Abstract
To define the prognostic factors for local control and overall survival among 100 consecutive patients with chordoma of the base of skull or upper cervical spine treated by fractionated irradiation combining proton and photon beams. Between December 1993 and August 2002, 100 patients (median age: 53 years [8 – 85], M/F sex ratio: 3/2) were treated by a combination of high-energy photons and protons. The proton component was delivered at the Centre de Protonthérapie d'Orsay (CPO) by a 201 MeV beam. The median total dose delivered to the tumor volume was 67 GyECo. With a median follow-up of 31 months [range: 0 – 87], 25 tumours relapsed locally. The 2- and 4-year local control rates were 86.3% (±3.9%) and 53.8% (±7.5%), respectively. According to multivariate analysis, at least 95% of the tumor volume encompassed by the 95% isodose (p = 0.048; RR: 3.4 95%CI [1.01 – 11.8]) and a minimal dose delivered into the tumor volume <56 GyECo (p = 0.042; RR: 2.3 95%CI [1.03 – 5.2]) were independent prognostic factors of local control. Ten patients died. The 2- and 5-year overall survival rates were 94.3% (±2.5%) and 80.5% (±7.2%), respectively. According to multivariate analysis, local tumor control (p = 0.005; RR: 21 95%CI [2.2 – 200]) was a prognostic factor of overall survival. For chordomas of the base of the skull and upper cervical spine treated by surgery and irradiation combining photons and protons, the quality of irradiation, reflected by homogeneity of the dose into the tumor volume, is a major factor of local control. Close attention must be paid to minimize the underdosed areas close to critical organs. The role of surgical resection remains paramount, and a trial of dose escalation would have to consider an increase in the dose to critical organs, especially as current results indicate the low toxicity of this treatment.
Chordoma is a rare slow-growing malignant tumor, which accounts for 1% to 4% of all malignant tumours of bone and mainly occurs in adults in the fifth and sixth decades, although it can occur at any age Citation[1], Citation[2]. The incidence rate is related to age and gender, as it is more frequent in males under the age of 40. Chordoma has mainly been described in the axial skeleton Citation[1]. An intracranial site appears to be more frequent in young women. No association has been observed with other cancers. The median survival is approximately 6 years, and the 5- and 10-year survival rates are approximately 70% and 40%, respectively, regardless of the site and treatment Citation[3]. Chordoma is a tumor with mainly local and regional extension. It tends to relapse locally, but it is considered to be a malignant tumor in the presence of documented metastases and must be treated as a malignant tumor. Treatment is intimately dependent on the constraints related to the immediate proximity of neurological structures. It is therefore very important for neurosurgeons or radiation oncologists to obtain local control and prevention of treatment-related side effects.
Surgery is the first-line treatment, but excision is often limited by adjacent critical anatomical structures. Recent progress in microsurgery has allowed improved, but not complete excision. Macroscopic postoperative tumor tissue is therefore frequent Citation[4].
Postoperative irradiation has been recommended to improve chordoma local control. However, due to the presence of critical organs close to the lesion, the photon irradiation dose must always be less than 60 – 70 Gy. Proton therapy allows an increased dose to the tumor with shielding of critical structures due to the ballistic characteristics of protons. Because of their heavy mass, they diffuse very slightly laterally away from the beam (narrow lateral penumbra). Moreover, they progressively deposit an increasing quantity of energy which stops suddenly at the end of the trajectory in the form of a peak (Bragg peak). The depth of the peak can be modulated and therefore adapted to the site and thickness of the tumor. For several years, proton therapy has therefore been the recommended treatment for chordomas regardless of their site.
Regular updates of series of patients treated for chordoma or chondrosarcoma at the Orsay proton therapy center have been published Citation[5–7]. We present a recent up-date of 100 patients treated for chordoma and discuss the conclusions that can be drawn from this experience.
Patients and methods
From December 1993 to January 2002, 103 patients were referred to the Centre de Protonthérapie d'Orsay for irradiation of a chordoma of the base of skull or upper cervical spine. This series includes 100 patients, 40 females and 60 males with a median age of 53 years [range: 8 – 85 years]. One patient re-irradiated after radiosurgery and 2 patients who did not complete treatment were excluded from this study (discontinuation at 22 Gy and death from an intercurrent cause at 40 Gy).
In 52 cases, the tumor was confined to the clivus, it involved the sphenoid sinus in 19 cases, the cavernous sinus in only two cases, the cavernous and sphenoid sinuses in five cases, the petrous bone in seven cases, and several sites in three cases. In 12 cases, the chordoma was localized in the cervical spine (C1–C4) (10 cases) or by extension from a clivus lesion (2 cases).
The median maximum diameter and volume were 46 mm [range: 14 – 94 mm] and 23 cm3 [range: 1 – 125 cm3], respectively. No difference in size or volume was observed according to the site of the lesion (base of skull or cervical spine).
Seventy patients were referred for irradiation immediately after the first surgical procedure and 30 patients were treated for a relapse, with or without another surgical procedure. Sixty-four patients had undergone one surgical operation, 35 had undergone two to four operations. Surgical data were not available for one patient. The last surgery was considered to be complete in 16 cases, incomplete in 75 cases and only a biopsy was performed in 9 cases. The mean interval between last surgery and irradiation was 9 months [range: 1 – 87 months].
Irradiation procedure was described previously Citation[5], Citation[7–9]. Briefly after implantation of three to five gold fiducial markers in the outer skull bone under local anesthesia, one or several individual customized thermoplastic immobilization was made for each patient. CT scan, with MRI was systematically performed to delineate the tumor and the organs at risk. Gross tumor volume (GTV) included the visible tumor or area deemed to contain tumor on imaging was manually delineated. Clinical target volume (CTV) for possible microscopic disease extension was anatomically generated around the GTV by expanding a 3D margin of 5 to 7 mm around the GTV and eventually manually corrected. A planning target volume (PTV1) encompassing CTV with a 3D margin from 3 to 9 mm was automatically defined for photon therapy. A PTV2 was also automatically defined for proton therapy with a 3D margin of 3 mm from the CTV. The dose prescribed to the CTV was 55 CGE (1 Gy proton = 1.1 Gy photon gamma) Citation[10]. The dose prescribed to the GTV was 67 or 71 CGE. The organs at the risk close to the tumor were specifically protected and dose limits were imposed (). All patients were irradiated with a combination of photons and protons. The median total dose delivered was 67 CGE [range: 60 – 71 CGE]. The median doses delivered by photons and by protons were 45 Gy [range: 29 – 55 Gy] and 22 CGE [range: 12 – 38 CGE], respectively. The photon dose was delivered by fractions of 1.8 Gy per day and protons were delivered by fractions of 1.8 CGE per day for children, and 2 CGE per day for adults. All beams were treated each day during photon therapy and one to three beams per day during proton therapy. The median duration of irradiation was 54 days [range: 45 – 119 days].
Table I. Recommended doses to the critical organs at CPO
The median clinical and radiological follow-up for all patients were 31 months [range: 0 – 87 months] (mean: 33.5 months) and 27 months [range: 0 – 86 months] (mean: 31.1 months), respectively and the median clinical follow-up for surviving patients was 33 months [range: 0 – 87 months] (mean: 34.1 months). One patient was lost to follow-up immediately after radiotherapy. After radiotherapy, all patients were reviewed by clinical examination and imaging every 6 months for the first 5 years and then annually.
Statistics
The overall and relapse-free survival rates were calculated according to the Kaplan-Meyer method. Local control was defined on clinical and radiological arguments: absence of increase in tumor mass on MRI, confirmed at 3 months and absence of suspicious clinical symptoms. In the case of clinical and radiological discordance, often corresponding to recurrence of symptoms with no changes on imaging, MRI was performed 3 months later and, in the case of a change on imaging, the relapse was considered at the date of recurrence of clinical symptoms. The curves were compared by the Log-Rank (Mantel-Cox) method. Multivariate analysis was performed according to the Cox method. Groups of values were compared by the nonparametric test (Mann-Whitney U test). The limit of significance was 0.05. Only parameters found to be significant on univariate analysis were included in multivariate analysis. Statistical analysis was performed by Statview 5.1 software (SAS industries).
Results
The minimum dose delivered to the GTV ranged from 44.5 to 68.5 CGE [median: 55.6 CGE, mean: 56.4 CGE], corresponding to 65% to 99% of the prescribed dose [median: 83%, mean: 84%]. The minimum dose delivered to 95% of the GTV ranged from 50 to 71 CGE [median: 62.7 CGE, mean: 62.4 CGE], corresponding to 81% to 102% of the prescribed dose [median: 93%, mean: 93%]. The percentage of GTV encompassed by the isodose 90% ranged from 71% to 100% [median: 98%, mean: 95%]. The percentage of GTV encompassed by the isodose 95% ranged from 58% to 100% [median: 92%, mean: 90%].
Overall survival
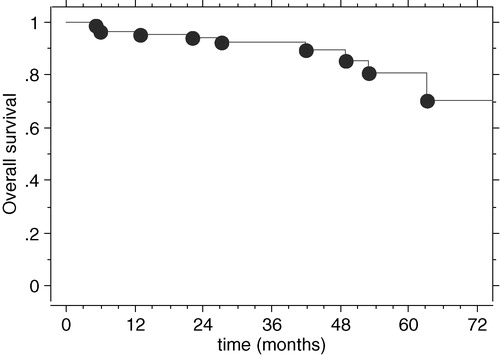
Nine patients died from progression of the chordoma and one died from intercurrent disease. The 2-, 4- and 5-year overall survival rates were 94.3% (±2.5%), 89.6% (±4.1%) and 80.5% (±7.2%), respectively (). Univariate analysis highlighted the following unfavorable prognostic factors: relapse (p < 0.0001), maximum tumor diameter >45 mm (p = 0.03), tumor volume >23 cm3 (p = 0.01) and less than 90% of the GTV encompassed by the 95% isodose of the total prescribed dose (p = 0.02). Patients older than 53 years old tended to have a lower overall survival rate (p = 0.07). Gender, number of surgical operations before irradiation, quality of last resection, indication for irradiation (first tumor or at relapse time), total dose, minimum dose and duration of irradiation were not prognostic factors.
On multivariate analysis, relapse was the only independent factor of overall survival (p = 0.005; RR: 21, 95%CI [2.2 – 200]. Five-year survival rates of patients whose tumor was locally controlled and those whose tumor was not controlled were 98.5% (±2.5%) and 52% (±15%), respectively.
Local control
Twenty-eight tumors relapsed locally, but no metastatic recurrence was observed. Seventeen relapses occurred in a volume that had received more than 55 CGE and 9 relapses occurred in a volume that had received less than 55 CGE including 3 cases in the nasal fossae (surgical incision in all 3 cases). The site of relapse could not be defined in two patients. The median interval between end of radiotherapy and relapse was 26 months [range: 3 – 71 months]. The 2- and 4-year local control rates were 86.3% (±3.9%) and 53.8% (±7.5%), respectively. Univariate analysis identified several unfavorable prognostic factors: dose delivered to 95% or 90% of GTV <95% of the total prescribed dose (p = 0.016 and p = 0.036, respectively), and minimum dose delivered to the tumor <56 CGE (p = 0.03). Any other parameters were not identified as prognostic factors.
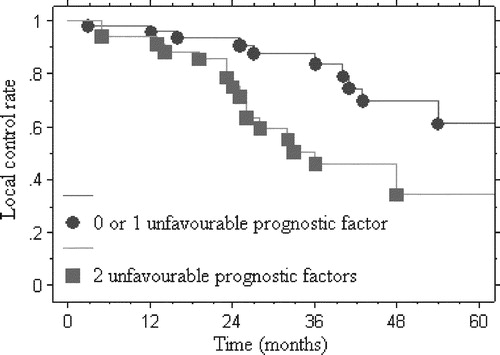
On multivariate analysis, a minimum dose to the tumor <56 CGE and less than 95% of the total prescribed dose in 95% of the GTV were independent unfavorable prognostic factors of local control (p = 0.048; RR: 3.4 95%CI [1.01 – 11.8] and p = 0.042; RR: 2.3 95%CI [1.03 – 5.2], respectively). Four-year local control rates of patients with 0 or 1 unfavorable prognostic factor and those with 2 unfavorable prognostic factors were 69.7% (±9.1%) and 34.2% (±12.4%), respectively ().
Figure 2. Curve of local control of patients with chordoma according to the number of unfavorable prognostic factors: 0 or 1 (less than 95% of the total dose prescribed in 95% of the GTV or minimum dose ≤56 CGE black circle •) and both (less than 95% of the total dose prescribed in 95% of the GTV and minimum dose ≤56 CGE black square ▪) (p < 0.004).
Complications
During irradiation and the 6 weeks after irradiation, all patients described the usual early side effects: asthenia, loss of appetite, transitory temporal and/or frontoparietal alopecia, mild erythema and sometimes nausea. No early side effect required discontinuation of irradiation or hospitalization.
Forty-two patients experienced one or more late complications. The median time to onset of these complications was 8 months [range: 2 – 43 months]. Eight patients presented visual disorders, 5 presented unilateral decreased vision by less than 5/10, two patients presented loss of vision of more than 5/10 and one diabetic patient became blind due to a chiasmatic lesion 8 months after the end of irradiation, as the chiasm had received a total dose of 48 CGE. Eleven patients presented clinical neuropsychological disorders: one case of chronic somnolence, 3 cases of severe, disabling depression, 6 cases of memory loss, including one case of anterograde amnesia requiring the assistance of a third person and one patient became bedridden. None of these patients developed necrosis or radiation-related leukoencephalopathy. One case of asymptomatic bilateral temporal necrosis was diagnosed on imaging. Maximum doses delivered to these temporal lobes were less than 30 CGE. Twenty-one patients reported decreased hearing with or without deterioration of the vocal audiogram. Hearing loss was unilateral in 16 cases and bilateral in 5 cases. A hearing aid was required in one case of bilateral hearing loss. Sixteen cases of deterioration of pituitary function were also observed, requiring complete hormone replacement therapy in nine cases, while eight cases presented partial pituitary dysfunction (TSH: 2 cases, ACTH: 3 cases, LH and FSH: 1 case, unspecified in 2 cases).
Discussion
In 1856, Virchow described, for the first time, a tumor of the clivus composed of “physaliphore” cells. In 1858, Müller found histologic similarities with the cells of the embryonic notochord that mainly give rise to the nucleus pulposus of intervertebral discs. However, it was only in 1894 that Ribbert and Steiner confirmed Müller's hypothesis using histologic and experimental arguments; by culturing primitive notochord remnants from a rabbit intervertebral disc, they obtained growth of a tumor which reproduced the histologic features of the chordoma described by Müller. However, the spinal chordoma arose from the vertebral bodies but never from the intervertebral discs.
This tumor mainly occurs in men, as confirmed in our series with a male predominance of 60%. Chordomas of the cervical spine occur earlier than skull base chordomas, at a mean age of 35 and 53 years, respectively, and these sites also classically occur earlier than sacral chordomas Citation[1]. This tumor mainly occurs at the two ends of the spine; according to Dahlin, 50% of chordomas arise in the sacrum, 37% are located at the base of the skull, and the others occur, in decreasing order, in the cervical, lumbar and thoracic spine Citation[1]. In our series, the cervical spine was involved in 12% of patients. Rosenberg et al. reported that 37% of tumors were misdiagnosed as chordoma, with a revised diagnosis of chondrosarcoma after second reading of 200 pathology results Citation[11]. Immunohistochemistry is very useful to distinguish between chordoma and chondrosarcoma, especially in the sphenoid. Rosenberg et al. found that chordomas were positive for cytokeratin, and the majority were also positive for epithelial membrane antigen (EMA) and carcinoembryonic antigen (CEA). In contrast, none of the chondrosarcomas stained for cytokeratin, EMA or CEA. Vimentin and S-100 were positive in more than 95% of chordomas and chondrosarcomas Citation[12].
The surgical treatment of chordoma of the base of skull remains a challenge for neurosurgeons, even with the development of microsurgery. Macroscopically complete resection is obtained in 62% to 79% of cases, but postoperative imaging often reveals residual tumor Citation[13–15]. Even when resection appears to be complete, the recurrence rates are between 12% and 60% Citation[14–16]. However, the majority of authors agree that surgery is the mainstay in the management of patients with chordoma Citation[17–19].
The mean duration of survival of untreated patients is estimated to be 28 months after onset of symptoms Citation[15]. The time to relapse after surgery, whether or not it is completed by irradiation, ranges from 2 to 3 years, but can be as long as 10 years Citation[15], Citation[20–27]. In our series, the median time to relapse for the base of the skull and cervical spine was 26 months and 13 months, respectively. These intervals, similar to those reported in other studies, can be easily explained by the fact that the majority of relapses occurred in volumes receiving a dose less than or equal to 55 CGE, because the tumor was situated close to a critical structure and was therefore deliberately underdosed. However, this dose can be considered to be insufficient and probably equivalent to no irradiation.
The survival and local control rates of the current series are comparable to those of previously published series using particle beams (). The results are also very similar to those reported by Debus et al., who used fractionated stereotactic irradiation with photons. The 2- and 5-year local control rates were 82% and 50%, respectively, while the overall survival rates were 97% and 82%, respectively Citation[28]. These results can be explained by the identical median dose in the two series, 66.6 Gy delivered in the series by Debus et al. and 67 CGE in our series. The results presented by Darmstadt team, which treated patients with 60 CGE of light ion irradiation were encouraging, but follow-up was still too short to allow any definite conclusions Citation[29], Citation[30].
Table II. Overall survival and local control rates of patients treated with proton irradiation for chordoma.
The prognostic factors for overall survival or local control are multiple. Age has been frequently reported Citation[15], Citation[24], Citation[31], Citation[32], as chordomas are more aggressive in children. They present a high mitotic activity, hypercellularity and a different pleomorphism from that observed in adults. The risk of metastatic spread appears to be higher in children than in adults Citation[32]. In a review of the literature, Borba et al. found that children under the age of 5 years had a very poor prognosis Citation[31], but Benk et al. reported a similar prognosis for young patients and adults Citation[24]. For adults, outcome also appears to differ according to age. Forsyth et al. reported 5- and 10-year overall survival rates of 75% and 63% for patients under the age of 40 years compared to 30% and 11% for older patients Citation[13]. In our series on univariate analysis, patients younger than 53 had a statistically better overall survival rate than older patients. Age may simply reflect surgical possibilities, as in our series the mean age of patients whose last resection was complete, incomplete or only consisted of biopsy was 50, 49 and 64 years (p = 0.053), respectively.
In our series, the patient's gender was not a prognostic factor for either overall survival or local control. The MGH study (Massachusetts General Hospital) showed that the 5- and 8-year local control rates for men and women were 81% and 65% (p = 0.035) and 75% and 17% (p = 0.01), respectively Citation[33], Citation[34]. The LLUMC team (Loma Linda University Medical Center) also found a less favorable outcome for women than for men, but without any statistically significant difference. Halperin et al., who studied all hypotheses able to explain these differences, concluded that they were probably secondary to a statistical artefact Citation[35].Tumor size has been reported to be a prognostic factor of overall survival or local control Citation[21], Citation[25], Citation[36]. In our series, the maximum tumor diameter was a prognostic factor for overall survival, but not for local control. However, the mean maximum diameter and the mean GTV were slightly higher for tumors which relapsed than for those that did not relapse: 49 mm and 46 mm (p = 0.6), and 36 mL and 29 mL (p = 0.4), respectively. These dimensions do not appear to be related to the quality of resection, which was not a prognostic factor for relapse or for overall survival: four relapses were observed among the 16 patients (25%) in whom the last resection was considered to be complete compared to 24 of the 84 patients (29%) with incomplete resection (p > 0.9). The fact that the bulkiest tumors relapsed more frequently could be due to the fact that they were adjacent to critical structures and the irradiation dose delivered could not be optimized because of the dose limitations imposed by these critical structures Citation[4] and could be related to the difficulty of obtaining dose homogeneity in these large volumes Citation[34]. Multivariate analysis of local control showed that the minimum dose received by the tumor, a corollary of dose constraints, and the tumor volume included in the 95% isodose are the major prognostic factors. The dosimetric prognostic factors of local control identified in our study confirm those reported by the Boston team Citation[34].
The Boston team showed that the role of dose in local control was clearly of primary importance, as this group reported that 6 of 26 relapses occurred in a volume that had received a high dose and 15 relapses occurred in a volume that had received a decreased dose because of the constraints imposed by critical structures Citation[23]. In our series, although the majority of relapses occurred in low-dose areas, some relapses occurred in volumes that had received 67 CGE. It was therefore decided to increase the irradiation dose to the tumor volume to 71 CGE, which corresponds to the nominal dose initially selected by the Boston proton therapy center. In our series, some relapses were observed along surgical incisions (nasal fossa) or in the clivus away from the initial tumor. Relapse along the surgical incision has been described for a long time. Many authors have reported cases of reseeding in the nasal fossa Citation[4], Citation[27], Citation[37], Citation[38]. These structures (when used for surgical access, including biopsy) and the clivus must therefore be entirely included in the CTV. However, the volumes at risk of relapse by reseeding remain difficult to define. These relapses seem to evolve more rapidly than the primary tumor and can occur away from the clivus, sometimes in the cervical spine (C1 or C2), and cannot be clearly distinguished from metastases. Some surgeons have suggested a “drop-cell” effect Citation[37], Citation[39]. These findings raise the problem of the limits of the CTV, which do not only depend on the natural extension of the disease, but also on the surgical procedure.
The dose tolerated by all critical structures has not been clearly established. It would be unacceptable to take any risks with single structures such as the optic chiasm, spinal cord or brainstem. However, our study shows a very low spinal cord complication rate for a dose of 55 CGE over the spine (1 case of transient Lhermitte syndrome). Other authors have reported similar findings Citation[40]. No brainstem complications were observed in this series of 100 patients, although we delivered doses of up to 63 CGE over this structure. The only complication of the optic chiasm was observed after a dose of less than 50 CGE. In the light of this low complication rate, it might be possible to increase our dose limits. In the tumor dose escalation protocols conducted in the MGH trials, the limit doses to critical structures were also increased Citation[40]. For the same tumor volume, the percentage of tumor volume encompassed by the 95% isodose can decrease from 99% to 86% depending on whether the tumor is situated away from or in contact with the chiasm. Dose escalation with protons is therefore only possible when the tumor is situated away from critical structures or when the dose to these structures is also increased. However, treatment must be optimized by close collaboration between surgeons and radiation oncologists Citation[41]. Interposition of inert tissue (e.g. fat tissue) can also help to exclude critical structures from the high-dose region, for example to separate the tumor from the brainstem. However, it has also been suggested that fat graft could be a cause of local failure due to tumor seeding at the site of fat harvesting Citation[37].
Relatively few complications were reported with the exclusive proton or combined photon-proton irradiation. Santoni et al. studied temporal lobe complications in 96 patients irradiated for chordoma or chondrosarcoma. The 2- and 5-year complication rates were 8% and 13%, respectively. Male gender was an unfavorable prognostic factor, although the mechanism has not been elucidated Citation[42]. Glosser et al. studied neuropsychologic function in 17 patients who received irradiation up to a dose of 66 CGE for tumors of the base of the skull. They did not observe any early or late cognitive side effects. However, some patients developed transitory disorders such as depression or anxiety Citation[43]. Debus et al. studied brainstem side effects in a series of 367 patients irradiated for chordoma or chondrosarcoma. They reported 17 cases of toxicity attributable to irradiation. The 10-year complication rate was 12% and the mean time to onset was 10 months. Ninety percent of disorders occurred during the first 3 years after treatment. The prognostic factors for this complication were the number of surgical operations before irradiation, an irradiation dose greater than 60 CGE to more than 0.9 mL of the brainstem and diabetes Citation[44]. Wenkel et al. reported a case of brainstem necrosis in a series of 45 patients irradiated with protons for meningioma. However, the dose received by the brainstem was higher than that recommended by the treatment protocol Citation[45]. Marucci et al. observed 13 grade 1 – 2 and 4 grade 3 spinal cord complications. The dose constraints to the front and middle of the spine were 55 to 58 CGE and 67 to 70 CGE, respectively. In this series, only the number of surgical procedures before irradiation was a prognostic factor for complications Citation[40].
Munzenrider and Liebsch reported 40% of pituitary complications after base of the skull irradiation with protons Citation[46]. Slater et al. showed that these complications occurred between 14 and 45 months after irradiation and that for 50% of patients who developed these complications, the dose delivered to the pituitary gland was greater than 67.6 CGE Citation[47]. In our series, 16 patients presented a pituitary gland insufficiency which required hormone replacement therapy; nine of these patients presented complete pituitary insufficiency. In our series, the complication rate was correlated with the dose up to 60 CGE, as the risk appeared to decrease beyond this dose. Pai et al. reported 5 year and 10-year rates of 72% and 84% for hyperprolactinemia, 30% and 63% for hypothyroidism, 29% and 36% for hypogonadism, and 19% and 28% for hypoadrenalism, respectively, for patients treated by proton therapy for tumors of the base of skull. Doses higher than 50 CGE to the pituitary axis and 70 CGE to the hypophyseal gland appeared to be prognostic factors for complications Citation[48].
The optic pathways are also radiosensitive organs and are often situated adjacent to the tumor. Habrand et al. showed that the visual complication rate was about 10% for a dose of 55 CGE and more than 20% for doses higher than 60 CGE Citation[49]. In a review of optic pathway complications, Kim et al. showed that the “patch” technique was a high-risk factor when contact between the two beams occurred at the optic nerves or adjacent to the chiasm Citation[50]. Wenkel et al. reported 4 ocular complications of the optic pathways, secondary to a high delivered dose Citation[45]. In our series, one patient developed chiasm necrosis although the delivered dose alone cannot account for this complication (48 CGE) and seven patients presented decreased visual acuity.
Irradiation-related hearing loss is a frequently described complication. However, the criteria for evaluation of hearing loss are not well defined Citation[46], Citation[51–53]. In our study, we preferred a more pessimistic evaluation by including both the patients’ complaints with or without audiogram changes, although these two parameters are not perfectly correlated. Shoenthaler et al. showed that, in 2/3 of patients who developed hearing loss, the inner ear had received an irradiation dose greater than 62.7 CGE Citation[52]. In our series, only one patient presented hearing loss requiring a hearing aid. For the other cranial nerves, Munzenrider and Liebsh estimated that the probability of cranial nerve palsies increased from 1% at a dose of 62 CGE to 5% at a dose of 72 CGE Citation[46]. Urie et al., who used a 3D planning system, studied the nervous complications in 27 patients and 594 delineated nervous structures. They found only 17 nerve lesions in 5 patients Citation[53].
Conclusion
Proton beam irradiation delivers high doses to chordomas of the base of the skull and upper cervical spine. Local control is correlated with the homogeneity of the dose delivered to the tumor volume. This homogeneity is particularly related to the intimate contact of the tumor with critical structures and seems to be independent of tumor volume. Consequently, the indication for repeat surgery must be systematically discussed at a multidisciplinary meeting if the tumor is in contact with critical structures in order to obtain an open space between these structures and the tumor to minimize the risk of underdosage of the tumor close to these critical structures. Considering the high doses delivered, this irradiation appears to be well tolerated and dose escalation to critical structures may be indicated to further increase the dose to the tumor. However, independent prognostic factors of local control and survival described in this study are “retrospective” and “non-predictive” factors and might be difficult to be used at the stage of dosimetry.
References
- Dahlin DC, MacCarthy CS. Chordoma: a study of fifty-nine cases. Cancer 1952; 5: 1170–8
- Higinbotham NL, Phillips RF, Farr HW, Hustu HO. Chordoma. Thirty-five-year study at Memorial Hospital. Cancer 1967; 20: 1841–50
- McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control 2001; 12: 1–11
- Hug EB, Loredo LN, Slater JD, DeVries A, Grove RI, Schaefer RA, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg 1999; 91: 432–9
- Noël G, Habrand JL, Mammar H, Pontvert D, Haie-Meder C, Hasboun D, et al. Combination of photon and proton radiation therapy for chordomas and chondrosarcomas of the skull base: the Centre de Protontherapie D'Orsay experience. Int J Radiat Oncol Biol Phys 2001; 51: 392–8
- Noël G, Habrand IL, Mammar H, Haie-Meder C, Pontvert D, Dederke S, et al. Highly conformal therapy using proton component in the management of meningiomas: preliminary experience of the centre de protonthérapie d'Orsay. Strahlenther Onkol 2002; 178: 480–5
- Noël G, Habrand IL, Jauffret, de Crevoisier. Radiation therapy for chordoma and chondrosarcoma of the base of the skull and the cervical spine: prognostic factors and patterns of failure. Strahlenther Onkol 2003; 179: 241–8
- Feuvret, L, Noël, G, Calugaru, V, Terrier, P, Habrand, JL. Chondromyxoid fibroma of the skull base: differential diagnosis and radiotherapy-two case reports and a review of literature. Acta Oncol 2005; in press.
- Noel G, Feuvret L, Calugaru V, Hadadi K, Baillet F, Mazeron JJ, et al. Chondrosarcomas of the base of the skull in Ollier's disease or Maffucci's syndrome--three case reports and review of the literature. Acta Oncol 2004; 43: 705–10
- Urano M, Verhey LJ, Goitein M, Tepper JE, Suit HD, Mendiondo O, et al. Relative biological effectiveness of modulated proton beams in various murine tissues. Int J Radiat Oncol Biol Phys 1984; 10: 509–14
- Rosenberg AE, Nielsen GP, Keel SB, Renard LG, Fitzek MM, Munzenrider JE, et al. Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol 1999; 23: 1370–8
- Rosenberg AE, Brown GA, Bhan AK, Lee JM. Chondroid chordoma--a variant of chordoma. A morphologic and immunohistochemical study. Am J Clin Pathol 1994; 101: 36–41
- Forsyth PA, Cascino TL, Shaw EG, Scheithauer BW, O'Fallon JR, Dozier JC, et al. Intracranial chordomas: a clinicopathological and prognostic study of 51 cases. J Neurosurg 1993; 78: 741–7
- Gay E, Sekhar LN, Rubinstein E, Wright DC, Sen C, Janecka IP, et al. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery 1995; 36: 887–96
- Menezes AH, Gantz BJ, Traynelis VC, McCulloch TM. Cranial base chordomas. Clin Neurosurg 1997; 44: 491–509
- Watkins L, Khudados ES, Kaleoglu M, Revesz T, Sacares P, Crockard HA. Skull base chordomas: a review of 38 patients, 1958–88. Br J Neurosurg 1993; 7: 241–8
- Al Mefty O, Borba LA. Skull base chordomas: a management challenge. J Neurosurg 1997; 86: 182–9
- Sekhar LN, Schramm VL, Jr, Jones NF. Subtemporal-preauricular infratemporal fossa approach to large lateral and posterior cranial base neoplasms. J Neurosurg 1987; 67: 488–99
- Sen CN, Sekhar LN, Schramm VL, Janecka IP. Chordoma and chondrosarcoma of the cranial base: an 8-year experience. Neurosurgery 1989; 25: 931–40
- Amendola BE, Amendola MA, Oliver E, McClatchey KD. Chordoma: role of radiation therapy. Radiology 1986; 158: 839–43
- Austin-Seymour M, Munzenrider J, Goitein M, Verhey L, Urie M, Gentry R, et al. Fractionated proton radiation therapy of chordoma and low-grade chondrosarcoma of the base of the skull. J Neurosurg 1989; 70: 13–7
- Austin-Seymour M, Munzenrider J, Linggood R, Goitein M, Verhey L, Urie M, et al. Fractionated proton radiation therapy of cranial and intracranial tumors. Am J Clin Oncol 1990; 13: 327–30
- Austin JP, Urie MM, Cardenosa G, Munzenrider JE. Probable causes of recurrence in patients with chordoma and chondrosarcoma of the base of skull and cervical spine. Int J Radiat Oncol Biol Phys 1993; 25: 439–44
- Benk V, Liebsch NJ, Munzenrider JE, Efird J, McManus P, Suit H. Base of skull and cervical spine chordomas in children treated by high-dose irradiation. Int J Radiat Oncol Biol Phys 1995; 31: 577–81
- Berson AM, Castro JR, Petti P, Phillips TL, Gauger GE, Gutin P, et al. Charged particle irradiation of chordoma and chondrosarcoma of the base of skull and cervical spine: the Lawrence Berkeley Laboratory experience. Int J Radiat Oncol Biol Phys 1988; 15: 559–65
- Castro JR, Linstadt DE, Bahary JP, Petti PL, Daftari I, Collier JM, et al. Experience in charged particle irradiation of tumors of the skull base: 1977–1992. Int J Radiat Oncol Biol Phys 1994; 29: 647–55
- Fagundes MA, Hug EB, Liebsch NJ, Daly W, Efird J, Munzenrider JE. Radiation therapy for chordomas of the base of skull and cervical spine: patterns of failure and outcome after relapse. Int J Radiat Oncol Biol Phys 1995; 33: 579–84
- Debus J, Schulz-Ertner D, Schad L, Essig M, Rhein B, Thillmann CO, et al. Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int J Radiat Oncol Biol Phys 2000; 47: 591–6
- Schulz-Ertner D, Haberer T, Scholz M, Thilmann C, Wenz F, Jakel O, et al. Acute radiation-induced toxicity of heavy ion radiotherapy delivered with intensity modulated pencil beam scanning in patients with base of skull tumors. Radiother Oncol 2002; 64: 189
- Schulz-Ertner D, Nikoghosyan A, Thilmann C, Haberer T, Jakel O, Karger C, et al. Results of carbon ion radiotherapy in 152 patients. Int J Radiat Oncol Biol Phys 2004; 58: 631–40
- Borba LA, Al Mefty O, Mrak RE, Suen J. Cranial chordomas in children and adolescents. J Neurosurg 1996; 84: 584–91
- Coffin CM, Swanson PE, Wick MR, Dehner LP. Chordoma in childhood and adolescence. A clinicopathologic analysis of 12 cases. Arch Pathol Lab Med 1993; 117: 927–33
- Suit HD, Goitein M, Munzenrider J, Verhey L, Davis KR, Koehler A, et al. Definitive radiation therapy for chordoma and chondrosarcoma of base of skull and cervical spine. J Neurosurg 1982; 56: 377–85
- Terahara A, Niemierko A, Goitein M, Finkelstein D, Hug E, Liebsch N, et al. Analysis of the relationship between tumor dose inhomogeneity and local control in patients with skull base chordoma. Int J Radiat Oncol Biol Phys 1999; 45: 351–8
- Halperin EC. Why is female sex an independent predictor of shortened overall survival after proton/photon radiation therapy for skull base chordomas?. Int J Radiat Oncol Biol Phys 1997; 38: 225–30
- O'Connell JX, Renard LG, Liebsch NJ, Efird JT, Munzenrider JE, Rosenberg AE. Base of skull chordoma. A correlative study of histologic and clinical features of 62 cases. Cancer 1994; 74: 2261–7
- Arnautovic KI, Al Mefty O. Surgical seeding of chordomas. J Neurosurg 2001; 95: 798–803
- Fischbein NJ, Kaplan MJ, Holliday RA, Dillon WP. Recurrence of clival chordoma along the surgical pathway. AJNR Am J Neuroradiol 2000; 21: 578–83
- Asano S, Kawahara N, Kirino T. Intradural spinal seeding of a clival chordoma. Acta Neurochir (Wien) 2003; 145: 599–603
- Marucci L, Niemierko A, Liebsch NJ, Aboubaker F, Liu MC, Munzenrider JE. Spinal cord tolerance to high-dose fractionated 3D conformal proton-photon irradiation as evaluated by equivalent uniform dose and dose volume histogram analysis. Int J Radiat Oncol Biol Phys 2004; 59: 551–5
- Carpentier A, Polivka M, Blanquet A, Lot G, George B. Suboccipital and cervical chordomas: the value of aggressive treatment at first presentation of the disease. J Neurosurg 2002; 97: 1070–7
- Santoni R, Liebsch N, Finkelstein DM, Hug E, Hanssens P, Goitein M, et al. Temporal lobe (TL) damage following surgery and high-dose photon and proton irradiation in 96 patients affected by chordomas and chondrosarcomas of the base of the skull. Int J Radiat Oncol Biol Phys 1998; 41: 59–68
- Glosser G, McManus P, Munzenrider J, Austin-Seymour M, Fullerton B, Adams J, et al. Neuropsychological function in adults after high dose fractionated radiation therapy of skull base tumors. Int J Radiat Oncol Biol Phys 1997; 38: 231–9
- Debus J, Hug EB, Liebsch NJ, O'Farrel D, Finkelstein D, Efird J, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys 1997; 39: 967–75
- Wenkel E, Thornton AF, Finkelstein D, Adams J, Lyons S, De La MS, et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys 2000; 48: 1363–70
- Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol 1999; 175(Suppl 2)57–63
- Slater JD, Austin-Seymour M, Munzenrider J, Birnbaum S, Carroll R, Klibanski A, et al. Endocrine function following high dose proton therapy for tumors of the upper clivus. Int J Radiat Oncol Biol Phys 1988; 15: 607–11
- Pai HH, Thornton A, Katznelson L, Finkelstein DM, Adams JA, Fullerton BC, et al. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys 2001; 49: 1079–92
- Habrand JL, Austin-Seymour M, Birnbaum S, Wray S, Carroll R, Munzenrider J, et al. Neurovisual outcome following proton radiation therapy. Int J Radiat Oncol Biol Phys 1989; 16: 1601–6
- Kim, J, Munzenride, JE, Maas, A, Finkelstein, D, Liebsch, N, Hug, E, et al., Optic neuropathy following combined proton and photon radiotherapy for base of skull tumors. Int J Radiat Oncol Biol Phys 1997;39:272, (abs. 2064).
- Hug EB, DeVries A, Thornton AF, Munzenride JE, Pardo FS, Hedley-Whyte ET, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol 2000; 48: 151–60
- Schoenthaler, R, Fullerton, B, Maas, A, Collier, JM, Liebsch, N, Hug, E, et al., Relationship between dose to auditory pathways and audiological outcomes in skull-base tumor patients receiving high-dose proton-photon radiotherapy. Int J Radiat Oncol Biol Phys 1996;36:291, (abs. 2026).
- Urie MM, Fullerton B, Tatsuzaki H, Birnbaum S, Suit HD, Convery K, et al. A dose response analysis of injury to cranial nerves and/or nuclei following proton beam radiation therapy. Int J Radiat Oncol Biol Phys 1992; 23: 27–39
- Igaki H, Tokuuye K, Okumura T, Sugahara S, Kagei K, Hata M, et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys 2004; 60: 1120–6