?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The horse chestnut leaf-miner (Cameraria ohridella Deschka & Dimić, Lepidoptera, Gracillariidae) is an invasive pest causing extensive damage to leaves of the horse-chestnut (Aesculus hippocastanum L.) in Europe. In Lithuania, C. ohridella invaded in 2002 causing wilting, browning and premature fall of A. hippocastanum leaves. The aim was to get a better understanding of possible linkages between foliar fungal communities and leaf-miner damage in A. hippocastanum. Leaves of A. hippocastanum, differentially damaged by C. ohridella, were collected in 10 sites in Lithuania. The fungal communities were described through DNA isolation and amplification using an ITS rRNA marker and Ion Torrent-sequencing. Clustering of 214,897 high-quality sequences resulted in 1017 non-singleton fungal taxa, among which Aureobasidium pullulans (28.2% of all fungal sequences), Endoconidioma populi (27.7%), Phoma fungicola (11.3%), Cladosporium ramotenellum (7.6%) and Cryptococcus sp. 2185_4 (5.0%) were most common. Correspondence analysis showed that fungal communities from heavily and slightly damaged leaves were largely intermingled, showing that in both types of samples fungal communities were similar. In conclusion, the study demonstrated that the phyllosphere of A. hippocastanum is inhabited by a high diversity of fungal species, the majority of which constitute generalist endophytes, epiphytes and saprotrophic fungi. The occurrence of common phyllosphere fungi was unrelated to the degree of damage by C. ohridella.
Introduction
The horse-chestnut (Aesculus hippocastanum L.) is native to the southern Balkans (northern Greece, Macedonia, Bulgaria and Albania), but was introduced elsewhere in Europe through common planting in urban areas (Aas and Riedmiller Citation1994). In Lithuania, an assessment carried out between 1992 and 2009 showed a deterioration in the health of A. hippocastanum over time and, associated it with severe incidents of horse chestnut leaf-miner (Cameraria ohridella) (Snieskiene et al. Citation2011). Cameraria ohridella is an insect pest that cause extensive damage to A. hippocastanum leaves in many European countries (Ivinskis and Rimšaitė Citation2006). The pest was first observed in Macedonia in the 1970s and described as a new species in 1986 (Simova-Tosic and Filev Citation1985; Deschka and Dimic Citation1986). Analysis of mitochondrial and microsatellite DNA confirmed a Balkan origin for the species (Valade et al. Citation2009). In Lithuania, C. ohridella was first observed in 2002 and has since rapidly spread throughout the country (Ivinskis and Rimšaitė Citation2006). Cameraria ohridella larvae feeding during the summer causes rapid wilting, browning and eventually premature leaf fall. Although severe infestation can threaten tree vitality, damaged trees usually survive. Yet, heavy damage and falling leaves reduce the aesthetic value of these trees as a component in urban greenery. Thus, the ongoing C. ohridella epidemic questions the suitability of A. hippocastanum in urban plantations. Cameraria ohridella has also proven to negatively impact A. hippocastanum reproduction, thereby resulting in a potential gradual decline of this tree species in its native range (Thalmann et al. Citation2003).
Due to sudden invasion of C. ohridella and a high interest in A. hippocastanum as an element of urban greenery, a better understanding of the biology and ecology of this pest is needed, as well as possible control measures. Conventional insecticides are unsuitable to control this insect since its larvae reside inside of the leaf. The use of systemic insecticides is also inappropriate, largely due to the negative impact in urban environment on human health, insects, and particularly bees. Thus, biological control would be preferable. Unfortunately, information regarding specialised parasites of C. ohridella is limited and partly discouraging. For example, while insectivorous birds have shown to reduce the density of larvae and pupae of C. ohridella by up to 40% (Mösch et al. Citation2018), the efficiency of parasitoids of C. ohridella was found to be below 6% as the parasitoid complex lacks specialists (Grabenweger et al. Citation2010). Monitoring natural infections of hibernating pupae of C. ohridella by entomopathogenic fungi showed that the rate of infections was also generally low (<7%) (Schemmer et al. Citation2016). Thus, effective biocontrol solutions are still to be discovered.
Plants are often colonised by numerous endophytic fungi, which grow within different tissues without causing apparent disease symptoms (Petrini Citation1991; Wilson Citation1995). Substances produced by fungal endophytes may be toxic to plant pathogens or act as repellents against insects or herbivores (Omacini et al. Citation2001; Arnold et al. Citation2003; Findlay et al. Citation2004). Other mechanisms of protection may include parasitism, competitive exclusion or stimulation of the defence metabolism of the host trees (Witzell et al. Citation2014 and references therein). Thus, endophytic fungi antagonistic to pest insects might be important as biocontrol agents. On the other hand, invasion and outbreak of pest insects can impact fungal communities in plant tissues, leading to the predominant establishment of pathogenic species (Menkis et al. Citation2015; Meyer et al. Citation2015; Willsey et al. Citation2017). Despite the potential importance, such processes associated with fungal communities in the phyllosphere of A. hippocastanum are largely unknown and require further attention.
The aim of the present study was to obtain a better understanding on fungal communities associated with leaves of A. hippocastanum differentially damaged by C. ohridella by high-throughput sequencing method. The study was expected to reveal the possible overall fungal biodiversity in the phyllosphere of A. hippocastanum including fungi occurring specifically in slightly and heavily damaged leaves. Such information on fungi in slightly damaged leaves may suggest potential biological control agents of the insect pest while in heavily damaged leaves – fungi that benefit from the damage or that are potentially introduced by the insect pest.
Materials and methods
Study sites and sampling
The study included 10 sites representing 40–50-year-old A. hippocastanum growing in urban plantations in Lithuania (, ). All study sites were situated in ca. 20,500 km2 land area with similar urban environments, elevation (between 59 m and 127 m a.s.l.) and climatic conditions (data not shown). Sampling was carried out in late June 2013. In each site, the trees suffered of C. ohridella damage. Thirty heavily damaged (80–100% of leaf area damaged) and 30 slightly damaged (0–15% damaged) whole leaves (approx. same size) per site were randomly sampled from different geographical orientations of the crown (approximately 3–5 m above the ground using telescopic secateurs) of different A. hippocastanum trees, resulting in 300 heavily and 300 slightly damaged leaves all together (). In both types of leaves, damages were due to mining by larvae of C. ohridella. Both heavily and slightly damaged leaves were collected from the trees growing within the same area of each site. Sampled leaves were individually packed into plastic bags, transported to the laboratory and stored at −20°C prior to DNA analysis.
Figure 1. Map of Lithuania showing 10 study sites in which heavily damaged and slightly damaged leaves of A. hippocastanum were sampled. Sites are numbered as in .
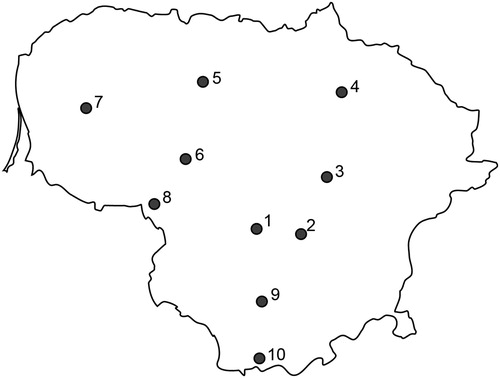
Table 1. Sampled heavily and slightly damaged leaves of A. hippocastanum, generated high-quality ITS rRNA fungal sequences and detected diversity of fungal taxa.
DNA isolation, amplification and sequencing
Prior to DNA isolation, each sample was freeze-dried at −60°C for 2 days, and no preceding surface sterilisation was conducted. Lyophilised leaves were cut into smaller fractions and 1 g (dry weight) was placed into a 2-mL screw-cap centrifugation tube together with glass beads, and homogenised using a Fast prep shaker (Precellys 24, Bertin Technologies, Rockville, MD). Genomic DNA was isolated from 300 samples of heavily damaged and 300 samples of slightly damaged A. hippocastanum leaves using Power Plant DNA Isolation Kits (MoBio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s recommendations. In addition, isolated DNA was purified using JETquick DNA Clean-Up System (Genomed, Löhne, Germany). In each sample, genomic DNA concentration was determined using a ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Diluted (1–10 ng/µl) genomic DNA samples were amplified separately using the primer pair gITS9 (5′-GAACGCAGCRAAIIGYGA-3′) (Ihrmark et al. Citation2012) and ITS4 (5′-xxxxxxxxTCCTCCGCTTATTGATATGC-3′) (White et al. Citation1990) containing eight bp sample identification barcodes denoted by x (a single barcode per each site and type of damage, i.e. 20 in total). Using this primer pair, amplified PCR products were estimated between 280 and 420 bp in size including larger parts of the 5.8S rRNA gene sequences, complete sequences of non-coding ITS2 rRNA region and partial sequences of the 28S rRNA gene. The PCR reactions, 45 µl in volume for each sample, were performed using a Veriti Thermal Cycler (Applied Biosystems, Carlsbad, CA, USA) using DreamTaq Green DNA polymerase (Fermentas, St. Leon-Rot, Germany). The PCR cycle parameters consisted of an initial denaturation at 95°C for 2 min, 27 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 45 s, followed by a final extension step at 72°C for 7 min. The PCR products were analysed on 1.5% agarose gels (Agarose D1, Conda, Madrid, Spain) under UV and amplicons were purified using QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). Purified PCR products concentration was determined using Quant-iT™ dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA, USA) and an equimolar mix of all PCR products was used for Ion Torrent sequencing. Construction of the sequencing library and sequencing using a 316 chip as a part of the larger sample was completed by NGI SciLifeLab (Uppsala, Sweden).
Bioinformatics
The sequences generated were subjected to quality control and clustering in the SCATA NGS sequencing pipeline (http://scata.mykopat.slu.se). Quality filtering of the sequences included the removal of short sequences (<200 bp), sequences with low read quality, primer dimers and homopolymers, which were collapsed to 3 bp before clustering. Sequences that were missing a barcode or primer were excluded. The primer and sample barcodes were then removed from the sequence, however, information on the sequence association with the sample was stored as meta-data. The sequences were then clustered into different taxa using single-linkage clustering based on 98.5% similarity. The most common genotype (real read) for clusters was used to represent each taxon. For clusters containing two sequences, a consensus sequence was produced. The fungal taxa were taxonomically identified using GenBank (NCBI) database and the Blastn algorithm (Altschul et al. Citation1997). The criteria used for identification were: sequence coverage >80%; similarity to taxon level 98–100%, similarity to genus level 94–97%. Sequences not matching these criteria were considered unidentified and were given unique names shown in and Supplementary Table 1. Representative sequences of all fungal non-singletons are available from GenBank under accession numbers MG827410 – MG828426.
Table 2. Relative abundance of the 20 most common fungal taxa directly sequences from heavily and slightly damaged leaves of A. hippocastanum sampled in 10 study sites in Lithuania.
Statistical analyses
Rarefaction analysis was performed using Analytical Rarefaction v.1.3 available at http://www.uga.edu/strata/software/index.html. Differences in richness of fungal taxa in heavily and slightly damaged leaves of A. hippocastanum (data pooled from all sites) was compared by non-parametric chi-square test (Magurran Citation1988). Comparisons of relative abundance (estimated as a number of sequences) of the 20 most dominant fungal taxa in heavily and slightly damaged leaves were analysed through one-way analysis of variance (ANOVA) and Tukey’s test, which provided confidence intervals for all pairwise differences between means (Chalmers and Parker Citation1989; Fowler et al. Citation1998). Distributional assumptions were checked for ANOVA. The statistics were computed using Minitab statistical software v. 18.1 (Minitab® Inc., State College, Pennsylvania, USA). Shannon diversity index, qualitative Sorensen similarity index and correspondence analysis (CA) in Canoco 4.5 (Shannon Citation1948; Magurran Citation1988; ter Braak and Smilauer Citation1998) were used to characterise the diversity and composition of fungal communities in different datasets: heavily damaged vs. slightly damaged leaves and different study sites. Since these indices provide quantitative or qualitative measures, they were widely used in community studies. Shannon’s index accounts for both abundance and evenness of the species and is calculated using the equationwhere pi is the proportion of individuals, ln is the natural logarithm, Σ is the sum of the calculations, and S is the number of species. Sorensen index measures similarity in species composition for two communities by the equation
where a is the number of species found in community one; b is the number of species in community two and c is the number of species shared by the two communities. In addition, the nonparametric Mann-Whitney test in Minitab was used to test if Shannon indexes were statistically similar or not.
Results
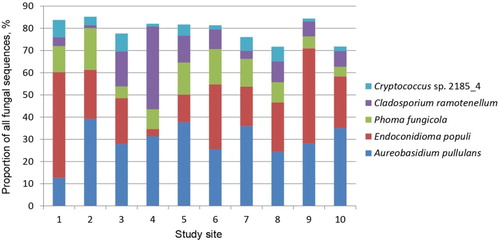
A total of 322,643 sequences was generated by Ion Torrent sequencing from 300 heavily damaged and 300 slightly damaged leaves of A. hippocastanum representing 10 study sites (, ). However, 107,746 (33.4%) sequences were of low quality and thus excluded from further analysis. Clustering of the remaining 214,897 high-quality sequences (316 bp on average) resulted in 1083 non-singleton contigs (at 98.5% similarity representing different taxa) and in 2403 singleton contigs, which were excluded from further analysis. Among the non-singletons, 1017 (93.9%) were representing fungi, 61 (5.6%) plants, 3 (0.3%) protists and 2 (0.2%) animals. Rarefaction analysis showed that fungal taxa detected in heavily and slightly damaged leaves vs. the number of sequences did not reach the asymptote, indicating that a potentially higher diversity of fungal taxa could be detected with increased sequencing effort. The detected fungi were 74.0% Ascomycota, 25.8% Basidiomycota, 0.1% Chytridiomycota and 0.1% Mucoromycota. Pooling the data from all sites showed that the absolute richness of fungal taxa was marginally higher in heavily damaged leaves (869 taxa out of 126,683 sequences) than in slightly damaged leaves (863 out of 87,241) and that 715 taxa were shared between both types of samples (). If the same number of sequences had been taken from each type of samples, the difference in chi-squared test was still not significant (p > 0.05). Information on the 20 most common fungal taxa representing 89.9% of all fungal sequences is shown in . ANOVA showed that the abundance of these fungal taxa did not differ significantly when heavily and slightly damaged leaves of different study sites were compared (p > 0.05). The most common fungi were A. pullulans (28.2% of all fungal sequences), E. populi (27.7%), P. fungicola (11.3%), C. ramotenellum (7.6%) and Cryptococcus sp. 2185_4 (5.0%), all of which occurred in all study sites at variable abundances (). The plant pathogen B. cinerea (0.6%) was one of the most common fungi detected at low abundances in both heavily and slightly damaged leaves of A. hippocastanum (). The remaining 997 fungal taxa were rare and their relative abundances varied between 0.001% and 0.2% (Supplementary Table 1).
Figure 2. Venn diagram showing the overall diversity of fungal taxa (data pooled from all sites) and nine most common fungi exclusively found in heavily and slightly damaged leaves of A. hippocastanum, and overlap between both types of samples. The fungal taxa are top-down listed in descending order of occurrence as in Supplementary Table 1.
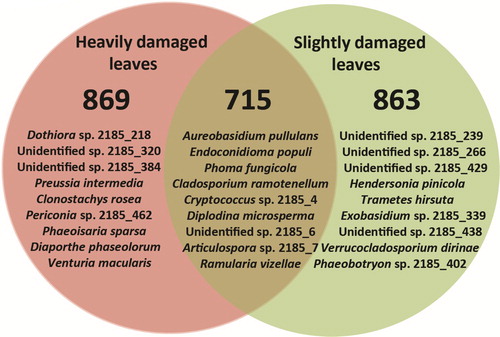
Figure 3. Relative abundance of the five most common fungal taxa (data from heavily damaged and slightly damaged leaves is pooled within each site) in 10 study sites. Sites are numbered as in .
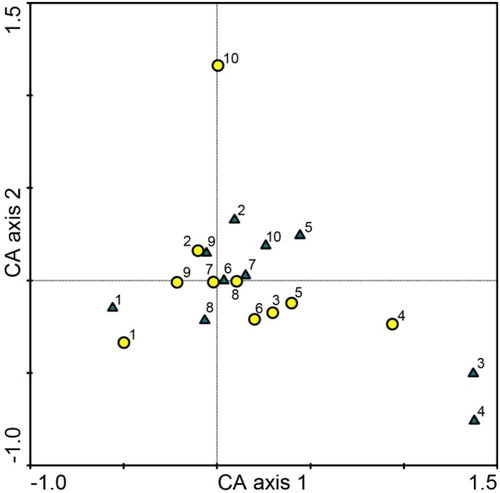
CA of fungal communities explained 20.3% variation on Axis 1 and 14.3% on Axis 2. Fungal communities from heavily and slightly damaged leaves of A. hippocastanum were largely intermingled (). Sorensen similarity index of fungal communities was high (0.83) when comparing both types of A. hippocastanum damaged leaves, and moderate to high (0.48–0.64) when comparing different study sites. Shannon diversity index of fungal communities was high and ranged between 1.9 and 2.7 in heavily damaged leaves, and between 2.1 and 3.3 in slightly damaged leaves of different study sites (). The nonparametric Mann–Whitney test showed that Shannon diversity indexes were statistically similar.
Discussion
The results demonstrated that richness of fungal taxa associated with the phyllosphere of A. hippocastanum was similar in both types of samples (, , Supplementary Table 1). Although there were some fungal taxa occurring exclusively in heavily and slightly damaged tissues (, Supplementary Table 1), these were relatively rare. However, dominant fungi in both heavily and slightly damaged leaves were often the same (). In agreement, CA that also incorporated abundance data, showed a proximal placement of fungal communities derived from both types of A. hippocastanum leaves (). Taken together, these observations suggest that despite the degree of damage from C. ohridella, the richness of fungal taxa and the structure of fungal communities in the phyllosphere of A. hippocastanum were similar. This demonstrates that fungi in the phyllosphere might have broad ecological plasticity. However, the possibility should not be excluded that more time is required for differentiation of fungal communities in each type of samples.
The results also revealed a variable abundance of common fungal taxa among individual study sites (), showing that despite the same ecological niche (leaves of A. hippocastanum), differences in environmental conditions of each study site and/or substrate quality have likely affected the abundance of associated fungal taxa. For example, Jumpponen and Jones (Citation2010) have also shown that fungal communities in the phyllosphere of Quercus macrocarpa are greatly influenced by site conditions. Fort et al. (Citation2016) have shown that the microclimate is among the major factors that might account for differences in foliar fungal communities between sites. Although the number of fungal taxa detected in our study was high (1017 fungal taxa) and comparable to similar phyllosphere fungi studies in different tree species (Jumpponen and Jones Citation2009; Menkis et al. Citation2015), rarefaction analysis demonstrated that additional sampling and sequencing would have resulted in higher richness of fungal taxa. Nevertheless, the abundance of such taxa can be expected to be low, thereby limiting the effect on fungal community structure.
The majority of dominant fungal taxa () appeared to be generalist endophytes, epiphytes, saprotrophs, and potentially opportunistic pathogens (Kirk et al. Citation2008). Among these, A. pullulans a ubiquitous black yeast-like fungus found in different environments. Commonly known as a naturally occurring epiphyte or endophyte, it subsists on a wide range of plant species without causing any apparent disease symptoms (Andrews et al. Citation2002). E. populi has also previously been reported within the phyllosphere of aspen and alder (Tsuneda et al. Citation2010). The present study therefore expands knowledge on its ecology and host range. P. fungicola has appeared in association with stem canker and fruit blight in pistachio (Chen et al. Citation2013), suggesting that under suitable conditions it can be pathogenic. Nevertheless, in the present study, the abundance of P. fungicola was similar in both heavily and slightly damaged leaves, indicating that its establishment was probably latent. C. ramotenellum is often characterised as an ubiquitous saprotroph (Zalar et al. Citation2007). D. microsperma was also one of most common fungi, which has been reported as the most abundant endophyte in healthy twigs of Salix fragilis (Mejía et al. Citation2011). Among the other more commonly detected fungi, B. cinerea is mainly known as a facultative parasite that usually causes disease to predisposed plants (Capieau Citation2004; Menkis et al. Citation2006). Several fungi of genus Taphrina were also detected (), and these are known to cause leaf and catkin curl diseases in plants (Lutzoni et al. Citation2004).
While a total of 154 (15.1% of all) fungal taxa were exclusively detected in heavily damaged leaves, their relative abundance was very low and comprised 0.2% of all fungal sequences (Supplementary Table 1). This suggests that these taxa were probably present as fungal propagules and/or broader establishment was restricted due to competition from dominant fungi. Interestingly, among these, 45 (4.4% of all taxa) could be identified at taxon level, 47 (4.6%) – at genus level and 62 (6.1%) remained unidentified (Supplementary Table 1), thereby limiting any ecological and functional role assessment. Nevertheless, the presence of fungi from genera Cryptococcus, Endoconidioma, Beauveria, Periconia, Dothiora, Parastagonospora, Cladosporium, Exobasidium, Erysiphe and Aspergillus that dominated this community (Supplementary Table 1), indicated that many of these were saprotrophs or pathogens. In comparison, there were 148 (14.6% of all) fungal taxa exclusively detected in slightly damaged leaves, comprising 0.2% of all fungal sequences (Supplementary Table 1). Among these, 46 (4.5% of all fungal taxa) could be identified at taxon level, 46 (4.5%) – at genus level and 56 (5.5%) remained unidentified (Supplementary Table 1), showing similar success of identification as compared to fungi exclusively detected in heavily damaged leaves. In slightly damaged leaves, among other common fungi we detected entomopathogen Isaria farinose and plant endophyte Preussia minima (Supplementary Table 1), which could be important for biocontrol of C. ochridella. Isaria farinosa was shown to cause infections in pupae of C. ohridella under the field and laboratory conditions (Schemmer et al. Citation2016). Preussia minima was shown to have strong enzymatic capacity, and thus, might modify chemical substances in plant tissues (Zaferanloo et al. Citation2014; Din et al. Citation2018).
Observations of the present and other studies demonstrate that the leaf area damaged by larvae of C. ochridella may vary significantly among different sites as well as among leaves within the same tree (Kopacka and Zemek Citation2017). Koskella et al. (Citation2017) have also found little explanation for the degree of C. ochridella incidence, when studying bark-associated bacterial communities and horse chestnut bleeding canker disease. In addition, other studies have demonstrated that phenolic compounds in leaves (Oszmianski et al. Citation2014; Paterska et al. Citation2017) and/or genetic background of Aesculus trees (Bacovsky et al. Citation2017) may partially explain their susceptibility to the pest. However, despite the present study included a number of trees with possibly different genetic backgrounds, within each site the overall degree of damage from C. ohridella was similar, indicating the need for more detailed studies.
In conclusion, the study demonstrated that the phyllosphere of A. hippocastanum is inhabited by a high diversity of fungal taxa, the most dominant of which constitute generalist endophytes, epiphytes and saprotrophic fungi. Further, occurrences of common phyllosphere fungi were unrelated to the degree of damage by C. ohridella, and instead, were likely determined by environmental conditions and/or substrate properties.
SFOR-2018-0012-File002.xlsx
Download MS Excel (215.7 KB)Acknowledgements
We thank Deanne Redr for the language revision.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aas G, Riedmiller A. 1994. Trees of Britain & Europe. London, UK: HarperCollinsPublishers.
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. doi: 10.1093/nar/25.17.3389
- Andrews JH, Spear RN, Nordheim EV. 2002. Population biology of Aureobasidium pullulans on apple leaf surfaces. Canad J Microbiol. 48:500–513. doi: 10.1139/w02-044
- Arnold AE, Mejia LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre A. 2003. Fungal endophytes limit pathogen damage in a tropical tree. PNAS. 100:15649–15654. doi: 10.1073/pnas.2533483100
- Bacovsky V, Vyhnanek T, Hanacek P, Mertelik J, Safrankova I. 2017. Genetic diversity of chestnut tree in relation to susceptibility to leaf miner (Cameraria ohridella Deschka & Dimic). Trees-Struct. Funct. 31:753–763. doi: 10.1007/s00468-016-1506-2
- Capieau K. 2004. Doctoral thesis: Biological control of grey mould in Swedish forest nurseries. Swedish University of Agricultural Sciences, Uppsala, ISBN 91-576-6709-8.
- Chalmers N, Parker P. 1989. Fieldwork and statistics for ecological projects, second edition. Dorchester: The Open University.
- Chen SF, Morgan DP, Michailides TJ. 2013. First report of Phoma fungicola associated with stem canker and fruit blight of pistachio in Arizona. Journal of Plant Pathology. 95:451.
- Deschka G, Dimic N. 1986. Cameraria ohridella sp. n. (Lep., Lithocolletidae) from Macedonia, Yugoslavia. Acta Entomologica Jugoslavica. 22:11–23.
- Din ZU, de Medeiros LS, Abreu LM, Pfenning LH, Jymeni DBL, Rodrigues E. 2018. Differential metabolism of diastereoisomeric diterpenes by Preussia minima, found as endophytic fungus in Cupressus lusitanica. Bioorganic Chemistry. 78:436–443. doi: 10.1016/j.bioorg.2018.04.003
- Findlay JA, Li GQ, Miller JD, Womiloju TO. 2004. Insect toxins from spruce endophytes. Canadian Journal of Chemistry. 81:284–292. doi: 10.1139/v03-044
- Fort T, Robin C, Capdevielle X, Deliere L, Vacher C. 2016. Foliar fungal communities strongly differ between habitat patches in a landscape mosaic. PeerJ. 4:e2656. doi: 10.7717/peerj.2656
- Fowler J, Cohen L, Jarvis P. 1998. Practical statistics for field biology. 2nd ed. Chichester: Wiley.
- Grabenweger G, Kehrli P, Zweimuller I, Augustin S, Avtzis N, Bacher S, Freise J, Girardoz S, Guichard S, Heitland W, et al. 2010. Temporal and spatial variations in the parasitoid complex of the horse chestnut leafminer during its invasion of Europe. Biol Invasions. 12:2797–2813. doi: 10.1007/s10530-009-9685-z
- Ihrmark K, Bodeker ITM, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, Strid Y, Stenlid J, Brandstrom-Durling M, Clemmensen KE, et al. 2012. New primers to amplify the fungal ITS2 region – evaluation by 454-sequencing of artificial and natural communities. Fems Microbiol Ecol. 82:666–677. doi: 10.1111/j.1574-6941.2012.01437.x
- Ivinskis P, Rimšaitė J. 2006. The horse-chestnut leafminer (Cameraria ohridella Deschka & Dimic 1986) (lepidoptera, Gracillariidae) in Lithuania. Acta Zool Lit. 16:1392–1657. doi: 10.1080/13921657.2006.10512749
- Jumpponen A, Jones KL. 2009. Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol. 184:438–448. doi: 10.1111/j.1469-8137.2009.02990.x
- Jumpponen A, Jones KL. 2010. Seasonally dynamic fungal communities in the Quercus macrocarpa phyllosphere differ between urban and nonurban environments. New Phytol. 186:496–513. doi: 10.1111/j.1469-8137.2010.03197.x
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. 2008. Dictionary of the fungi. Wallingford: CABI.
- Kopacka M, Zemek R. 2017. Spatial variability in the level of infestation of the leaves of horse chestnut by the horse chestnut leaf miner, Cameraria ohridella (Lepidoptera: Gracillariidae) and in the number of adult moths and parasitoids emerging from leaf litter in an urban environment. Eur J Entomol. 114:42–52. doi: 10.14411/eje.2017.007
- Koskella B, Meaden S, Crowther WJ, Leimu R, Metcalf CJE. 2017. A signature of tree health? Shifts in the microbiome and the ecological drivers of horse chestnut bleeding canker disease. New Phytol. 215:737–746. doi: 10.1111/nph.14560
- Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, et al. 2004. Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot. 91:1446–1480. doi: 10.3732/ajb.91.10.1446
- Magurran AE. 1988. Ecological diversity and its measurement. Princeton, NJ: Princeton University Press.
- Mejía LC, Castlebury LA, Rossman AY, Sogonov MV, White JF. 2011. A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host-associations, and a four-gene phylogeny. Stud Mycol. 68:211–235. doi: 10.3114/sim.2011.68.10
- Menkis A, Marčiulynas A, Gedminas A, Lynikienė J, Povilaitienė A. 2015. High-throughput sequencing reveals drastic changes in fungal communities in the phyllosphere of Norway spruce (Picea abies) following invasion of the spruce bud scale (Physokermes piceae). Microb Ecol. 70:904–911. doi: 10.1007/s00248-015-0638-z
- Menkis A, Vasiliauskas R, Taylor AFS, Stenström E, Stenlid J, Finlay R. 2006. Fungi in decayed roots of conifer seedlings from forest nurseries, afforested clearcuts and abandoned farmland. Plant Pathol. 55:117–129. doi: 10.1111/j.1365-3059.2005.01295.x
- Meyer JB, Gallien L, Prospero S. 2015. Interaction between two invasive organisms on the European chestnut: does the chestnut blight fungus benefit from the presence of the gall wasp? Fems Microbiol Ecol. 91(10):1–10.
- Mösch S, Eilers EJ, Hommes M. 2018. Biocontrol of Cameraria ohridella by insectivorous birds in different landscape contexts. BioControl. 63:215–225. doi: 10.1007/s10526-017-9857-1
- Omacini M, Chaneton EJ, Ghersa CM, Muller CB. 2001. Symbiotic fungal endophytes control insect host-parasite interaction webs. Nature. 409:78–81. doi: 10.1038/35051070
- Oszmianski J, Kalisz S, Aneta W. 2014. The content of phenolic compounds in leaf tissues of white (Aesculus hippocastanum L.) and red horse chestnut (Aesculus carea H.) colonized by the horse chestnut leaf miner (Cameraria ohridella Deschka & Dimic). Molecules. 19:14625–14636. doi: 10.3390/molecules190914625
- Paterska M, Bandurska H, Wyslouch J, Molinska-Glura M, Molinski K. 2017. Chemical composition of horse-chestnut (Aesculus) leaves and their susceptibility to chestnut leaf miner Cameraria ohridella Deschka & Dimic. Acta Physiol Plant. 39(16):1–16.
- Petrini O. 1991. Fungal endophytes of tree leaves. In: Andrews J, Hirano S, editors. Microbial ecology of leaves. New York: Springer Verlag. p. 179–197.
- Schemmer R, Chladekova P, Medo J, Barta M. 2016. Natural prevalence of entomopathogenic fungi in hibernating pupae of Cameraria ohridella (Lepidoptera: Gracillariidae) and virulence of selected isolates. Plant Prot. Sci. 52:199–208. doi: 10.17221/110/2015-PPS
- Shannon CE. 1948. A mathematical theory of communication. Bell Syst Tech J. 27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
- Simova-Tosic D, Filev S. 1985. Contribution to the knowledge of leaf miners of horse chestnut. Zastita Bilja. 36:235–239.
- Snieskiene V, Stankeviciene A, Zeimavicius K, Balezentiene L. 2011. Aesculus hippocastanum L. state changes in Lithuania. Pol J Environ Stud. 20:1029–1035.
- ter Braak CJF, Smilauer P. 1998. Canoco reference manual and user’s guide to Canoco for Windows: software for canonical community ordination, Version 4. Ithaca, NY: Microcomputer Power.
- Thalmann C, Freise J, Heitland W, Bacher S. 2003. Effects of defoliation by horse chestnut leafminer (Cameraria ohridella) on reproduction in Aesculus hippocastanum. Trees. 17:383–388. doi: 10.1007/s00468-003-0249-z
- Tsuneda A, Hambleton S, Currah RS. 2010. Endoconidioma populi from aspen and alder: phylogeny, and variations in cleistopycnidial morphology and their ecological implications. Botany. 88:675–684. doi: 10.1139/B10-043
- Valade R, Kenis M, Hernandez-Lopez A, Augustin S, Mari Mena N, Magnoux E, Rougerie R, Lakatos F, Roques A, Lopez-Vaamonde C. 2009. Mitochondrial and microsatellite DNA markers reveal a Balkan origin for the highly invasive horse-chestnut leaf miner Cameraria ohridella (Lepidoptera, Gracillariidae). Mol Ecol. 18:3458–70. doi: 10.1111/j.1365-294X.2009.04290.x
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editor. PCR protocols: a guide to methods and applications. San Diego: Academic Press, Inc; p. 315–322.
- Willsey T, Chatterton S, Carcamo H. 2017. Interactions of root-feeding insects with fungal and oomycete plant pathogens. Front Plant Sci. 8:6. doi: 10.3389/fpls.2017.01764
- Wilson D. 1995. Endophyte – the evolution of a term, and clarification of its use and definition. Oikos. 73:274–276. doi: 10.2307/3545919
- Witzell J, Martín JA, Blumenstein K. 2014. Ecological aspects of endophyte-based biocontrol of forest diseases. In: Verma V, Gange A, editors. Advances in endophytic research. New Delhi: Springer; p. 321–333.
- Zaferanloo B, Bhattacharjee S, Ghorbani MM, Mahon PJ, Palombo EA. 2014. Amylase production by Preussia minima, a fungus of endophytic origin: optimization of fermentation conditions and analysis of fungal secretome by LC-MS. BMC Microbiol. 14:12. doi: 10.1186/1471-2180-14-55
- Zalar P, de Hoog GS, Schroers HJ, Crous PW, Groenewald JZ, Gunde-Cimerman N. 2007. Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Stud Mycol. 58:157–183. doi: 10.3114/sim.2007.58.06