Abstract
Objective: European cancer survival rates vary widely. System factors, including whether or not primary care physicians (PCPs) are gatekeepers, may account for some of these differences. This study explores where patients who may have cancer are likely to present for medical care in different European countries, and how probability of presentation to a primary care clinician correlates with cancer survival rates.
Design: Seventy-eight PCPs in a range of European countries assessed four vignettes representing patients who might have cancer, and consensus groups agreed how likely those patients were to present to different clinicians in their own countries. These data were compared with national cancer survival rates.
Setting: A total of 14 countries.
Subjects: Consensus groups of PCPs.
Main outcome measures: Probability of initial presentation to a PCP for four clinical vignettes.
Results: There was no significant correlation between overall national 1-year relative cancer survival rates and the probability of initial presentation to a PCP (r = −0.16, 95% CI −0.39 to 0.08). Within that there was large variation depending on the type of cancer, with a significantly poorer lung cancer survival in countries where patients were more likely to initially consult a PCP (lung r = −0.57, 95% CI −0.83 to −0.12; ovary: r = −0.13, 95% CI −0.57 to 0.38; breast r = 0.14, 95% CI −0.36 to 0.58; bowel: r = 0.20, 95% CI −0.31 to 0.62).
Conclusions: There were wide variations in the degree of gatekeeping between countries, with no simple binary model as to whether or not a country has a “PCP-as-gatekeeper” system. While there was case-by-case variation, there was no overall evidence of a link between a higher probability of initial consultation with a PCP and poorer cancer survival.
European cancer survival rates vary widely, and health system factors may account for some of these differences.
The data from 14 European countries show a wide variation in the probability of initial presentation to a PCP.
The degree to which PCPs act as gatekeepers varies considerably from country to country.
There is no overall evidence of a link between a higher probability of initial presentation to a PCP and poorer cancer survival.
KEY POINTS
Introduction
There is wide variation in the cancer survival rates across Europe [Citation1,Citation2]. For example, in the United Kingdom, over 6000 deaths a year that occurred within 5 years of diagnosis would have been avoided if survival in Britain had matched the mean for Europe [Citation3,Citation4], representing 6–7% of all deaths due to cancer. The variation in European 1-year relative cancer survival rates is even higher than that for 5-year survival. While 1-year relative survival rates for cancer can be affected by lead-time and over-diagnosis biases [Citation5,Citation6], they are generally taken to be an indicator of more advanced disease at diagnosis [Citation4,Citation7]. Analysis of the EUROCARE-5 data [Citation8] shows that the 1-year relative survival rate for all cancer sites varies from 60.0 to 80.5% between registries, with large variation even within EUROCARE’s five main European regions. Some studies have suggested that there are also differences between countries in cancer stage at the time of starting treatment [Citation9–11]. The survival and stage differences raise the question as to how much the differing diagnostic pathways in those countries affect the speed of diagnosis. While recent overall cancer survival trends show improvement [Citation12], there is little narrowing in the national differences [Citation13].
Healthcare systems considered to have a primary care gatekeeper system tend to have a significantly lower 1-year relative cancer survival than systems without such gatekeeper functions [Citation14]. However, achieving more timely cancer diagnoses in primary care poses a considerable challenge [Citation15]. A primary care physician (PCP) will see only a small number of new cancers each year. Half of patients with malignancies present in primary care with evolving and undifferentiated symptoms [Citation16] that are much more likely to be interpreted as something other than cancer. Even classical “red flag” symptoms like dysphagia and rectal bleeding have positive predictive values of less than 6% [Citation17].
The Örenäs Research Group is a collaborative group of researchers from 20 European countries that investigates how primary care factors influence the varying European cancer survival rates. Discussion within the group suggested that, in some countries that are considered to have PCPs as gatekeepers, some patients bypass the PCP. Conversely, in other countries where patients can consult specialists without PCP referral, the majority of adult patients still present to PCPs. In addition, a previous study shows that there can be different levels of gatekeeping, for instance with some PCPs being gatekeepers for all patients except for children, or women with gynaecological problems [Citation18]. This study was therefore designed to find out where patients with possible cancer symptoms are likely to present in different European countries, and how that correlates with national 1-year relative cancer survival rates.
Material and methods
Study design
The study used a case-based questionnaire, completed by consensus groups from 14 European countries. Four vignettes were included in the questionnaire. Each of these vignettes gave the patient’s presenting symptoms, previous medical history, medication, clinical findings and other relevant information. Two of the vignettes were designed and validated by the International Cancer Benchmarking Partnership (ICBP) [Citation19], and used with permission. Minor changes were made to make the vignettes relevant to the study format, which was designed by eight members of the Örenäs Research Group. This group also piloted the questionnaire with local colleagues. The clinical scenarios were written in English and placed in an on-line questionnaire. All participants used this single questionnaire.
The vignettes were:
A 62-year-old male smoker with chronic obstructive pulmonary disease who now has a two-week history of a productive cough, positive predictive value for lung cancer (PPV): 3.6% [Citation20];
A 53-year-old woman with lower abdominal pain and abdominal distension, PPV for ovarian cancer: 3.1% [Citation21];
A 35-year-old breastfeeding woman with an abnormal nipple discharge and eczematous changes around the nipple, PPV for breast cancer below 1.2% [Citation22];
A 22-year-old man with coeliac disease who now has abdominal pain, rectal bleeding and diarrhea (no evidence to estimate PPV for colorectal cancer available).
Respondents were asked to agree the probability that each patient would initially present to each of the following:
A PCP/general practitioner;
A practice nurse (i.e. a nurse working in a primary care practice);
A specialist doctor outside a hospital;
A specialist doctor in a hospital;
A specialist nurse outside a hospital;
A specialist nurse in a hospital;
A hospital emergency department.
For each vignette there was also space for free-text entries.
Selection of study subjects and information gathering
As PCPs were thought to have the best overview of where patients may initially present, the Örenäs Research Group lead in each centre invited local key informant PCPs to join a consensus group. During the consensus group meetings, members were asked to complete the questionnaire individually, then the results were pooled and discussed until a group consensus was achieved for each vignette. Each of the local leads entered their consensus group’s responses into an on-line survey questionnaire. MH downloaded and analysed the survey results.
Outcomes and analysis
For each centre, the probability of presentation of each vignette to each type of clinician was noted. The mean probabilities were then calculated for each of the clinicians that were considered in the questionnaire, as well as for each of the four vignettes. Probability values for PCP/practice nurse, specialist doctor inside/outside hospital and specialist nurse inside/outside hospital pairs were added together to give composite values for each. Three countries each had Örenäs leads in two different regional centres; for these, the mean probability values for each pair of centres were used. Data on national 1-year relative survival for each of the four cancers were downloaded from the EUROCARE-5 database [Citation8]. Pearson’s correlation coefficients for each set of probabilities with their national 1-year relative survival rates were then calculated.
Results
Study population
Örenäs Research Group members from 17 centres in 14 countries completed the on-line survey (). These included at least three countries in each of Central, Eastern, Northern and Southern Europe. The countries represented ranged from those with the highest EUROCARE-5 1-year cancer relative survival ranking to those with the lowest, and there were at least three countries in each survival quartile [Citation8,Citation12]. Some responses related to regions and others to whole countries. A total of 78 clinicians participated, with a median of four (range 3–9) in each consensus group.
Table 1. List of participating consensus groups, with national 1-year relative cancer survival rates.
Outcomes of the study
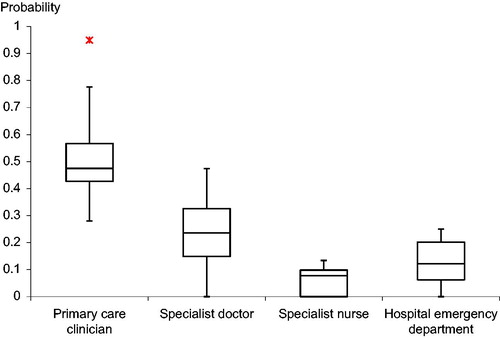
shows the mean probability of presentation to each type of clinician for each participating country. The probabilities of presentation to each clinician group are given in and the range of variations is summarised in . The median overall probability across all the surveyed countries of initial presentation to a primary care clinician (a PCP or a practice nurse) was 0.48, though with a wide variation (range 0.28–0.95). Patients were less likely to present directly to specialist physicians than to PCPs, but again with a wide variation (median probability = 0.24, range 0–0.47). There was a low overall probability of initial consultation with a specialist nurse in all the countries studied (median probability = 0.08, range 0–0.13), but a wider variation in the probability of an initial presentation at an emergency department (median probability = 0.12, range 0–0.25).
Table 2. Mean probability of presentation to each clinician for each participating country.
Table 3. Probability of presentation to each clinician group for all four vignettes combined and correlation between the probability of initial presentation to each clinician group and each country’s 1-year overall relative cancer survival.
also shows the correlations between the probability of initial presentation to each clinician group and each country’s 1-year overall relative cancer survival. For primary care clinicians and specialist physicians there was no significant correlation. However, there was a significant positive correlation for probability of presentation to a specialist nurse, and a significant negative correlation for probability of presentation to an emergency department.
shows, for each individual vignette, the correlation between the probability of initial presentation to each clinician group and that country’s individual cancer 1-year relative cancer survival. It is shown graphically for primary care clinicians in . For the patient with possible lung cancer, there was a significant negative correlation between the probability of initial presentation to a primary care clinician and each country’s 1-year relative lung cancer survival rate. This compared with a significant positive correlation between survival and the probability of initial presentation to a specialist physician. There was no significant correlation with the probability of initial presentation to a specialist nurse or an emergency department.
Figure 2. Correlations for individual vignettes between probability of initial presentation to a primary care clinician and national 1-year relative cancer survival rates, with 95% confidence intervals.
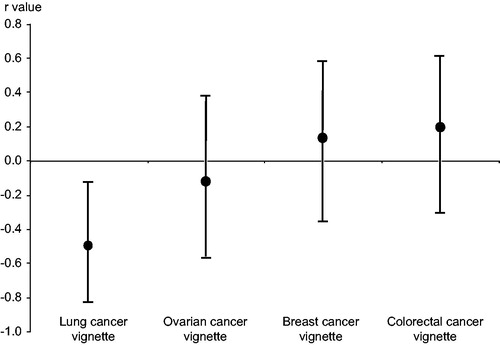
Table 4. Correlations, for individual vignettes, between probability of initial presentation to each clinician group and national cancer 1-year relative cancer survival.
For the patient with possible ovarian cancer, there was a very small, non-significant negative correlation with 1-year relative ovarian cancer survival rates for presentation to a primary care clinician, and a negligible correlation with the probability of initial presentation to a specialist physician. While there was only a probability of presentation to a nurse specialist in three countries, this was associated with an improved, but not statistically significant, 1-year relative survival. For presentation to an emergency department there was a reasonably large, but non-significant, negative correlation.
For the vignette with possible breast cancer, there were no significant correlations between national 1-year relative breast cancer survival rates and the probabilities of initial presentation for any of the clinician groups. Similarly, for the patient with possible colorectal cancer, there were no significant correlations.
Discussion
Statement of principal findings
The data from this primary care-based vignette study show a wide variation in the probability of initial presentation to a primary care clinician (a PCP or a practice nurse). The data show no significant overall evidence of a link between consensus group estimates of higher probability of initial presentation to a primary care clinician and altered cancer survival. However, there was case-by-case variation, with a significantly poorer lung cancer survival in countries where patients are more likely to initially consult a PCP.
Strengths and weaknesses of the study
The study gathered data from 17 primary care research centres in 14 European countries, with at least three centres in each of Eastern, Southern, Northern and Central Europe. All centres used an identical set of vignettes and questions.
While the use of consensus groups is a recognised methodology for probability estimations [Citation23], there may have been bias in consensus group membership selection, and a special interest in cancer survival rates may itself have caused bias. The PCPs in the groups may have had an inaccurate view of where patients in their jurisdictions were likely to present. However, recent real-life data on the route that patients take through the UK healthcare system before receiving a cancer diagnosis [Citation24,Citation25] show results that are similar to the consensus findings in our study. While the consensus groups were small, there is evidence that small group size maximises decision accuracy [Citation26], and that the degree of consensus increases with decreasing group size [Citation27].
The questionnaire used a Likert scale to report the probability of initial presentation to a clinician, but these probabilities were estimates by the consensus groups: participants from different centres may have interpreted the task differently because of differences in their cultural values and languages, and they may have had other social or health system factors, such as national levels of healthcare spending [Citation28], that acted as confounders. Not all European countries were included in the survey, and there may have been a type II error, i.e. the study may have been underpowered to detect small significant differences. The EUROCARE-5 survival rate data may have been affected by information bias. While the questionnaire gave “PCP” and “Internal medicine specialist” as separate options, some PCPs are also qualified as internal medicine specialists in some of the countries represented. However, each clinical scenario also gave a free-text entry option to allow participants to identify and comment on these issues.
Findings in relation to other studies
This study suggests that the “PCP as gate-keeper” model does not necessarily map across to how patients initially seek help, and in some of the countries where PCPs do not have a gatekeeper function, more than half of presentations are still likely to be to a primary care clinician. A recent ICBP study demonstrated a correlation between the readiness of primary care practitioners to investigate symptoms indicative of cancer and improved cancer survival rates [Citation29], and the way in which different healthcare systems support primary care in cancer diagnosis by quick and easy access to investigations may be a factor in speed of cancer diagnosis [Citation30]. There has been a call for better understanding of interactions between health system factors and professional behaviour, so that outcomes can be improved [Citation31]. Two recent studies suggest that there is a relationship between the medical system and physicians’ readiness and opportunity to refer based on a suspicion [Citation29,Citation31]. Many non-clinical factors are likely to have a significant impact on referral decisions; these include levels of gatekeeping responsibility, funding systems, access to special investigations, fear of litigation, and relationships with specialist colleagues [Citation18]. It may also be that a formal gatekeeping system introduces an asymmetrical relationship between the patient and the PCP which can result in patients self-restricting their care-seeking [Citation32], whereas the knowledge that a patient can, if wished, independently seek specialist advice may affect both patients’ and PCPs’ decision-making.
Our finding of a link between the probability of presentation to a primary care clinician and poorer survival for patients who may have lung cancer could be due to a variety of factors. It may be that primary care clinicians have poorer access to X-ray facilities than their specialist colleagues, and there is evidence that lung cancer patients presenting to hospital in the UK without a suspicious chest X-ray are less likely to have specialist care or histological confirmation of their cancer, and they have lower rates of active treatment [Citation33]. There is also evidence that, where a chest X-ray for a patient with lung cancer does originate in primary care, there is an earlier stage at diagnosis [Citation34]. There may be confounding factors: for instance, inequality in the treatment given to lung cancer patients could be due to variations in access to oncology services, with evidence for longer survival in patients whose first hospital attendance is at a radiotherapy centre [Citation35]. Also, there is a significant variation between the lung cancer referral guidelines in different jurisdictions [Citation36].
While this study showed a significant positive correlation between overall cancer survival rates and likelihood of initial consultation with a specialist nurse, that likelihood was low in all countries, so the importance of this link is unclear. However, while the role and competencies of specialist nurses are diverse across Europe [Citation37], it is thought that they have knowledge of, and insight into, the entire patient pathway, as well as high levels expertise for the patient groups for which they care [Citation37,Citation38].
It is known that patients with cancer who present as an emergency experience higher short-term mortality compared with non-emergency presentations, even when age, stage, and co-morbidity are accounted for [Citation39,Citation40]. This was reflected in our finding of a significant negative correlation between probability of an initial presentation at an emergency department and national 1-year relative cancer survival rates. Higher levels of emergency department cancer presentations could be due to delayed recognition of sinister symptoms by patients or their physicians, unavailability of non-emergency routes to care, or a complex interaction between these [Citation41], while lower levels of presentation to emergency departments may indicate good quality of cancer care in general [Citation42].
Implications for clinical policy and research
The ICBP study [Citation29] found a link between the readiness of primary care practitioners to investigate symptoms indicative of cancer and cancer survival rates, but found no specific health system features that consistently explained these findings. While it has been suggested that countries with a gatekeeper system have a significantly lower 1-year relative cancer survival than systems without such gatekeeper functions [Citation14], our study identified wide variations in the degree of gatekeeping between countries, with no simple binary model as to whether or not a country has a “PCP-as-gatekeeper” system. Further research on how system factors affect cancer survival rates is needed.
Conclusion
This vignette-based study provides information on how patients who may have cancer are likely to seek help initially, how that varies across 14 European countries, and how it relates to 1-year relative cancer survival rates. Although there was case-by-case variation, we found no overall evidence of a link between a higher probability of initial presentation to a primary care clinician and altered cancer survival.
Ethical approval
University of Bath ethical approval date: 24 November 2014; REACH registration number: EP 14/15 66.
Acknowledgements
The authors would like to thank the Örenäs Research Group collaborators who organised and ran local consensus groups, and provided the ensuing data: Isabelle Auger-Aubin (Université Paris Diderot, Paris, France); Jopseph Azuri (Tel Aviv University, Tel-Aviv, Israel); Erika Baum (Universität Marburg, Marburg, Germany); Krzysztof Buczkowski (Nicolaus Copernicus University, Torun, Poland); Nicola Buono (Caserta, Italy); Kiril Elenski (Plovdiv, Bulgaria); May-Lill Johansen (University of Tromsø, Tromsø, Norway); Peter Murchie (University of Aberdeen, Aberdeen, Scotland); Ulrike Naumann (Oakhill Surgery, Radstock, UK); Jolanta Sawicka-Powierza (Medical University of Bialystok, Bialystok, Poland); Emmanouil Smyrnakis (Aristotle University of Thessaloniki, Thessaloniki, Greece); Peter Vedsted (Danish Research Centre for Cancer Diagnosis in Primary Care (CaP) Aarhus, Denmark); and Birgitta Weltermann (Universitätsklinikum Essen, Essen, Germany).
The authors wish to thank Peter Vedsted (Danish Research Centre for Cancer Diagnosis in Primary Care (CaP) Aarhus, Denmark) and Gordon Taylor (University of Bath, UK) for their helpful comments on the manuscript.
Two of the clinical vignettes were used by kind permission of the ICBP. Dr Peter Murchie and Dr Rhona Auckland, University of Aberdeen, kindly provided the other two vignettes.
Disclosure statement
The authors have declared no conflicts of interest in respect of this work and its publication.
References
- Møller H, Linklater KM, Robinson D. A visual summary of the EUROCARE-4 results: a UK perspective. Br J Cancer. 2009;101:S110–S114.
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2014;26:62038–62039.
- Abdel-Rahman M, Stockton D, Rachet B, et al. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer. 2009;101:S115–SS24.
- Richards MA. The size of the prize for earlier diagnosis of cancer in England. Br J Cancer. 2009;101:S125–S1S9.
- Carter JL, Coletti RJ, Harris RP. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ. 2015;350:g7773.
- Zahl PH, Jorgensen KJ, Gotzsche PC. Overestimated lead times in cancer screening has led to substantial underestimation of overdiagnosis. Br J Cancer. 2013;109:2014–2019.
- Woods LM, Coleman MP, Lawrence G, et al. Evidence against the proposition that “UK cancer survival statistics are misleading”: simulation study with national cancer registry data. BMJ. 2011;342:d3399.
- EUROCARE (ed). EUROCARE-5. Milan IaISdSR, Italy: Istituto Nazionale Tumori; 2014.
- Sant M. Differences in stage and therapy for breast cancer across Europe. Int J Cancer. 2001;93:894–901.
- Sant M, Allemani C, Capocaccia R, et al. Stage at diagnosis is a key explanation of differences in breast cancer survival across Europe. Int J Cancer. 2003;106:416–422.
- Imperatori A, Harrison RN, Leitch DN, et al. Lung cancer in Teesside (UK) and Varese (Italy): a comparison of management and survival. Thorax. 2006;61:232–239.
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5-a population-based study. Lancet Oncol. 2014;15:23–34.
- Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the international cancer benchmarking partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138.
- Vedsted P, Olesen F. Are the serious problems in cancer survival partly rooted in gatekeeper principles? Br J Gen Pract. 2011;61:3–512.
- Foot C, Harrison T. How to improve cancer survival: explaining England’s relatively poor rates. London: The King’s Fund; 2011. p. 30.
- Nielsen TN, Hansen RP, Vedsted P. Symptom presentation in cancer patients in general practice. Ugeskr Laeger. 2010;172:2827–2831.
- Jones R, Latinovic R, Charlton J, et al. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using general practice research database. BMJ. 2007;334:1040.
- Harris M, Frey P, Esteva M, et al. How health system factors influence referral decisions in patients that may have cancer: European symposium report. J Cancer Res Ther. 2016;4:7–10.
- Rose PW, Hamilton W, Aldersey K, et al. Development of a survey instrument to investigate the primary care factors related to differences in cancer diagnosis between international jurisdictions. BMC Fam Pract. 2014;15:122.
- Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101 Suppl 2:S80–S86.
- Hamilton W, Peters TJ, Bankhead C, et al. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ. 2009;339:b2998.
- Walker S, Hyde C, Hamilton W. Risk of breast cancer in symptomatic women in primary care: a case-control study using electronic records. Br J Gen Pract. 2014;64:e788–e793.
- Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2:i–iv.
- National Cancer Intelligence Network. Routes to diagnosis 2006–2013, preliminary results. London: Public Health England; 2015.
- Elliss-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer–determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107:1220–1226.
- Kao AB, Couzin ID. Decision accuracy in complex environments is often maximized by small group sizes. Proc Biol Sci. 2014;281: 20133305.
- Hare A. A study of interaction and consensus in different sized groups. Am Sociol Rev. 1952;17:261–267.
- Kringos DS, Boerma W, van der Zee J, et al. Europe’s strong primary care systems are linked to better population health but also to higher health spending. Health Aff (Millwood). 2013;32:686–694.
- Rose PW, Rubin G, Perera-Salazar R, et al. Explaining variation in cancer survival between 11 jurisdictions in the international cancer benchmarking partnership: a primary care vignette survey. BMJ Open. 2015;5:e007212.
- Rubin G, Vedsted P, Emery J. Improving cancer outcomes: better access to diagnostics in primary care could be critical. Br J Gen Pract. 2011;61:317–318.
- Brown S, Castelli M, Hunter D, et al. How might healthcare systems influence speed of cancer diagnosis: a narrative review. Soc Sci Med. 2014;116:56–63.
- Andersen RS, Vedsted P, Olesen F, et al. Does the organizational structure of health care systems influence care-seeking decisions? A qualitative analysis of Danish cancer patients’ reflections on care-seeking. Scand J Prim Health Care. 2011;29:144–149.
- Melling PP, Hatfield AC, Muers MF, et al. Lung cancer referral patterns in the former Yorkshire region of the UK. Br J Cancer. 2002;86:36–42.
- Ades AE, Biswas M, Welton NJ, et al. Symptom lead time distribution in lung cancer: natural history and prospects for early diagnosis. Int J Epidemiol. 2014;43:1865–1873.
- Jack RH, Gulliford MC, Ferguson J, et al. Geographical inequalities in lung cancer management and survival in South East England: evidence of variation in access to oncology services? Br J Cancer. 2003;88:1025–1031.
- Nicholson BD, Mant D, Neal RD, et al. International variation in adherence to referral guidelines for suspected cancer: a secondary analysis of survey data. Br J Gen Pract. 2016;66:e106–e113.
- Dury C, Hall C, Danan JL, et al. Specialist nurse in Europe: education, regulation and role. Int Nurs Rev. 2014;61:454–462.
- Read C. Time for some advanced thinking? The benefits of specialist nurses. Health Serv J 2015 (Suppl.)3–4.
- McPhail S, Elliss-Brookes L, Shelton J, et al. Emergency presentation of cancer and short-term mortality. Br J Cancer. 2013;109:2027–2034.
- Parekh R, Wilson-Theaker W, Sibanda V, et al. Demography and survival in lung cancer patients presenting to the accident and emergency department. Eur Respir J 2014;44: P2738.
- Padgett DK, Brodsky B. Psychosocial factors influencing non-urgent use of the emergency room: a review of the literature and recommendations for research and improved service delivery. Soc Sci Med. 1992;35:1189–1197.
- Thoresen CK, Sandvik H, Hunskaar S. Cancer patients’ use of primary care out-of-hours services: a cross-sectional study in Norway. Scand J Prim Health Care. 2016;34:232–239.