% (approx. 35 m3 h−1). Powders were stored in a desiccator till further use. Depending on the used spraying concentrations, powders were named SPH 1%, SPH 10%, and SPH 20%. Each type of powder was prepared in triplicate.
2.5. Morphology and size analysis
Cylindrical microparticles were applied as dispersions in ethyl acetate onto silica wafers and air dried. Spray-dried particles were distributed onto conductive carbon discs. After sputter coating with gold (Quorum Q150R ES; Quorum Technologies Ltd., East Grinstead, UK), yielding a coating of 10–15 nm, samples were imaged with an EVO HD15 scanning electron microscope (Zeiss, Oberkochen, Germany) at an accelerating voltage of 5.0 kV for cylindrical particles and 3.5 kV for spherical particles. Geometric particle dimensions were measured by ZEN 2012 (blue edition) software (Zeiss, Oberkochen, Germany). For spherical microparticles 600 particle diameters and for cylindrical microparticles 600 lengths and 300 diameters were determined. Each three different batches were analyzed for size analysis.
2.6. Confocal laser scanning microscopy (CLSM) imaging
Microparticles were applied on a microscope glass slide together with immersion oil and covered by a cover slip. Imaging was performed by a LSM 710 AxioObserver confocal microscope with an EC Plan-Neofluar 100x oil objective (Zeiss, Oberkochen, Germany). Excitation was performed at 488 and 561 nm and detection wavelength ranges of at 493–556 and 566-685 nm were used, respectively, in order to visualize gelatin particles loaded with FITC-dextran (Khan and Schneider Citation2013) and rhodamine B which was added to label the matrix.
2.7. Determination of nanoparticle content
A calibration curve was created with a GNP nanosuspension of known concentration. Samples were analyzed by an infinite 200 fluorescence spectrophotometer (Tecan, Männedorf, Switzerland) at an excitation wavelength of 488 nm and detection at 525 nm. Powders were accurately weighed and dispersed in water by vortexing until absence of visible aggregates. Complete disaggregation into nanoparticles was assumed. The contained GNP were quantified by measuring the fluorescence.
2.8. Evaluation of aerodynamic properties
The aerodynamic properties of the powders such as mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), and fine particle fraction (FPF) were analyzed by Next Generation Impactor (Copley Scientific, Nottingham, UK) experiments as described previously (Torge et al. Citation2017). A coating of Brij® 35/ethanol/glycerol (6/34/60) was applied to each impactor cup (two droplets for small cups, four droplets for large cups), distributed with a sponge and let for evaporation for 10 min. The pre-separator was filled with a defined volume of water (10 mL). Size 3 hard gelatin capsules were loaded with powder (approximately 20 mg for spray-dried powder and 1.5 mg for cylindrical microparticles) and positioned in a HandiHaler® (Boehringer Ingelheim, Ingelheim, Germany). Gas flow was set to 60 L min−1 by a flowmeter (M1A, Copley Scientific, Nottingham, UK). The capsule was punctured inside the HandiHaler® and the powder was aerosolized by applying a 4 s gas flow by a vacuum pump and critical flow controller (both Erweka, Heusenstamm, Germany). The powder that impacted in the induction port and the cups was subsequently collected by rinsing with water. The powder amounts in all different NGI parts were quantified by fluorescence spectroscopic measurements (infinite 200, Tecan, Männedorf, Switzerland) at λex = 565 nm and λem = 625 nm. The cumulative mass fractions were determined for all stages and translated into probit values. Subsequently, the probit values were plotted against the log cutoff diameter of the respective stages. For linear regression, the curve points including a log cutoff diameter of 0.7 (as the cut off diameter of 5 µm is relevant for FPF calculation) and a probit of 5 (relevant for MMAD calculation) were used. The MMAD is defined as the diameter that corresponds to a cumulative mass-weighted fraction of 50% (hence a probit of 5). The GSD was calculated using the following Equationequation (1)(1)
(1) :
(1)
(1)
d84 and d16 are defined as diameters corresponding to the 84% and 16% percentiles of the cumulative mass-weighted aerodynamic particle size distribution. To calculate the FPF, the corresponding probit for a cutoff diameter of 5 µm (hence log cutoff diameter of 0.7) was translated into the respective cumulative mass fraction. By definition the FPF is the powder fraction released from the capsule with an aerodynamic diameter below 5 µm. Thus, the mass of particles below 5 µm that was determined in the NGI cups was related to the complete powder mass released from the capsule (powder found in cups, pre-separator and induction port).
2.9. Redispersibility behavior
Powder was distributed on a silica wafer and covered by a water droplet. After 5 min, the drop was carefully removed by a tissue. The wafer was washed once by water and air dried. Samples were imaged after sputter coating with gold (Quorum Q150R ES, Quorum Technologies Ltd., East Grinstead, UK) with an EVO HD15 SEM (Zeiss, Oberkochen, Germany; accelerating voltage of 5.0 kV).
3. Results and discussion
3.1. Nano- and microparticle characterization
FITC dextran labeled gelatin nanoparticles (GNP) were prepared with a size of about 300 nm and a zeta potential of −15 mV, demonstrating the electrostatic stabilization of the nanosuspension under aqueous conditions (). Then the GNP were embedded into microparticles. The template-assisted technique was applied to prepare cylindrical microparticles and spray drying was performed to produce spherical microparticles. For both, mannitol and leucine were utilized as water soluble matrix excipients.
Table 1. Physicochemical characterization of gelatin nanoparticles (GNP).
Cylindrical microparticles were prepared using template membranes with three different pore diameters (CYL 1 µm, CYL 3 µm, and CYL 5 µm). The template-assisted technique yielded microparticles of homogeneous size and a diameter depending on the used template membrane pore diameter (). The maximal particle length is determined by the thickness of the membrane (10 µm). The mean length of all types of cylindrical particles was below 10 µm (). The mean diameters were as well smaller than the theoretical diameters that are defined by the pore diameter of the used template membrane. The smaller resulting diameter and length is most probably due to the reversible swelling behavior of gelatin nanoparticles in aqueous media (Khan and Schneider Citation2013; Sahoo et al. Citation2015). Upon drying, a shrinking of the GNP inside the pores is expected due to loss of water. In consequence, the formed cylindrical microparticles display dimensions smaller than the membrane pores. Furthermore, the excipient solution used for interconnecting the nanoparticles fills a larger volume than the excipients in dried state. The swelling of the nanoparticles explains as well the need of multiple filling cycles to reach a sufficient rod length. Despite the multiple filling steps, cylindrical particles were not completely filled inside as observed in CLSM imaging (). In CYL 1 µm already several voids can be seen, while CYL 5 µm are mostly hollow. In most cylindrical microparticles GNP (green fluorescence) and matrix excipients (red fluorescence) are co-localized. Though, few particles show only red fluorescence (, CYL 3 µm). This can presumably be attributed to the fact, that some pores were not filled by nanoparticles and in consequence contained only excipients from the interconnection step.
Figure 1. Cylindrical and spherical microparticles visualized by scanning electron microscope. The size of CYL particles depended on the used template membrane. An increasing feed concentration resulted in increased particle diameters for spray-dried particles (SPH). Scale bar represents 10 µm.
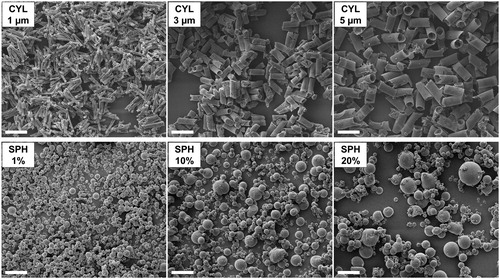
Figure 2. Confocal laser scanning microscope images: cylindrical microparticles were partially hollow and few CYL particles consisted only of excipients. Spherical microparticles were mostly solid and showed a co-localization of GNP and excipients. Scale bar 10 µm.
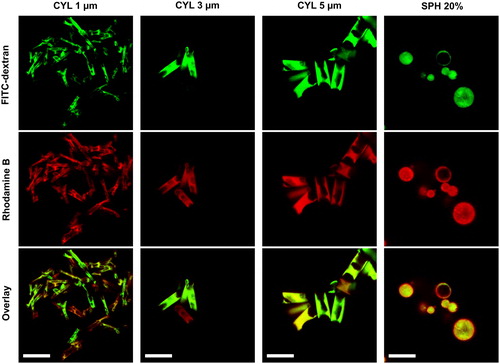
Table 2. Geometric and aerodynamic properties and nanoparticle (NP) content of microparticles.
All cylindrical particles show a narrow size distribution, defined by a Span value <1 ().
Only the CYL 1 µm particles are fibers per definition, as they display an aspect ratio (ratio of length to diameter) over 3. Fibers are defined by an elongated, approximately cylindrical shape, a length over 5 µm and an aspect ratio above 3 (Sturm and Hofmann Citation2009; Su and Cheng Citation2006).
Spray drying was performed as second technique for the preparation of nano-embedded microparticles. The feed concentration upon spray drying is known to have an influence on the resulting particle size (Bohr, Ruge, and Beck-Broichsitter Citation2014). To evaluate, how easily the size of spray-dried particles can be influenced, a combination of GNP with mannitol and leucine was spray dried in three different liquid feed concentrations. According to the used concentration, spray-dried powders were named SPH 1%, SPH 10% and SPH 20%. Larger spherical particles resulted with increasing feed concentration. The obtained median diameters ranged between 1.3 and 1.8 µm; however, with high Span values indicating a broad size distribution (). In CLSM images mostly solid and few hollow particles were observed (). Co-localization of red and green fluorescence indicated an embedding of GNP into the matrix.
The distribution of nanoparticles and matrix was homogeneous for spherical particles. In comparison, cylindrical particles lacking the nanoparticle fluorescence signal were found although in small fractions. The template-assisted technique results thus in particles that show more homogeneous sizes, but less homogeneous nanoparticle content.
By using suitable templates, the size of cylindrical nano-embedded microparticles can be easily tuned. The resulting microparticles show narrow size distributions with Span values of max. 0.22 for particle diameters and 0.80 for cylinder lengths (). In comparison, several parameters influence the size and morphology of spray-dried particles, such as feed concentration, feed and gas flow rate and inlet temperature. Reaching specific targets such as a desired MMAD and fine particle fraction is complex. Theoretical modeling to design particles is possible, however requires a profound knowledge about the droplet size distribution in the atomizer depending on feed and gas flow rate (Hoe et al. Citation2014). In practice, particle sizes are mostly optimized experimentally, such as by statistical design of experiments, requiring several experiments to achieve a certain target size. In this work only the feed concentration was varied, as it has a pronounced effect on the particle size. The range of concentrations that could be utilized was limited by the solubility of mannitol and by the risk of high product loss for low concentrations. The variation of the feed concentration within a reasonable range resulted in an only relatively small range of median particle sizes (1.3–1.8 µm). At the same time, the size distribution was broad with Span values of 1.08–2.13.
In comparison to spray drying, the template-assisted technique features the advantage of easily tunable particles within a relatively wide range. Resulting particles show a homogeneous shape and narrow size distribution.
3.2. Aerodynamic properties
Next generation impactor (NGI) experiments were performed to evaluate the aerodynamic properties. The deposition does not only depend on the aerodynamic particle size of the particles, but also on other parameters such as airway geometries, including pathological changes such as bronchial obstruction. Furthermore, the inhalation flow and volume influences the successful aerosolization of the powder and transport of particles into the lungs, while an exhalation occurring too early might prevent particles from deposition and lead to their exhalation or deposition in upper lung regions. For the comparison of the different cylindrical and spherical microparticles, standard conditions were used for the NGI experiments, taking into account a certain gas flow rate and volume.
The mass median aerodynamic diameters (MMAD) of cylindrical microparticles prepared from different template membranes ranged from 3.5 to 7.4 µm (). Particles with a MMAD of 5–10 µm are reported to deposit mainly in large conducting airways and the oropharyngeal region (Labiris and Dolovich Citation2003). Hence, the CYL 5 µm particles might be used to target the upper airways. However, a pronounced product loss is expected in the oropharynx due to large particle sizes. CYL 5 µm are thus not considered as appropriate for pulmonary application. A MMAD of 1–5 µm is associated with a deposition in small airways and in the alveolar region. Especially particles with a MMAD smaller than 3 µm deposit in the alveoli (Labiris and Dolovich Citation2003). CYL 1 µm and CYL 3 µm particles are hence both considered as suitable to target the conducting airways, with CYL 3 µm probably depositing mostly in larger airways while CYL 1 µm are expected to reach smaller airways close to the alveolar region. By using templates with pore diameters below 1 µm or with smaller membrane thickness, a targeting of the alveolar region might be enabled.
For spray-dried spherical particles, the obtained MMAD values covered only a range from 3.1 to 4.2 µm (). All SPH powders were hence appropriate for a targeting of the conducting airways. Though, it would not be possible to target other lung regions without modifying further spray drying parameters or the device. By varying the feed concentration, the geometric size and thus also the MMAD could not be further influenced. It is noteworthy, that the MMAD values determined here are most probably not the MMAD values of the primary particles, but of particle aggregates that remain depending on the powder cohesiveness and dispersion method.
The distribution width of the aerodynamic diameter is described by the geometric standard deviation (GSD). With increasing diameter of cylindrical particles, the GSD decreased. For spherical particles in comparison, the GSD increased with increasing sphere diameter (). These opposing tendencies correspond to the trend observed for Span values of the geometric sizes. Cylindrical microparticles showed by trend lower GSD values than spherical particles. For CYL 5 µm even a monodisperse distribution was achieved. Usually, most pharmaceutical aerosols are polydisperse (GSD >1.5) (Newman et al. Citation2009). By further advancing the template-assisted technique, improvements in terms of the particle homogeneity and a further reduction of the GSD are expected. Powders with low GSD might enable a targeting of certain lung regions with a reduced drug exposure for the rest of the lungs. A targeting for pulmonary delivery might be of interest for inhalative cancer treatment. In contrast, the therapy of bacterial infections benefits from the distribution of antibiotics throughout the lungs to reach all sites of infection (Labiris and Dolovich Citation2003).
Fine particle fractions were comparable with around 30% for CYL 1 µm, SPH 10% and SPH 20%. Higher FPF was reached for SPH 1%. The FPF decreased with increasing diameter for CYL particles. The fine particle fraction might still be improved for cylindrical microparticles. Due to the air drying of the dispersed particles, densely packed pellets were formed. The low powder amounts did not allow application of conventional disaggregation methods. With an appropriate technique, powders might be homogenized and fine particle fraction could be increased due to lower amount of aggregates. Also the MMAD values would be lower, due to better dispersion of particle aggregates.
Overall, CYL 1 µm particles show comparable aerosolization properties as SPH 10% particles; however, with the advantage of a higher NP content and thus higher possible drug load (). When comparing CYL 1 µm with SPH 10% particles, it can be seen that the aerodynamic diameter not only depends on the geometric diameter, but also on the shape of the particles. The aerodynamic diameter (dae) is described by the following formula (2):
(2)
(2)
with dgeo being the aerodynamic diameter, ρ the particle density, and χ the shape factor (Dailey Citation2010). For non-spherical particles the shape factor is usually <1 (Dailey Citation2010). CYL 1 µm have a comparable aerodynamic diameter as SPH 10%, despite having an about 3.5 times larger length in comparison to the geometric diameter of the spherical particles, due to their elongated shape. The hollow character of the cylindrical particles is also expected to have an impact on the aerodynamic diameter, due to reduced density.
The NGI is a state-of-the-art device and well-established for the testing of pharmaceutical aerosols. Nevertheless, there is a lack of experience regarding the evaluation of aspherical particles. Due to divergences in the deposition in comparison to spherical particles, the NGI might not be the best option to test aerodynamic properties of elongated particles. Interception is a relevant deposition mechanism for cylindrical particles (Asgharian and Yu Citation1989). Interception occurs mainly at bifurcations with a diameter corresponding approximately to the fiber length, when particles are propelled out of the airflow (Su and Cheng Citation2006). In the NGI, the particles follow the airstream in a zig-zag-pattern through the NGI. The angles in this flow pattern are smaller than bends in bifurcations. It is possible that cylindrical particles are propelled out from the airflow in these bends and are not any more able to follow the airstream. Hence, particles might deposit in stages with cutoff diameters that are larger than their actual aerodynamic diameter. The real MMAD of the cylindrical particles might be lower than the values obtained experimentally. An evaluation of available impactor devices is necessary to validate the suitability for aspherical particles. Especially a study comparing the in vivo deposition with in vitro data would be of interest. Deposition models such as human tracheobronchiolar casts or airway replica, modeling the airways in anatomically correct dimensions, were presented by Sussman, Cohen, and Lippmann (Citation1991) and Su and Cheng (Citation2006). These models are promising options for an evaluation of the aerodynamic behavior of cylindrical particles as they take the anatomical dimensions of the lungs into account.
3.3. Nanoparticle content
For nano-embedded microparticles, the amount of nanoparticles per microparticles is of interest. Higher nanoparticle contents enable as well higher drug amounts to be transported into the lungs. Low excipient contents are favored for inhalations as dry powders, as only limited powder amounts can be inhaled with one application.
For spray drying, the nanoparticle to excipient ratio initially present in the spraying feed is expected to correspond to the ratio found in the particles. In comparison, an excess of the materials is used in the template-assisted technique, so that the final ratio in the particles is not known. To determine the contained nanoparticle amount in both cylindrical and spherical microparticles, gelatin nanoparticles were quantified by fluorescence spectrophotometric measurements. The determined nanoparticle content of spherical particles was as expected close to the initially used nanoparticle concentration of 5% (). Higher nanoparticle contents would not have been possible for the SPH 20% sample, as a minimum volume of water was required for redispersion of nanoparticles during purification. For SPH 1% and SPH 10%, higher nanoparticle concentrations could have been used, but were kept constant for better comparability. In general, low nanoparticle contents are advisable for nano-embedded microparticles containing GNP, as proteins have been reported to show accumulation at the air-liquid interface of droplets in the spray dryer (Sham et al. Citation2004). Due to the drying heat, irreversible aggregation might occur. High excipient amounts are recommended to prevent nanoparticle aggregation and to facilitate redispersibility of nanoparticles. For nano-embedded microparticles containing PLGA nanoparticles and mannitol as matrix material it has been previously described, that a maximum nanoparticle content of 20% is advisable to ensure rapid disintegration (Torge et al. Citation2017).
Higher nanoparticle contents were reached for cylindrical microparticles (). CYL 1 µm showed the highest nanoparticle amount, which is probably due to the higher degree of filling for CYL 1 µm in comparison to the mostly hollow CYL 3 µm and CYL 5 µm. For cylindrical particles with larger diameter, more voids are present that can be filled by the interconnection excipients. In the template-assisted technique no heat is involved, therefore the risk for irreversible aggregation of nanoparticles is expected to be lower than for spray drying.
In summary, higher drug loading might be achieved for cylindrical microparticles, as higher nanoparticle contents were reached in comparison to spherical microparticles.
3.4. Disintegration behavior
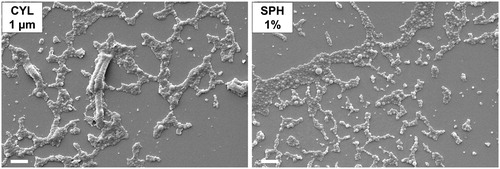
It is essential that nano-embedded microparticles disintegrate easily and nanoparticles can be released to benefit from their advantages such as mucus and biofilm penetration. The redispersion of nanoparticles was investigated by imaging the powders after incubation with a droplet of water. Spherical microparticles were completely disintegrated and only free nanoparticles were observed after incubation with water (). The good disintegration behavior can be related to the high content of mannitol, which separated nanoparticles from each other. In comparison, cylindrical microparticles did not disintegrate completely. Remnants of the microparticles were found. The incomplete disintegration can be explained by the fact, that upon microparticle preparation by template-assisted technique, nanoparticles are partially in direct contact with each other without being separated by excipient bridges. Further improvement of the technique is required to enable full disintegration of the microparticles.
3.5. Comparison of the preparation methods
The preparation of cylindrical nano-embedded microparticles by template-assisted technique is a novel preparation method. Hence, the feasibility of scale-up and the expenditure of time for the preparation are of high interest.
Spray drying in comparison is already well established in pharmaceutical research and production. By using a bench top spray dryer, several grams of powder can be produced in less than 1 h. Spray dryer are available in different dimensions, so that a scale-up to production scale can be done. However, particles with different sizes and morphologies need to be expected upon a change of the device (Littringer et al. Citation2013). Hence, further series of experiments are required to transfer an established preparation to another spray dryer device.
The preparation of particles by template-assisted technique requires several days due to overnight drying steps. In this work we used template membranes with a diameter of 25 mm that had to be processed each individually. The mean yield per membrane ranges only from 0.29 mg for CYL 5 µm to 0.55 mg for CYL 1 µm. Hence, a further development of the preparation technique is indispensable to enable a scale-up.
For the sake of completeness, it needs to be stated that there is already an advanced template-based technology for the preparation of aspherical particles available: the PRINT-technology (Particle Replication in Non-wetting Templates). Polymeric micromolds with micro- or nanocavities of a certain shape are spontaneously filled by capillary forces, the formed particles are then extracted from the template (Garcia et al. Citation2012). However, to our knowledge, this technique has never been applied to embed polymeric nanoparticles into a water-soluble matrix. The PRINT-technology is deemed not to be suitable to prepare nano-embedded, redispersible microparticles. For the filling of the polycarbonate membranes, several filtration steps were necessary to concentrate the nanoparticles in the pores. Using only capillary forces for filling would not have been sufficient. The special requirements for a filling of a template with nanoparticles need to be considered for a successful upscale of the method.
Theoretically speaking, it might be possible to prepare cylindrical microparticles in a continuous process using large membranes moving through different stations for filling, drying and interconnection. Nondestructive approaches for the release of microparticles would be of special interest. By circumventing the necessity to dissolve the template membranes, a reuse of the membranes would be enabled and high consumption of organic solvents could be avoided. A nondestructive microparticle release might be achieved by pulling the microparticles out of the membrane with an adhesive material or by pushing them out by applying pressure (Pourasghar Citation2016).
A lot of progress is still required to render possible, that cylindrical nano-embedded microparticles can find their way to application.
4. Conclusion
Cylindrical nano-embedded microparticles are a novel drug delivery system for the transport of nanoparticles into the lungs, while spray drying of spherical microparticles is already well established. The preparation of aspherical nano-embedded microparticles by template-assisted technique is still far away from an industrial application. However, cylindrical microparticles display promising features. The size of the particles can be easily tuned in a broad range by utilizing appropriate templates. In consequence, the MMAD can also be influenced. In combination with a narrow size distribution, a targeting of a narrow range of airway generations might be possible. In addition, higher drug loadings might be reached for cylindrical particles compared to spherical particles in the future, provided that the disintegration behavior of cylindrical particles can be further improved.
In summary, cylindrical nano-embedded microparticles are a promising drug delivery system for the inhalative application, requiring further progress to render a future implementation in pharmaceutical industry and application possible.
Additional information
Funding
References
- Asgharian, B., and C. P. Yu. 1989. A simplified model of interceptional deposition of fibers at airway bifurcations. Aerosol Sci. Technol. 11(1):80–88. doi: 10.1080/02786828908959301.
- Bohr, A., C. A. Ruge, and M. Beck-Broichsitter. 2014. Preparation of nanoscale pulmonary drug delivery formulations by spray drying. Adv. Exp. Med. Biol. 811:183–206. doi: 10.1007/978-94-017-8739-0_10.
- Carvalho, T. C., J. I. Peters, and R. O. Williams, 3rd. 2011. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 406(1–2):1–10. doi: 10.1016/j.ijpharm.2010.12.040.
- Champion, J. A., and S. Mitragotri. 2006. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 103(13):4930–4934. doi: 10.1073/pnas.0600997103.
- Dailey, L. A. 2010. Inhalative verabreichung von proteinen und peptiden. In Innovative arzneiformen—Ein lehrbuch für studium und praxis, ed. K. Mäder and U. Weidenauer, Stuttgart: Wissenschaftliche Verlagsgesellschaft Stuttgart.
- Elzoghby, A. O. 2013. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control Release 172(3):1075–1091. doi: 10.1016/j.jconrel.2013.09.019.
- Garcia, A., P. Mack, S. Williams, C. Fromen, T. Shen, J. Tully, J. Pillai, P. Kuehl, M. Napier, J. M. Desimone, and B. W. Maynor. 2012. Microfabricated engineered particle systems for respiratory drug delivery and other pharmaceutical applications. J. Drug Deliv. 2012:941243. doi: 10.1155/2012/941243.
- Hadinoto, K., and W. S. Cheow. 2014. Nano-antibiotics in chronic lung infection therapy against Pseudomonas aeruginosa. Colloids Surf. B: Biointerfaces 116:772–785. doi: 10.1016/j.colsurfb.2014.02.032.
- Hoe, S., J. W. Ivey, M. A. Boraey, A. Shamsaddini-Shahrbabak, E. Javaheri, S. Matinkhoo, W. H. Finlay, and R. Vehring. 2014. Use of a fundamental approach to spray-drying formulation design to facilitate the development of multi-component dry powder aerosols for respiratory drug delivery. Pharm. Res. 31(2):449–465. doi: 10.1007/s11095-013-1174-5.
- Khan, S. A., and M. Schneider. 2013. Improvement of nanoprecipitation technique for preparation of gelatin nanoparticles and potential macromolecular drug loading. Macromol. Biosci. 13(4):455–463. doi: 10.1002/mabi.201200382.
- Kohler, D., M. Schneider, M. Kruger, C. M. Lehr, H. Mohwald, and D. Wang. 2011. Template-assisted polyelectrolyte encapsulation of nanoparticles into dispersible, hierarchically nanostructured microfibers. Adv. Mater. 23(11):1376–1379. doi: 10.1002/adma.201004048.
- Labiris, N. R., and M. B. Dolovich. 2003. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 56(6):588–599. doi: 10.1046/j.1365-2125.2003.01892.x.
- Lippmann, M. 1990. Effects of fiber characteristics on lung deposition, retention, and disease. Environ. Health Perspect. 88:311–317. doi: 10.2307/3431093.
- Littringer, E. M., R. Paus, A. Mescher, H. Schroettner, P. Walzel, and N. A. Urbanetz. 2013. The morphology of spray dried mannitol particles—The vital importance of droplet size. Powder Technol. 239:162–174. doi: 10.1016/j.powtec.2013.01.065.
- Mohwald, M., S. R. Pinnapireddy, B. Wonnenberg, M. Pourasghar, M. Jurisic, A. Jung, C. Fink-Straube, T. Tschernig, U. Bakowsky, and M. Schneider. 2017. Aspherical, nanostructured microparticles for targeted gene delivery to alveolar macrophages. Adv. Healthc. Mater. 6(20):1700478. doi: 10.1002/adhm.201700478.
- Newman, S., P. Anderson, P. Byron, R. Dalby, and J. Peart. 2009. Respiratory drug delivery: Essential theory and practice. Richmond, VI: Respiratory Drug Delivery Online.
- Pott, F. 1987. Problems in defining carcinogenic fibres. Ann. Occup. Hyg. 31(4B):799–802.
- Pourasghar, M. 2016. Entwicklung einer methode zur nicht-destruktiven freisetzung von nanostrukturierten mikrostäbchen aus einer porösen matrize. In Life science engineering, 83. Gießen: Technische Hochschule Mittelhessen.
- Ruge, C. A., A. Bohr, M. Beck-Broichsitter, V. Nicolas, N. Tsapis, and E. Fattal. 2016. Disintegration of nano-embedded microparticles after deposition on mucus: A mechanistic study. Colloids Surf. B: Biointerfaces 139:219–227. doi: 10.1016/j.colsurfb.2015.12.017.
- Sahoo, N., R. K. Sahoo, N. Biswas, A. Guha, and K. Kuotsu. 2015. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int. J. Biol. Macromol. 81:317–331. doi: 10.1016/j.ijbiomac.2015.08.006.
- Sham, J. O. H., Y. Zhang, W. H. Finlay, W. H. Roa, and R. Löbenberg. 2004. Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung. Int. J. Pharm. 269(2):457–467. doi: 10.1016/j.ijpharm.2003.09.041.
- Sturm, R., and W. Hofmann. 2009. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J. Hazard. Mater. 170(1):210–218. doi: 10.1016/j.jhazmat.2009.04.107.
- Su, W. C., and Y. S. Cheng. 2006. Deposition of fiber in a human airway replica. J. Aerosol Sci. 37(11):1429–1441. doi: 10.1016/j.jaerosci.2006.01.015.
- Sussman, R. G., B. S. Cohen, and M. Lippmann. 1991. Asbestos fiber deposition in a human tracheobronchial cast. I. Experimental. Inhal. Toxicol. 3(2):145–160. doi: 10.3109/08958379109145281.
- Torge, A., P. Grutzmacher, F. Mucklich, and M. Schneider. 2017. The influence of mannitol on morphology and disintegration of spray-dried nano-embedded microparticles. Eur. J. Pharm. Sci. 104:171–179. doi: 10.1016/j.ejps.2017.04.003.
- Tsapis, N., D. Bennett, B. Jackson, D. A. Weitz, and D. A. Edwards. 2002. Trojan particles: Large porous carriers of nanoparticles for drug delivery. Proc. Natl. Acad. Sci. U. S. A. 99(19):12001–12005. doi: 10.1073/pnas.182233999.
- Tscheka, C., M. Hittinger, C. M. Lehr, N. Schneider-Daum, and M. Schneider. 2015. Macrophage uptake of cylindrical microparticles investigated with correlative microscopy. Eur. J. Pharm. Biopharm. 95:151–155. doi: 10.1016/j.ejpb.2015.03.010.
- Ungaro, F., I. d'Angelo, A. Miro, M. I. La Rotonda, and F. Quaglia. 2012. Engineered PLGA nano- and micro-carriers for pulmonary delivery: Challenges and promises. J. Pharm. Pharmacol. 64(9):1217–1235. doi: 10.1111/j.2042-7158.2012.01486.x.