Abstract
Copyright © 2020 American Association for Aerosol Research
EDITOR:
Introduction
The recent e-cigarette, or vaping, product use–associated lung injury (EVALI) crisis led to hospitalization and death in US youth and young adults (Blount et al. Citation2020; US Food and Drug Administration (US FDA) Citation2019). Most EVALI cases were apparently linked to the vaping of e-liquids that contained vitamin E acetate (VEA) and tetrahydrocannabinol (THC) (Blount et al. Citation2020). At the same time, not all vapers who inhaled VEA- or THC-containing aerosol have developed EVALI symptoms; therefore other factors, such as an individual’s health status, differences in puffing behaviors, or types of vaping devices, may be important.
One of the distinct properties of both VEA and THC is a low vapor pressure (Lovestead and Bruno Citation2017; PubChem Citation2020) that may result in generation of aerosol particles of a small size in the low-submicron (<200 nm) or nano-size (<100 nm) range. Compounds with a low vapor pressure when heated to a high temperature can create critical supersaturation, leading to nucleation (Oxtoby Citation1992) while at the same time, because of lack of the surrounding molecules that support particle condensational growth (Bunz and Koyro Citation2000), aerosol size is reduced. Small particles penetrate deep into the lungs depositing in the alveolar region (Heyder et al. Citation1986). Also, the aerosol may include toxic chemical constituents that could be formed from chemical reactions of VEA or THC at the high temperatures used in vaping (Wu and O'Shea Citation2020; Lanzarotta et al. Citation2020). While reactions involving the common e-liquid solvents propylene glycol (PG) and vegetable glycerol (VG) at corresponding vaping conditions have been studied extensively and showed presence of toxic compounds in e-cigarette emissions (Khlystov and Samburova Citation2016; Talih et al. Citation2019; Son et al. Citation2020; Uchiyama et al. Citation2020), the products formed from VEA or THC (and temperature of their vaping) are largely unknown.
The objective of this study was to assess particle size distribution of VEA aerosolized using several types of vaping pens under two puffing regimens and to conduct preliminary chemical analysis of the VEA aerosol to determine if thermal degradation took place during vaping. While we fully recognize that compounds such as THC or other components present in e-liquids could also contribute to lung injury, it is important to investigate VEA, which appears to be strongly correlated with clinical presentations of EVALI (Blount et al. Citation2020) and showed pulmonary damage during in vivo study (Bhat et al. Citation2020).
Materials and methods
The study used four vaping pens (Kind Dab, Kind Discreet, Kind Mist, and Joyetech eVic, Figure S1, in the online supplementary information [SI]) to generate aerosol from pure VEA (≥96% HPLC grade; MilliporeSigma, Burlington, Massachusetts, US) at different heating power, and under two puffing flow rates. Three vaping pens were chosen from one of the popular brands (Kind) that allowed for the adjustment of heating power and the ability to disassemble the device so that the internal geometry of the vaping pen could be measured. The fourth selected vaping pen (Joyetech eVic) allowed for noninvasive measurements of the coil temperature (see SI). Kind Discreet and Kind Mist pens had ceramic coils, Kind Dab had a titanium coil, and the Joyetech eVic used a stainless steel coil. All vaping pens are bottom type coils and were used in a vertical position to provide better coverage of the coils by the liquid. Because no puffing topography data relevant to VEA/THC vaping were available (Carrara, Giroud, and Concha-Lozano Citation2019), we applied a puffing regimen that was previously used for e-cigarette tests (Mikheev et al. Citation2018). Eight square-shaped 5-s puffs with 60-s inter-puff intervals at a flow rate of 20 or 40 mL/s were performed per test. Tests were conducted in triplicate, except for two conditions for which tests were performed in duplicate.
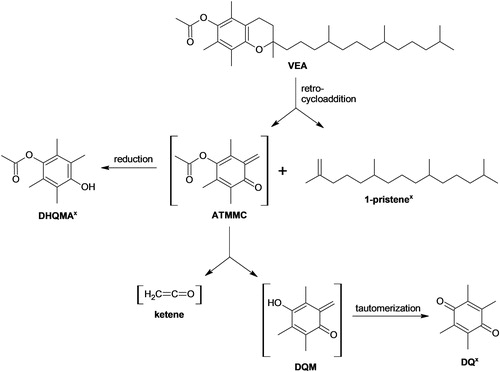
Figure 1. Putative reaction scheme showing VEA degradation reactions that may occur under vaping conditions. The “x” symbol indicates a compound that was tentatively identified by GC × GC-TOFMS analysis of samples generated using the Joyetech eVic device. Brackets denote a reactive intermediate for which the presence was inferred although no direct evidence for the compound was observed.
Aerosol size distribution characterization
The Differential Mobility Spectrometer (DMS500) in combination with the Smoking Cycle Simulator (SCS) (both manufactured by Cambustion Ltd, Cambridge, UK) was used to measure aerosol size distribution in real time under controlled puffing topography. DMS500 provides count median diameter (CMD) measurements at high particle size resolution (38 size classes) within wide particle size and concentration ranges (5 nm to 1 µm, and up to 9 orders of magnitude, respectively). The Electrical Low Pressure Impactor (ELPI+; Dekati Ltd, Kangasala, Finland), which collects aerosol on 14 size fractions (6 nm to 10 µm), was applied simultaneously with DMS500 in a similar manner as described earlier (Mikheev et al. Citation2018) for one of the test conditions (Joyetech eVic at 8.4 W heating power and 20 mL/s puffing flow rate) to sample aerosol on aluminum foils (placed on the impactor stages). Aerosol collected on the foils was used for gravimetric mass median aerodynamic diameter (MMAD) determination and then delivered for chemical analysis. CMD data measured by DMS500 were calculated to MMAD (Hatch and Choate Citation1929) and compared with ELPI + data.
Chemical analysis of samples collected on ELPI + foils
Aerosol samples from the Joyetech eVic collected on ELPI + foils, as described above, were extracted (see the SI) and analyzed by two-dimensional gas-chromatography–time-of-flight mass-spectrometry (GC × GC-TOFMS) for non-targeted chemical analysis on a LECO Pegasus 4D instrument (LECO; St Joseph, MI, US), using acquisition parameters indicated in SI). One aerosol-blank control sample was generated by collecting room air on 14 ELPI + foils under the same conditions used for VEA aerosol collection. Three liquid control samples were prepared by adding 4.5 µL of VEA to each of three foils (per sample) that were placed within a loose stack of 14 foils (total) inside a glass jar. The aerosol-blank control sample and the liquid control samples were extracted (see SI) and analyzed in the same fashion as the test aerosol samples.
Table 1. DMS500 data obtained on different vape pens varying heating power level and puffing flow rate.
The data were evaluated by comparing the chromatographic retention times and the mass spectra of detected signals from the three test aerosol samples against the four control samples. A difference was noted when a detected signal was observed consistently for the test aerosol samples and was absent from all control samples. When possible, tentative compound identities were assigned for the differentiating signals by matching mass spectra to the National Institute of Standards and Technology 17 Mass Spectral Library.
Results
Aerosol size distribution measurements
shows DMS500 data obtained during these tests: particle number concentration (PNC), CMD, and geometric standard deviation (GSD). Each test showed log-normal aerosol size distribution (e.g., Figure S3, SI) with PNC ranged from 107 to 108 particles/cm3. Aerosol size is dependent upon the device type, heating power, and puffing topography. For all tested devices and conditions, CMD varied within 50–200 nm. As flow rate increased, all devices showed CMD decreasing and PNC increasing for most of the tests. MMAD calculated from CMD obtained by DMS500 was comparable with MMAD obtained from ELPI + data, with average values of 370 nm and 308 nm, respectively.
GC × GC-TOFMS results
There were 18 signals detected exclusively in the three test aerosol samples as compared against the four control samples, with each sample showing >1000 signals. Of the 18 unique signals, three were tentatively identified as 1-pristene, durohydroquinone monoacetate (DHQMA), and duroquinone (DQ) (Figures S4–S6, SI). The identities of these compounds were not confirmed through analyzing authentic standards. In each test aerosol sample, the signal tentatively identified as 1-pristene had the largest response; comparison with the VEA signal suggested that approximately 5–10% of the VEA degraded during aerosolization.
As shown in , the possible presence of 1-pristene and DHQMA is consistent with the formation of 4-acetoxy-2,3,5-trimethyl-6-methylene-2,4-cyclohexadienone (ATMMC) as an intermediate in the degradation of VEA. Duroquinone methide (DQM) and ketene are also believed to be intermediates, as suggested by the tentative identification of DQ. Although we have no direct evidence for ATMMC, DQM, and ketene, they are expected to be too reactive to be detected via the analysis of extracts from foil-collected samples.
Discussion
Aerosol size distribution data
The most striking relationship was the strong influence of the puffing flow rate on the aerosol size. Increasing the flow rate from 20 to 40 mL/s led to substantial decreases in particle size for three of the four tested vape-pens. Particle size at a given concentration of the surrounding vapor is defined by condensational growth time. All four vaping devices were taken apart, and internal diameter and length of the aerosol delivery channel (tube between the heated coil and vape-pen outlet) were measured for three of the tested devices. (Kind Dab was excluded due to complicated internal geometry). The aerosol residence (growth) time for both flow rates was calculated. The flow rate adjustment from 20 to 40 mL/s changed the calculated residence time for Kind Mist, Kind Discreet, and Joyetech eVic from 0.011 to 0.005 s, from 0.015 to 0.008 s, and from 0.040 to 0.020 s, respectively (Table S2, SI). Shorter residence times were correlated with smaller particle sizes, showing that use of the same vape-pen by people with different styles of vaping could generate different sizes of particles, leading to different deposition patterns and potentially to different health implications. A higher puffing flow rate could lead to smaller than 50 nm particle size, hence allowing even deeper penetration into the alveolar region.
Unlike PG/VG-based e-cig emissions, VEA aerosol consists of a hydrophobic compound, suggesting that particles will not grow significantly by absorbing surrounding moisture as they flow through the human respiratory system and that aerosol dissolution will be slow after particles are deposited in the lungs. These factors may lead to particle delivery to the deepest regions of the alveoli and difficulties clearing the deposited aerosol from the lungs through normal physiological processes.
GC × GC-TOFMS data
The tentatively identified products formed during VEA vaping () are DHQMA, which, to our knowledge, has not yet been reported as a VEA degradation product; and DQ and 1-pristene, which were previously identified as degradation products from vaping VEA with a NEXUS P-1 mini vaping device (Wu and O'Shea Citation2020). The observed thermal degradation of VEA is consistent with the known thermal degradation of vitamin E to form DQ and 1-pristene under Curie-point pyrolysis conditions at 610 °C (Goossens et al. 1984). In our investigation, the temperature of the Joyetech eVic heated coil was observed to reach approximately 500–600 °C (see SI).
Of the three tentatively identified products, DQ may present concerns associated with pulmonary toxicity because quinones are potent oxidants. Previous in vitro studies demonstrated cytotoxic effects toward human bronchial epithelial cells from naphthoquinone and phenanthrenequinone (Koike, Yanagisawa, and Takano Citation2014).
The three highly reactive posited intermediates—ATMMC, DQM, and ketene—may also be of pulmonary toxicity concern. ATMMC and DQM are quinone methides, a reactive compound class that can alkylate amino acids (Bolton, Turnipseed, and Thompson Citation1997) and DNA nucleosides (Lewis et al. Citation1996). For example, the metabolic formation of a quinone methide may be important in the pulmonary toxicity of butylated hydroxytoluene (Mizutani, Yamamoto, and Tajima Citation1983). Ketene is a powerful acetylating reagent (Eck 1973) that is highly toxic when inhaled (Wooster, Lushbaugh, and Redemann Citation1947). The putative reaction scheme in shows ketene formation directly as a product of ATMMC, consistent with one suggested pathway proposed by Wu and O’Shea (2020). While ketene may also be formed directly from the aryl acetates VEA (Wu and O'Shea Citation2020) or DHQMA, we have no evidence to support those pathways because no signals consistent with the expected concurrently generated products (vitamin E and durohydroquinone, respectively) were observed.
Conclusions
VEA aerosol showed small particle size that, depending on the puffing flow rate, was as small as ∼50 nm CMD. That aerosol size is unusually small in comparison with any of the previously known aerosol/smoke generation products designed for human inhalation (e.g., combustible cigarettes, PG/VG-based e-cigarettes, medical nebulizers). Thermal degradation of VEA as a result of aerosolizing was detected and could potentially form toxic compounds. Both small aerosol size and thermal degradation of VEA may have important health implications.
Limitations
Only four vaping pens under a limited range of puffing conditions were studied. Because no data were available on THC vaping topography, only two moderately low flow rates (20 mL/s and 40 mL/s) that are typical for e-cigarette vaping were applied. Chemical product identities were not confirmed by analyzing authentic standards. A single chemical component (VEA) was aerosolized whereas synergistic effects of combined mixtures (e.g., including PG, VG, THC, or other chemicals) may also occur.
Supplemental Material
Download MS Word (1.2 MB)Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Disclosure statement
The authors declare no competing financial interest.
Additional information
Funding
References
- Bhat, T. A., S. G. Kalathil, P. N. Bogner, B. C. Blount, M. L. Goniewicz, and Y. M. Thanavala. 2020. An animal model of inhaled vitamin E acetate and EVALI-like lung injury. The New England Journal of Medicine 382 (12):1175–7. doi:10.1056/NEJMc2000231.
- Blount, B. C., M. P. Karwowski, P. G. Shields, M. Morel-Espinosa, L. Valentin-Blasini, M. Gardner, M. Braselton, C. R. Brosius, K. T. Caron, D. Chambers, et al. 2020. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. The New England Journal of Medicine 382 (8):697–705. doi:10.1056/NEJMoa1916433.
- Bolton, J. L., S. B. Turnipseed, and J. A. Thompson. 1997. Influence of quinone methide reactivity on the alkylation of thiol and amino groups in proteins: Studies utilizing amino acid and peptide models. Chemico-Biological Interactions 107 (3):185–200. doi:10.1016/S0009-2797(97)00079-3.
- Bunz, H., and M. Koyro. 2000. Growth characteristics of aerosols in a laminar flow tube reactor. Journal of Aerosol Science 31:934–5. doi:10.1016/S0021-8502(00)90945-5.
- Carrara, L., C. Giroud, and N. Concha-Lozano. 2019. Development of a vaping machine for the sampling of THC and CBD aerosols generated by two portable dry herb cannabis vaporisers. Medical Cannabis and Cannabinoids: 1–10. Published by S. Karger AG, Basel. doi:10.1159/000505027.
- Eck, H. 1973. Acetylations with ketene. Chemiker-Zeitung 97:62–67.
- US Food and Drug Administration (US FDA). 2019. Lung illnesses associated with use of vaping products.
- Hatch, T., and S. P. Choate. 1929. Statistical description of the size properties of non uniform particulate substances. Journal of The Franklin Institute 207 (3):369–87. doi:10.1016/S0016-0032(29)91451-4.
- Heyder, J., J. Gebhart, G. Rudolf, C. F. Schiller, and W. Stahlhofen. 1986. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. Journal of Aerosol Science 17 (5):811–25. doi:10.1016/0021-8502(86)90035-2.
- Khlystov, A., and V. Samburova. 2016. Flavoring compounds dominate toxic aldehyde production during E-Cigarette vaping. Environmental Science & Technology50 (23):13080–5. doi:10.1021/acs.est.6b05145.
- Koike, E., R. Yanagisawa, and H. Takano. 2014. Toxicological effects of polycyclic aromatic hydrocarbons and their derivatives on respiratory cells. Atmospheric Environment 97:529–36. doi:10.1016/j.atmosenv.2014.04.003.
- Lanzarotta, A. C., T. Falconer, R. A. Flurer, and R. A. Wilson. 2020. Hydrogen bonding between tetrahydrocannabinol and vitamin e acetate in unvaped, aerosolized, and condensed aerosol E-liquids. Analytical Chemistry 92 (3):2374–8. doi:10.1021/acs.analchem.9b05536.
- Lewis, M. A., D. G. Yoerg, J. L. Bolton, and J. A. Thompson. 1996. Alkylation of 2'-deoxynucleosides and DNA by quinone methides derived from 2,6-di-tert-butyl-4-methylphenol. Chemical Research in Toxicology 9 (8):1368–74. doi:10.1021/tx960115+.
- Lovestead, T. M., and T. J. Bruno. 2017. Determination of cannabinoid vapor pressures to aid in vapor phase detection of intoxication. Forensic chemistry 5:79–85. doi:10.1016/j.forc.2017.06.003.
- Mikheev, V. B., A. Ivanov, E. A. Lucas, P. L. South, H. O. Colijn, and P. I. Clark. 2018. Aerosol size distribution measurement of electronic cigarette emissions using combined differential mobility and inertial impaction methods: Smoking machine and puff topography influence. Aerosol Science and Technology 52 (11):1233–48. doi:10.1080/02786826.2018.1513636.
- Mizutani, T., K. Yamamoto, and K. Tajima. 1983. Isotope effects on the metabolism and pulmonary toxicity of butylated hydroxytoluene in mice by deuteration of the 4-methyl group. Toxicology and Applied Pharmacology 69 (2):283–90. doi:10.1016/0041-008X(83)90310-1.
- Oxtoby, D. W. 1992. Homogeneous nucleation: Theory and experiment. Journal of Physics: Condensed Matter 4 (38):7627–50. doi:10.1088/0953-8984/4/38/001.
- PubChem. 2020. National Center for Biotechnology Information. (-)-alpha-Tocopherol, CID = 1742129. PubChem Database. NIH U.S. National Library of Medicine 2020: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Tocopherol.
- Son, Y., C. Bhattarai, V. Samburova, and A. Khlystov. 2020. Carbonyls and carbon monoxide emissions from electronic cigarettes affected by device type and use patterns. IJERPH 17 (8):2767. doi:10.3390/ijerph17082767.
- Talih, S., R. Salman, R. El-Hage, E. Karam, N. Karaoghlanian, A. El-Hellani, N. Saliba, and A. Shihadeh. 2019. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tobacco Control 28 (6):678–80. doi:10.1136/tobaccocontrol-2018-054616.
- Uchiyama, S., M. Noguchi, A. Sato, M. Ishitsuka, Y. Inaba, and N. Kunugita. 2020. Determination of thermal decomposition products generated from E-cigarettes. Chemical Research in Toxicology 33 (2):576–83. doi:10.1021/acs.chemrestox.9b00410.
- Wooster, H., C. Lushbaugh, and C. Redemann. 1947. The inhalation toxieity of ketene and of ketene dimer. Journal of Industrial Hygiene and Toxicology 29 (1):56–7.
- Wu, D., and D. O'Shea. 2020. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proceedings of the National Academy of Sciences of the United States of America 117 (12):6349–55. doi:10.1073/pnas.1920925117.
