Abstract
A simple gas-phase method has been developed for producing size- and composition-controlled nanoparticles of binary alloys. The process includes the formation and classification of aerosol nanoparticles of one material and the subsequent condensation of a controlled shell of another. The shell thickness is controlled by the evaporation temperature of the second material. Here we study the Au–Ga system with particle compositions ranging from pure Au to 50 atomic percent Ga. Transmission electron microscopy was used to study the morphology, composition, and structure of the generated particles.
INTRODUCTION
Metal and semiconductor nanoparticles, made in the gas phase or by chemical methods, are used in various investigations in mesoscopic electronics and physics where control over particle size and quality are important. Individually, nanoparticles can function as building blocks in single electron devices (CitationThelander et al. 2001). In greater numbers, they may be included in electronic (CitationOstraat et al. 2001) or magnetic (CitationSun et al. 2000) memory devices, or used as seeds for nanowhisker growth (CitationOhlsson et al. 2001). It is reasonable to assume that the number of applications will increase further if nanoparticles can be made of alloys with specified compositions.
Binary alloys and core-shell nanoparticles have been produced by several methods. Some recent examples include: Au–Pd synthesized in reverse micelles (CitationWu et al. 2001); Ru–Pt in solution (CitationPan et al. 1999); Cu–Zn by wire explosions (CitationWang et al. 2001); Co–Ni by both ion implantation and sol-gel methods (CitationFernandez et al. 2001); Fe–Al by laser ablation of a bulk alloy (CitationPithawalla et al. 2001); carbon-covered iron nanoparticles by photolysis of ferrocene (CitationElihn et al. 1999); Cd–Se nanocrystals in a microfluidic reactor (CitationChan et al. 2003); and Au–TiO2 and Au–SiO2 nanoparticles in a spray flame reactor (CitationMädler et al. 2003). Apart from the fundamental research presented in these representative works, some of them include specific applications such as catalysis (CitationPan et al. 1999; CitationWang et al. 2001; CitationWu et al. 2001) or magnetic storage (CitationFernandez et al. 2001; CitationPithawalla et al. 2001).
As an example, the Au–Ga alloy system is interesting in that it forms a eutectic in the bulk; the melting point has a local minimum at 341°C for the composition Au0.66Ga0.34. Another attractive feature is that evaporation of Ga can be done at moderate temperatures, which simplifies the investigation. An example of a specific application of alloy nanoparticles is to improve the growth of nanowhiskers. Currently pure Au aerosol particles are used as catalysts for growing nanowhiskers epitaxially (CitationOhlsson et al. 2001). The growth is initiated by the formation of an alloy between the seed particle and the substrate. We believe that this alloy formation, and hence the growth process, can be simplified if we begin with alloy aerosol nanoparticles instead of pure Au particles, similar as described for II–VI nanowires (CitationGrebinski 2004). In this article, we present a simple aerosol-based method for making binary alloy and core-shell nanoparticles. In principle, the method can be extended to ternary and higher order alloys as well as multishell (“onion”) particles.
EXPERIMENTAL
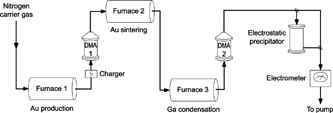
The process includes two main steps, which are depicted in . The first process step used an evaporation/condensation generator (CitationScheibel and Porstendörfer 1983) to produce Au particles by a method described previously (CitationMagnusson et al. 1999). Gold vapor was evaporated from gold pieces placed in a ceramic boat inside a tube furnace. Ultrapure particle-free nitrogen gas was used as a carrier gas for the formation of the Au aerosol. As the vapor-laden gas cooled down leaving the hot zone, nanoparticles of various sizes were formed due to homogeneous nucleation, condensation, and subsequent coagulation into agglomerates. In our experiments, the temperature of evaporation of the gold was set to 1850°C, ensuring a sufficient number concentration of gold nanoparticles in the size range 20–60 nm. After charging by a β-emitting radioactive source (63Ni), the particles were sorted by the first differential mobility analyzer (DMA; CitationKnutson and Whitby 1975), which selected particles with a narrow electrical mobility range from the gold particle size distribution. The aerosol flow was 1.68 l/min, and the sheath flow in the DMAs was 10 l/min. The flow of chosen particles was then directed towards a second furnace, which was used to sinter (compact) the agglomerate particles. The temperature of sintering was set to 600°C, a temperature sufficiently high for complete compaction of the agglomerate particles (CitationMagnusson et al. 1999).
The second step of the process was condensational addition of another element onto the generated Au cores. For this purpose, a ceramic boat containing Ga metal had been placed inside a third furnace downstream of the sintering furnace. The Ga vapor was transported out of the evaporation furnace together with the flow of the (sintered) Au aerosol. As the vapor left the hot zone it became supersaturated and condensed upon the Au particles. Through heterogeneous nucleation, Au–Ga compound particles were thus formed with the Au cores acting as condensation nuclei. The condensation process in the experiments is somewhat similar to the one occurring in a condensation nucleus counter (in order to count particles passing through such an instrument, supersaturated alcohol vapor is condensed upon particles, enlarging them into optically detectable sizes; CitationAgarwal and Sem 1980). A second DMA was placed after the Ga evaporation furnace to measure the change in the particle size distribution due to Au sintering and Ga condensation.
The sintered Au nanoparticles carried one negative charge each and retained their charge throughout the process. The negative charging was intentionally chosen because thermal recharging is observed at temperatures above 500°C (CitationMagnusson et al. 1999), which increases the number of negative charges on the particles. Another reason for choosing the negative polarity is that it has a higher charging probability (CitationWiedensohler 1988), thus increasing the yield of generated particles. The resulting particle size was determined from the position of the peak in the mobility distribution measured by the second DMA.
The final aerosol was fed into an electrometer to measure the particle concentration. A typical current was 500 fA, which corresponds to a number concentration of about 105 particles per cubic centimeter.
The morphology, composition, and structure of the generated aerosol nanoparticles were investigated by transmission electron microscopy (TEM). For size determination and indicative chemical analysis by TEM, a JEM-2000FX LaB6 microscope was used at 160 kV with a point resolution of 0.27 nm. More sophisticated measurements were made with a JEOL-3000F field-emission microscope at 300 kV with a point resolution of 0.16 nm. For the analyses by TEM and X-ray energy dispersive spectrometry (XEDS), particles were collected directly on holey carbon grids in an electrostatic precipitator (CitationDeppert et al. 1996b). With a collection voltage of 6 kV, the area of the deposition spot was ∼2 cm2, i.e., a particle concentration of 1 μm−2 was reached in 1 min. Previous observations have shown that there exists a difference in the particle diameter determination by the DMA and TEM measurements, respectively (CitationDeppert et al. 1996a). Therefore, a calibration was performed in order to compare the different particle diameters in the present experiments accurately. Pure Au particles of different diameters were selected by the second DMA and deposited for TEM investigations. Their sizes determined by the two methods were compared, resulting in a 10% smaller diameter measured with the TEM. The TEM investigations presented here included nanoparticles generated with initial Au cores of a mean diameter of 39 nm after sintering, while the amount of added Ga was varied.
Experiments similar to those reported here have been performed using the same experimental setup but with In instead of Ga placed in the third furnace (CitationMagnusson 2001).
RESULTS AND DISCUSSION
We studied in detail the increase in the particle diameter due to condensational growth of Ga on Au nanoparticles. The dependence on evaporation temperature and Au core diameter were both examined. In order to investigate the influence of thermal recharging, which could complicate the resulting particle size determination, mobility distributions were obtained by the second DMA.
shows examples of mobility scans of 39 nm Au particles (sintered at 600°C in the second furnace) after passage through the Ga evaporation furnace kept at different temperatures. Note that the distributions are presented as electrometer counts as a function of mobility diameter, which implies that the mobility scans do not represent number concentrations for multiply charged particles. For temperatures lower than about 700°C, there is only one major peak. This peak corresponds to the mobility distribution of singly charged particles. Note that as the evaporation temperature is increased, the mean of the mobility distribution is approximately constant until about 550°C and then begins to increase. Small observable shifts in this temperature range lie within fluctuations in the experimental conditions. At temperatures above 700°C, more peaks appear in the mobility distributions at lower mobility diameters, corresponding to particles carrying multiple negative charges. The Au particles carry initially only one charge, but after passing the Ga furnace the particles can acquire more charges, a phenomenon observed earlier for temperatures exceeding 600°C but still not elucidated (CitationMagnusson et al. 1999). This process becomes more pronounced towards higher temperatures, as can be seen in . For example, in the mobility scan at 939°C, four peaks can be seen. Assuming that the peak at about 45 nm corresponds to singly charged particles, the observed peaks at lower mobility diameters are in good agreement with calculations of the position of the doubly, triply, and quadruply charged peaks, indicated by the dotted lines. No other combination of number of charges and particle diameters fits the experimental data. Note that the position of the singly charged peak indicates a particle decrease compared to 791°C, an observation that will be discussed in detail in forthcoming sections. In the mobility scan labeled as 1037°C, the recharging process is strongly affecting the mobility distribution, making it impossible to determine the diameter of the resulting particles from the on-line aerosol measurement.
FIG. 2 Mobility distributions of Au–Ga alloy aerosol nanoparticles measured by the second DMA for different temperatures in the Ga evaporation furnace. The peak concentration of the 25°C distribution corresponds to 7 × 105 cm−3.
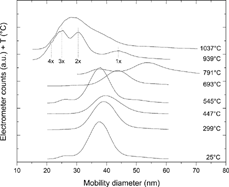
In order to investigate the effect of the recharging process, particles with diameters corresponding to two of the peaks at lower diameters in the mobility scan at 939°C in (labeled 2× and 3×) were deposited for TEM analysis. Taking the observed discrepancy between the DMA and TEM size determination of 10% into account, the TEM analysis revealed that these particles were of approximately same size: 45 nm (2×) and 42 nm (3×). These values are in good agreement with the singly charged peak measured by the DMA, 43 nm (). Thus, it was concluded that the peaks appearing as small diameters in the mobility scans recorded at elevated temperatures were a result of thermal recharging in the Ga evaporation furnace. This result also proves that it is possible to use the DMA as an on-line measurement of the alloy diameter, since the diameter can be determined from the position of the singly charged peak, either by itself or in agreement with peaks of higher charges. It was also observed that the deposited particles were composed of Au and Ga, as expected.
shows the mean diameter of the resulting particles, measured as the position of the maximum of the size distribution obtained by the second DMA, as a function of the temperature in the Ga evaporation furnace. The data points represent the growth behavior for sintered Au particles with three different diameters (28, 39, and 47 nm) as Ga is condensed onto them. For clarity, data points for sintered Au particles passing an empty third furnace are not included because they exhibit a straight line within the experimental fluctuations. For simplification of the coming discussion, the diagram has been divided into four separate regions, denoted A, B, C, and D, and separated by dotted lines. Furthermore, eye guidelines (the solid lines in the figure) have been added to help with visualization of the experimental data. From room temperature up to about 600°C (region A), there is no significant increase in the diameter. This indicates that none or only a small amount of Ga is condensed onto the Au particles. This is expected, since the vapor pressure of Ga is low at these temperatures (as an example, at 600°C the vapor pressure of Ga is 5× 10−3 Pa; CitationCRC 1997). Diameter variations in this temperature range are likely caused by fluctuations in the experimental conditions. Between 600°C and about 800°C (region B), however, there is a significant diameter increase. As the Ga vapor pressure is increased, a substantial amount of material is condensed onto the Au particles (similar to the process occurring in a condensation nucleus counter), resulting in the observed diameter increase. The increase is close to 20 nm for all three initial Au core sizes. Thus, the shell thickness is independent of initial core particle diameter. This is reasonable because the number of impinging atoms per unit surface area is independent of size due to constant vapor pressure at a given temperature.
FIG. 3 Growth behavior of Au–Ga alloy aerosol nanoparticles, with initially three different Au core diameters, 28 nm (▪), 39 nm (•), and 47 nm (▴). The data is presented as the mobility diameter measured by the second DMA, as a function of the Ga evaporation furnace temperature. Note that eye guidelines (the solid lines in the figure) have been added to help with visualization of the experimental data.
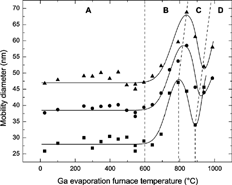
At a certain temperature, the number of evaporated Ga atoms will be so high that the formation of Ga primary particles by homogenous nucleation will compete with the process of Ga condensation onto a passing Au particle. Initially, this competition results in a lower diameter of the resulting particle, which can be seen in region C, . However, as the temperature is increased even further, the number of Ga atoms as well as particles created is increased, making it more probable for primary Ga particles to collide with the already generated Au–Ga particles. At a certain temperature, this coagulation process results in a significant growth of the Au–Ga particles, resulting in an increased diameter growth, as can be observed in region D. In principle it should be possible to suppress the production of Ga nanoparticles by increasing the Au particle number concentration and thus change the observed growth behavior. Also, other furnace designs allowing for a higher degree of control of the temperature gradient, and thus the supersaturation of Ga, could help suppress the nucleation of Ga vapor.
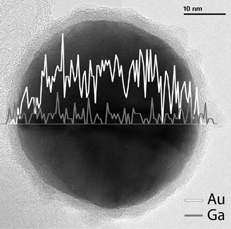
In order to confirm the aerosol measurements, TEM analysis was performed on the particles produced by evaporating Ga onto 39 nm Au core particles at different temperatures. Examples of resulting micrographs are presented in . For temperatures below 600°C, no significant shell can be seen on the Au core. Between 600°C and 800°C, a thin layer is observed, with thickness increasing with temperature. In those cases where the shell could be examined, only Ga was detected in it by XEDS analysis. The maximum Ga content for the entire particle found was 50 atomic percent. shows an example of a XEDS line scan of a particle where the Ga signal is constant over the entire particle, whereas the gold signal is only detected from the core with a maximum at the center. Since the shell is made of Ga and the incident electron beam excites both the shell and core of the particles, the XEDS signals from Ga from the shell and the core cannot be distinguished. In other words, it is difficult to clarify whether the core is pure Au or a combination of Au and Ga. However, the constant Ga signal might be an indication of a pure Au core. The line scan was made with the JEOL-3000F microscope operating in STEM-mode with a probe size of 0.6 nm.
FIG. 4 TEM micrographs of initially 39 nm Au particles, which have passed the Ga evaporation furnace at different temperatures (see text for discussion).
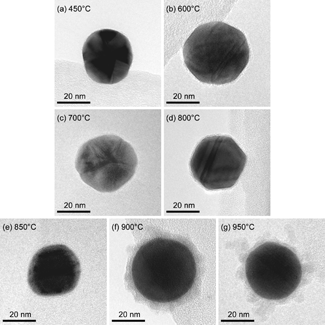
FIG. 5 XEDS line scan of a Au–Ga particle made with the Ga furnace at 900°C. The scan was collected for a period of 95 s, and the graph shows counts distributed over the diameter of the particle.
From it can be seen that at 850°C the shell thickness is smaller than at 800°C, supporting the previous discussion about the competing Ga particle formation. At 900°C, the occurrence of granular structure outside the core/shell particles can be observed, which is even more evident in the micrograph of particles created at 950°C. We associate this granular structure to primary Ga particles adsorbed to the core/shell particles after collisions in the gas phase. The diameters of the adsorbed clusters are only a few nanometers, and XEDS analysis showed that they consisted of pure Ga.
To verify the production of the primary Ga particles in the third furnace, an additional experiment was performed. The aerosol parameters were identical to the previous experiments, but no Au particles were generated. Since only a negligible fraction of any Ga particles formed in the third furnace are charged, they cannot be observed in the second DMA measurement. Therefore, TEM grids were placed in the outlet end of the Ga evaporation furnace, which was set to 800°C, 900°C, and 950°C, respectively, in order to allow for thermophoretic deposition of particles. Each experiment was run for 2 h in order to achieve sufficient coverage of particles on the grids. The resulting TEM images can be seen in . At 800°C, only a few agglomerates can be found on the grid, but at 900°C and 950°C particles are present in increasing numbers, with sizes corresponding to the particles found in at the same Ga temperatures. The XEDS analysis revealed that the particles were composed of Ga. In conclusion, the results of the TEM analyses support the aforementioned discussion on the particle growth process based on the aerosol measurements, which offers an explanation for the observed growth behavior presented in .
FIG. 6 TEM micrographs of Ga particles formed by homogeneous nucleation in the Ga evaporation furnace at different temperatures.

From the low melting temperature of Ga one could have expected that the condensing Ga would wet the Au core particle and form a spherical shell, but this was not found. The observed shell of Ga agglomerates may indicate the possibility of Ga particles having an oxidized surface preventing the merging of the otherwise liquid particles.
In the case of indium (CitationMagnusson 2001), the growth behavior of In condensing upon Au was observed, and just as for the Au–Ga nanoparticles, core/shell particles could be formed at certain experimental conditions.
From the phase diagrams of the Au–Ga and Au–In systems, one would expect complete alloying of the nanoparticles to occur, at least at the highest temperatures used. The TEM investigations revealed mainly core/shell particles in the Au–Ga case and mainly alloyed particles in the Au–In case, a difference that is not understood. It would perhaps be helpful to gain further understanding of alloying by processing the nanoparticles in a fourth furnace, which was not possible within the scope of this work.
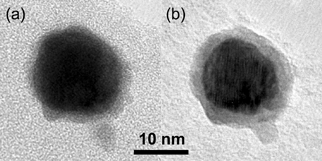
It should be noted that the energy of the electron beam could alter the properties of the as-produced particles. As an illustration we report an interesting phenomenon during XEDS investigation of Au–In particles. After 200 s in the electron beam the particles had segregated (). From the difference in contrast between core and shell in the segregated particles, we assume that the core is Au and the shell is In. It was not possible to decide whether the segregation was complete or if alloys with different compositions were present. Electron beam–driven reorganization in Au nanoparticles has been studied before (CitationSmith et al. 1986), but the mechanism behind it is still not clear.
FIG. 7 TEM images of one Au–In particle (a) before and (b) after a 200 s XEDS measurement. Intense electron radiation has made the particle segregate into a Au core with an In shell.
Au–Ga aerosol nanoparticles produced with the method presented here were used as catalysts to grow GaAs nanowires epitaxially from a GaAs substrate. Preliminary results show that it is possible to use Au–Ga nanoparticles as catalysts, even though more experiments are needed to investigate in detail whether using these particles is more advantageous than pure Au particles. Similar tests were also performed using Au–In aerosol nanoparticles as growth seeds for InAs nanowires, with similar results as in the Au–Ga case.
SUMMARY
We have developed a method for producing size-selected alloy nanoparticles. As a test system, we used size-selected Au particles as cores and condensationally added Ga. DMA, TEM, and XEDS measurements were used to determine the particle size and composition and to study the creation process. Under certain conditions, core/shell particles could be formed. The present method allows us to produce nanoparticles that would be difficult to make by other methods. It also produces large enough amounts of particles for the intended applications.
Acknowledgments
This work was carried out at the Nanometer Structure Consortium at Lund University and supported by the Swedish Foundation for Strategic Research (SSF) and the Swedish Research Council (VR). The authors would also like to thank Brent Wacaser for valuable comments during the final preparation of the manuscript.
REFERENCES
- Agarwal , J. K. and Sem , G. J. 1980 . Continuous Flow Single-Particle-Counting Condensation Nucleus Counter . J. Aerosol Sci. , 11 : 343 – 357 .
- Chan , E. M. , Mathies , R. A. and Alivisatos , A. P. 2003 . Size-Controlled Growth of CdSe Nanocrystals in Microfluidic Reactors . Nano Lett. , 3 : 199 – 201 .
- CRC . 1997 . CRC Handbook of Chemistry and Physics , Boca Raton , FL : CRC Press .
- Deppert , K. , Bovin , J.-O. , Malm , J.-O. and Samuelson , L. 1996a . A New Method to Fabricate Size-Selected Compound Semiconductor Nanocrystals: Aerotaxy . J. Cryst. Growth , 169 : 13 – 19 .
- Deppert , K. , Schmidt , F. , Krinke , T. J. , Dixkens , J. and Fissan , H. 1996b . Electrostatic Precipitator for Homogeneous Deposition of Ultrafine Particles to Create Quantum-Dot Structures . J. Aerosol Sci , 27 : S151 – S152 .
- Elihn , K. , Otten , F. , Boman , M. , Kruis , F. E. , Fissan , H. and Carlsson , J. O. 1999 . Nanoparticle Formation by Laser-Assisted Photolysis of Ferrocene . Nanostruct. Mater. , 12 : 79 – 82 .
- Fernandez , C. D. , Sangregorio , C. , Mattei , G. , De , G. , Saber , A. , Lo Russo , S. , Battaglin , G. , Catalano , M. , Cattaruzza , E. , Gonella , F. , Gatteschi , D. and Mazzoldi , P. 2001 . Structure and Magnetic Properties of Alloy-Based Nanoparticles Silica Composites Prepared by Ion-implantation and Sol-gel Techniques . Mat. Sci. Eng. C , 15 : 59 – 61 .
- Grebinski , J. W. , Richter , K. L. , Zhang , J. , Kosel , T. H. and Kuno , M. 2004 . Synthesis and Characterization of Au/Bi Core/Shell Nanocrystals: A Precursor towards II–VI Nanowires . J. Phys. Chem. B , 108 : 9745 – 9751 .
- Knutson , E. O. and Whitby , K. T. 1975 . Aerosol Classification by Electrical Mobility: Apparatus, Theory and Applications . J. Aerosol Sci. , 6 : 443 – 451 .
- Magnusson , M. H. 2001 . Metal and Semiconductor Nanocrystals for Quantum Devices. Solid State Physics , Lund , , Sweden : Lund University .
- Magnusson , M. H. , Deppert , K. , Malm , J.-O. , Bovin , J.-O. and Samuelson , L. 1999 . Gold Nanoparticles: Production, Reshaping, and Thermal Charging . J. Nanopart. Res. , 1 : 243 – 251 .
- Mädler , L. , Stark , W. J. and Pratsinis , S. E. 2003 . Simultaneous Deposition of Au Nanoparticles During Flame Synthesis of TiO2 and SiO2 . J. Mater. Res. , 18 : 115 – 120 .
- Ohlsson , B. J. , Björk , M. T. , Magnusson , M. H. , Deppert , K. , Samuelson , L. and Wallenberg , L. R. 2001 . Size-, Shape-, and Position-Controlled GaAs Nano-whiskers . Appl. Phys. Lett. , 79 : 3335 – 3337 .
- Ostraat , M. L. , De Blauwe , J. W. , Green , M. L. , Bell , L. D. , Brongersma , M. L. , Casperson , J. , Flagan , R. C. and Atwater , H. A. 2001 . Synthesis and Characterization of Aerosol Silicon nanocrystal Nonvolatile Floating-gate Memory Devices . Appl. Phys. Lett. , 79 : 433 – 435 .
- Pan , C. , Dassenoy , F. , Casanove , M. J. , Philippot , K. , Amiens , C. , Lecante , P. , Mosset , A. and Chaudret , B. 1999 . A New Synthetic Method Toward Bimetallic Ruthenium Platinum Nanoparticles; Composition Induced Structural Changes . J. Phys. Chem. B , 103 : 10098 – 10101 .
- Pithawalla , Y. B. , El-Shall , M. S. , Deevi , S. C. , Strom , V. and Rao , K. V. 2001 . Synthesis of Magnetic Intermetallic FeAl Nanoparticles from a Non-magnetic Bulk Alloy . J. Phys. Chem. B , 105 : 2085 – 2090 .
- Scheibel , H. G. and Porstendörfer , J. 1983 . Generation of Monodisperse Ag- and NaCl-aerosols with Particle Diameters Between 2 and 300 nm . J. Aerosol Sci. , 14 : 113 – 126 .
- Smith , D. J. , Petford-Long , A. K. , Wallenberg , L. R. and Bovin , J.-O. 1986 . Dynamic Atomic-level Rearrangements in Small Gold Particles . Science , 233 : 872 – 875 .
- Sun , S. H. , Murray , C. B. , Weller , D. , Folks , L. and Moser , A. 2000 . Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices . Science , 287 : 1989 – 1992 .
- Thelander , C. , Magnusson , M. H. , Deppert , K. , Samuelson , L. , Poulsen , P. R. , Nygård , J. and Borggreen , J. 2001 . Gold Nanoparticle Single-electron Transistor with Carbon Nanotube Leads . Appl. Phys. Lett. , 79 : 2106 – 2108 .
- Wang , Q. , Yang , H. B. , Shi , J. L. and Zou , G. T. 2001 . Preparation and Characterization of Nanocrystalline Powders of Cu–Zn alloy by Wire Electrical Explosion Method . Mat. Sci. Eng. A , 307 : 190 – 194 .
- Wiedensohler , A. 1988 . An Approximation of the Bipolar Charge-distribution for Particles in the Sub-micron Size Range . J. Aerosol Sci. , 19 : 387 – 389 .
- Wu , M. L. , Chen , D. H. and Huang , T. C. 2001 . Synthesis of Au/Pd Bimetallic Nanoparticles in Reverse Micelles . Langmuir , 17 : 3877 – 3883 .