Abstract
Tumors are angiogenesis dependent. Preclinical studies have shown that well-tolerated continuous low dose, i.e. metronomic, chemotherapy can exert significant antiangiogenic effects pe rse and thereby a greater antitumor influence than conventional chemotherapy with high, spaced-out bolus doses. There are however, no means of quantitatively assessing the antiangiogenic effect of chemotherapy in tumors. We therefore used a surrogate tumor-free, non-surgical rat mesentery model and quantitatively studied the dose effect of metronomic treatment with cisplatin, cyclophosphamide, doxorubicin, fluorouracil and paclitaxel on VEGF-A-mediated angiogenesis, a characteristic of tumors. Cyclophosphamide and paclitaxel treatment exerted significant dose-dependent antiangiogenic effects, whereas doxorubicin treatment produced insignificant effects. By contrast, metronomic cisplatin and fluorouracil treatment occasionally significantly stimulated angiogenesis in a dose-dependent, non-linear manner. To our knowledge, this is the first report of metronomic chemotherapy stimulating angiogenesis in vivo. The data suggest that the angiogenic response to cisplatin, cyclophosphamide, fluorouracil and paclitaxel was significantly influenced by the presence of antioxidants in the vehicles or when co-treated with N-acetylcystein, a widely used free-radical scavenger. The data relating to the metronomic scheduling were compared with bolus treatment data for the identical agent formulations in the same experimental model. Cisplatin, cyclophosphamide and paclitaxel caused approximately the same overall, agent-specific angiogenesis-modulating effects following metronomic and bolus treatments. Moreover, apparently secondary delayed effects of chemotherapy affected capillary sprouting.
The progress of conventional chemotherapy has been modest in terms of curing or significantly prolonging the lives of patients with cancer, particularly those with advanced-stage or metastatic disease Citation[1], Citation[2]. Tumor growth depends on angiogenesis Citation[3], Citation[4]. As has recently been reported in preclinical studies, the frequent or continuous so-called antiangiogenic Citation[5] or metronomic Citation[6] scheduling of low, well-tolerated doses of certain chemotherapeutic agents exerts a marked antiangiogenic effect. The metronomic chemotherapy in fact yields a superior antitumor effect compared with conventional chemotherapy that typically employs the highest possible dose without causing life-threatening levels of toxicity at intervals of two to four weeks Citation[5], Citation[7], Citation[8].
While the primary target of metronomic treatment is the angiogenically activated normal genomically stable vascular endothelial cell in tumor blood vessels, the primary target of conventional chemotherapy is the rapidly dividing genomically unstable mutagenic tumor cell. Because of tumor cell muations, development of drug-resistance is a major threat in conventional chemotherapy. Metronomic chemotherapy has the additional advantage of being less acutely toxic than conventional scheduling, yielding a lower accumulated dose, therefore making more prolonged treatment possible. Support for metronomic therapy also comes from mathematical modeling studies Citation[9]. Following metronomic chemotherapy, the apoptosis of vascular endothelial cells precedes the apoptosis and necrosis of tumor cells, even when the tumor has been made drug resistant Citation[10]. The prolonged interval between conventional chemotherapy courses provides an opportunity for new endothelial cells to be recruited, either from existing vasculature or from circulating progenitor endothelial cells, allowing tumor angiogenesis to proceed. There is, however, no reliable assay to monitor and quantify changes in tumor angiogenesis in vivo, which constitutes a serious impediment to assessing the effects of putative antiangiogenic drugs or treatments Citation[11], Citation[12].
Tumor angiogenesis uses the same signaling pathways as non-tumor angiogenesis Citation[13]. We have therefore studied the influence of chemotherapeutics on VEGF-A-mediated angiogenesis per se in tumor-free tissue, using the non-surgical rat mesenteric-window assay Citation[14–16]. Notably, VEGF-A is a pivotal angiogenic factor in most, if not all, tumors Citation[17], Citation[18] and also appears to be a mediator of angiogenic pathways related to other angiogenic factors Citation[19]. The assay that was used here permits the true quantitative assessment of a variety of objective angiogenesis variables and robust statistical analysis Citation[20–22]. It is noteworthy that this model closely mirrors the directly recorded growth-retarding and indirectly assessed antiangiogenic effect of metronomic chemotherapy in a syngeneic rat cancer model Citation[15]. We believe that an approach of this kind, that permits the detailed assessment of the dose-related angiogenesis-modulating effects per se of chemotherapeutic agents, may be a useful and perhaps necessary complement to preclinical studies in tumor models.
Clearly, the metronomic scheduling approach is promising in future clinical oncology, which calls for careful systematic studies of the antiangiogenic effects of chemotherapy in relevant preclinical models. It is noteworthy that certain promising antitumor effects by metronomic-like scheduling have recently been observed in clinical studies Citation[2], Citation[23–26] although dosing is as yet considered poorly defined. We report that low-dose; well-tolerated metronomic chemotherapy drug-specifically affects VEGF-A-mediated angiogenesis in terms of microvessel network growth, pattern formation, as well as the number and length of capillary sprouts.
Material and methods
Animals
Adult male out-bred Sprague-Dawley rats (B & K Universal, Sollentuna, Sweden) were acclimatized to a standardized environment for seven days, fed ad libitum and randomly allocated to weight-matched groups with two animals per cage Citation[14]. At the start of the experiments, the mean body weight varied between 210 and 227 g. Body weight was monitored twice weekly. The controls increased by approximately 55–70 g a week. Because of this relatively rapid growth, chemotherapy-related weight-gain retardation is a sensitive surrogate evaluation of toxicity, which also includes systemic well-being, anorexia and failure to thrive. Gauging body-weight gain is important, as low toxicity, allowing long-term continuous treatment, is inherent to metronomic chemotherapy. The local Animal Ethics Committee approved the study. The ethical guidelines that were followed meet the standards required by the UKCCCR guidelines Citation[27].
Angiogenesis treatment
Recombinant rat VEGF164 (564-RV/CF; R&D Systems Ltd., UK), the predominant isoform of VEGF-A, was diluted to 96 pmol/ml, frozen and thawed and a volume of 5 ml was injected i.p. Citation[28]. VEGF at this dose was given twice daily for 4.5 days, i.e. from Monday morning (Day 0) to Friday morning (Day 4). The treatment causes vigorous angiogenesis in the mesenteric test tissue, peaking around Day 21 Citation[28]. It is within this time frame of microvessel network proliferation that chemotherapy was given (see below).
Like most normal adult tissues, the present test tissue, i.e. the thin membranous mesentery (see below), is natively vascularized and lacks significant physiologic angiogenesis. Surgery that invariably induces wound-healing-related angiogenesis was not used. As a result, angiogenesis that resembles that occurring in tissues affected by clinically relevant angiogenesis diseases, including tumors, can be studied using this assay.
Chemotherapy
Initial dose-finding experiments using continuous infusion
We performed a dose-finding experiment with each agent using continuous infusion for seven consecutive days, continuous infusion being the extreme of metronomic scheduling. The doses were based on data in the literature and our own findings in previous experiments Citation[14], Citation[15]. The intention was to find doses that would diminish the weight gain by at most 10–15% at sacrifice, as compared with the rapidly growing vehicle control animals.
Metronomic treatment using continuous infusion
The following commercial cytotoxics and, in parentheses, the respective vehicle control formulation (dilution identical to that of the tested compound) were used for the continuous infusion experiments: cisplatin, Platinol® from Bristol-Myers Squibb (NaCl 9 mg/ml, HCl ad pH 2.5); cyclophosphamide, Orion, (NaCl 9mg/ml) or Sendoxan®, ASTA Medica (mannitol 50mg/ml); doxorubicin, Doxorubicin Nycomed® (NaCl 9 mg/ml, HCl ad pH 3.0); fluorouracil, Fluracedyl®, Nycomed (aqua, NaOH ad pH 8.9); and paclitaxel, Taxol®, Bristol-Myers Squibb (Ethanol:Cremophor® EL, 50:50 vol.). The doses are expressed as mg/kg/week.
On Day 6 after the start of the i.p. angiogenic treatment, Alzet® osmotic minipumps (Models 2001 or 2ML1; Alzet® Osmotic Pumps, Mountain View, CA, USA) were completely filled under sterile conditions with a cytotoxic agent or its vehicle that were released at a constant rate for seven consecutive days. One day later, after being stored in sterile 0.9% NaCl (w/v) saline overnight at 37°C, the pumps were surgically implanted s.c. on the back of rats that had been anesthetized with inhaled isoflurane (Forene®). The skin incision was immediately sutured. The animals were sacrificed on Day 14. The animals did not show any sign of illness and no animal was lost.
Bolus treatment
A single i.v. injection was given on day seven after the start of the angiogenic i.p. treatment with VEGA-A, i.e. seven days before sacrifice, as described in detail elsewhere Citation[14].
N-Acetylcystein (NAC) treatment
NAC, a widely used antioxidant Citation[29], Citation[30], and a treatment of choice in cases of acetaminophen toxicity in man as it acts, in part, by replenishing hepatic stores of glutathione, was continuously infused s.c. at 64 and 192 mg/kg/day on Days 7–14 using an Alzet® pump. NAC was administered alone or in combination with either an infusion of the saline vehicle or a cytotoxic agent, using two pumps, in animals that had received angiogenic i.p. treatment with VEGF-A on Days 0–4.
Angiogenesis quantification
Four membranous (“window”-like) parts of the mesentery from the most distal part of the mesentery, close to the ileocecal valve, were examined after being spread on objective slides Citation[28]. Normally, this tissue measures only 5–10 µm in thickness and forms a uniform, almost translucent membrane. The surrounding fatty tissue distinctly delineates each window. The test tissue is vascularized like most normal adult tissues. The entire vasculature of each intact mesenteric window was visualized immunohistochemically using a primary monoclonal antibody against rat endothelium, i.e. mrc ox-43 (Biosource International, Camarillo, CA, USA) labeling vascular endothelium in all tissues of the rat except that of brain capillaries Citation[31], Citation[32] (). This allows the straightforward identification of even the smallest microvessels in the intact network, in contrast to the immunohistochemical identification of microvessels in microtome sections of solid tissues and tumors in which the extent of epitope expression in capillary endothelial cells for various markers is not possible to control in detail Citation[33]. Moreover, chemotherapy possibly influences the expression of epitopes for immunohistochemical markers on endothelial cells Citation[34].
Figure 1. Immunohistochemical visualization of microvessels in the mesenteric window of a rat receiving a continuous infusion for seven days of paclitaxel at 16 mg/kg/w (a) and cremophore vehicle (b). Arrows indicate an intersection, while arrowheads indicate interconnecting loops (not all intersections and loops are indicated) and asterisks indicate sprouts although the field of view is not from the edge of the microvascular tree. Note the lower density of microvessels in a. The distance between two adjacent lines is 10 µm.
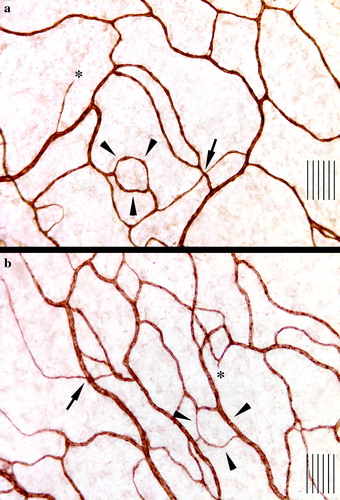
Microscopic morphometry and computerized image analysis were employed in a blind fashion. First, the total area of each mesenteric window was measured. The following variables were then measured objectively in the test tissue as described in detail elsewhere Citation[20], Citation[22], Citation[28], Citation[35]: the percentage of vascularized area (VA), a measurement of the spatial extension of the network; microvascular length (MVL), a composite measurement of microvessel density; the total microvascular length (TMVL = VA X the mean MVL/treatment group); the length of individual microvessel segments (Le. MS, the actual distance between two successive branching points); the number of MS per unit tissue volume (No. MS); the number of microvessel branching points per unit tissue volume (No. BP); the number of capillary sprouts per unit tissue volume (No. SP) and their individual length (Le. SP) at the edge of the expanding network. All Le. MSs and Le. SPs were pooled separately and ranked according to size in each treatment group. For statistical comparisons of the treatment groups, we focused on the shortest and the longest microvessel segments and sprouts Citation[22], Citation[35]. The fact that only sprouts at the periphery of the expanding network were recorded suggests that the shortest sprouts primarily represent the youngest sprouts and the longest primarily the oldest sprouts.
To our knowledge, the techniques used here objectively and quantitatively to assess microvessel variables, such as spatial extension, density, various aspects of pattern formation and the number and length of individual capillary sprouts in situ in a vascularized tissue in vivo, are unrivalled.
Statistics
The non-parametric Mann-Whitney U-test for unpaired (two-tailed) observations was used. A mean of four windows per animal was used as independent data for each variable in the mesenteric window. The criterion for statistical significance was p ≤ 0.05.
Results
Effect of metronomic chemotherapy on physiologic body-weight gain and size of the mesenteric test tissue
In the initial dose-finding experiments, the following doses were continuously infused for seven consecutive days and the percentage body weight at sacrifice compared with vehicle controls is given in parentheses: cisplatin 0.4 mg/kg/w (99%) and 2.0 mg/kg/w (94%); cyclophosphamide 40 mg/kg/w (98%) and 200 mg/kg/w (87%); doxorubicin 2 mg/kg/w (97%) and 10 mg/kg/w (87%); fluorouracil 24 mg/kg/w (97%) and 120 mg/kg/w (92%); paclitaxel 2.8 mg/kg/w (97%) and 4.4 mg/kg/w (93%).
In the subsequent angiogenesis experiments, the chemotherapy reduced the rate of body-weight gain dose dependently in an approximately linear fashion, at most up to 15% (data not shown). In no case did the treatment reduce the body weight in absolute terms. All the animals appeared to be normal and behaved normally. No chemotherapy affected the area of the individual mesenteric windows analyzed (data not shown), allowing a direct comparison between control and test groups in terms of the variables measured.
Dose-related angiogenesis-modulating effects of metronomic chemotherapy
The effects of cisplatin, cyclophosphamide, doxorubicin, fluorouracil and paclitaxel were assessed in terms of VA, MVL and TMVL and graphically presented in terms of TMVL for all in . Cyclophosphamide and paclitaxel dissolved and diluted in antioxidant-containing vehicles (mannitol and cremophor respectively) significantly inhibited angiogenesis in a roughly dose-dependent, linear fashion within the tested range of 50–200 mg/kg/w and 2.5–22 mg/kg/w respectively; however, no significant angiogenesis-modulating effect occurred when saline was used as the vehicle (). Continuous monotherapy for 7 days with cremophor did not significantly affect angiogenesis compared with continuous monotherapy with saline for the same period: mean±SEM of TMVL for cremophore was 12.81±1.90 and 11.34±1.35 for saline (p = 0.56).
Figure 2. Effect on VEGF-A-mediated angiogenesis following metronomic chemotherapy with cyclophosphamide (two experiments), doxorubicin (four experiments) and paclitaxel (three experiments). The agents were infused continuously as monotherapy or in the case of doxorubicin in combination with continuously infused N-acetylcystein (NAC) at 192 mg/kg/day or 64 mg/kg/day for seven consecutive days before sacrifice. Mean ± SEM data for total microvascular length (TMVL) that is a composite measurement of vascularized area (a measurement of microvessel spatial extension) times the mean of microvascular length (a measurement of microvessel density). Each treatment group comprised 10–14 animals and was compared to vehicle control. Data expressed as percentage of control. P-values of statistically significant effects compared with controls are indicated. Significant dose-dependent differences in individual experiments are given as asterisks: * in the case of cyclophosphamide and ** in the case of paclitaxel.Note that the significant antiangiogenic effect of cyclophosphamide and paclitaxel was observed only when the commercially available antioxidants mannitol and cremophor EL were used as vehicle and not when saline was used as control vehicle. Doxorubicin, on the other hand, did not in any of the four experiments demonstrate a significant angiogenesis-modulating influence, not even when co-treated with NAC, a potent antioxidant, at two doses. For the effect on sprouting, see . For comparison ith bolus treatment, see Fig. 4.
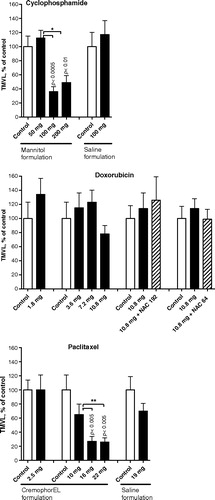
Figure 3. Proangiogenic effect on VEGF-A-mediated angiogenesis by cisplatin (four experiments) and fluorouracil (five experiments) when infused continuously as monotherapy and when co-treated with the free-radical scavenger N-acetylcystein (NAC) at 192 mg/kg/day for seven consecutive days before sacrifice. Mean ± SEM data for total microvascular length, TMVL (vascularized area times the mean of microvascular length) is shown as percentage of saline vehicle control. Each treatment group comprised 10–14 animals. Figures indicate statistically significant effects compared with controls. P-values for differences between different doses of either cytoxic or due to NAC co-treatment are also indicated.No significant antiangiogenic effect was observed following metronomic treatment with cisplatin and fluorouracil. Instead, a significant proangiogenic influence was induced by cisplatin in three of four experiments and with fluorouracil in one of five experiments. It is noteworthy that the co-treatment with NAC significantly reduced the angiogenic response of fluorouracil, while it significantly and fully normalized the proangiogenic effect of cisplatin. For the effect on capillary sprouting, see . For effect on microvessel and sprout growth and pattern formation, see .
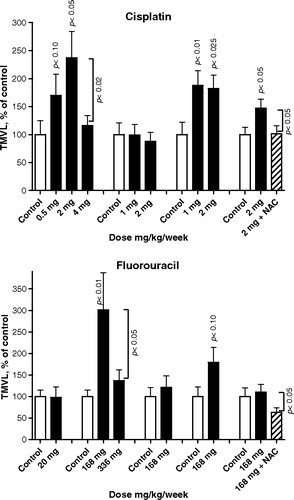
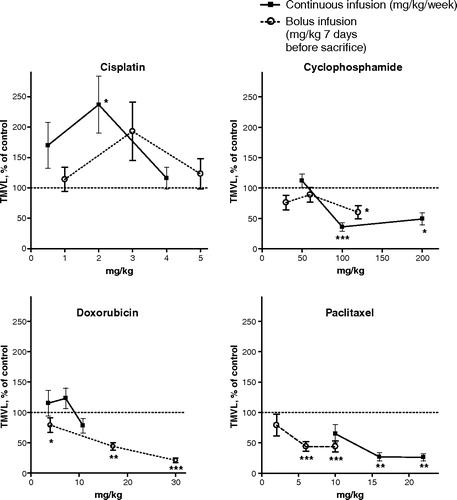
Figure 4. Data showing total microvascular length, TMVL, mean ± SEM. The chemotherapy was given either metronomically as a continuous infusion (filled squares) on Days 7 to 14 after the start of the angiogenic VEGF-A-treatment (lasting Days 0–4) or as a single bolus injection (open circles) on Day 7, i.e. 7 days before sacrifice. Each graph compares two separate dose-response experiments. On the abscissa the dose is expressed as mg/kg/week for continuous infusion or as mg/kg×1 seven days before sacrifice for i.v. bolus treatment. Detailed bolus data are published in part in (14). On the ordinate the data are expressed as percentage of the corresponding vehicle control. The vehicles were the same in the bolus and the metronomic scheduling except for the highest bolus doxorubicin dose for which 0.75 mg/ml para-hydroxibenzoate, 37.5 mg/ml lactos was used as vehicle. Each treatment group comprised 10–14 animals, apart from the doxorubicin control group which comprised 8 animals.Data points labelled with asterisks denote statistically significant differences compared to the respective control (* p < 0.05, ** p < 0.01 and *** p < 0.005). In terms of TMVL, cisplatin, cyclophosphamide and paclitaxel exerted significant or almost significant changes that were similar with either type of administration schedule. By contrast, while doxorubicin bolus treatment significantly suppressed angiogenesis no such effect was observed following the metronomic treatment. Note also the general trend to a proangiogenic effects, i.e. values above respective control experiment (100% dotted line), for cisplatin and the antiangiogenic effects for cyclophosphamide and paclitaxel (and for doxorubicin bolus treatment), i.e. values below the 100% dotted line.
In contrast, doxorubicin did not significantly affect angiogenesis in the tested dose range of 1.8–10.8 mg/kg/w, even when co-treated with the antioxidant NAC at two doses ().
Notably, cisplatin and fluorouracil occasionally exerted a significant, clearly non-linear, dose-dependent proangiogenic effect (). Co-treatment with NAC significantly and fully normalized the proangiogenic effect of cisplatin, while NAC significantly suppressed the angiogenic response when co-treated with fluorouracil ().
When fluorouracil was co-treated with NAC, the microvascular pattern and capillary sprouts were altered in numerous ways compared with the fluorouracil + saline control. In fact, in the presence of NAC, No. MS, No. BP and No. SP were significantly reduced, while the longest Le. MS, those of the 90–100 percentile, lengthened significantly, as did the longest sprouts, i.e. those of the 90–100 percentile ().
Table I. The effect of on microvessel growth and pattern formation and on capillary sprouting in VEGF-A-mediated angiogenesis following metronomic treatment with fluorouracil (168 mg/kg/w) as mono-therapy or when co-treated with N-acetylcystein, NAC (192 mg/kg/day).Number of microvessel segments per tissue unit volume, No. MS; number of microvessel branching points per tissue volume unit, No. BP; length of individual microvessel segments (i.e. the actual distance between two successive branching points), Le. MS; number of capillary sprouts per unit tissue volume, No. SP; and length of individual sprouts, Le. SP. Figures in bold and related p-values indicate significant difference compared with fluorouracil + saline vehicle. There was no significant difference in statistical terms between the fluorouracil + saline group and the saline + saline group. Each treatment group comprised 12–14 animals. Not done, N.D. Lengthening of Le. MS or Le. SP, [+]. Mean±SEM. When NAC was combined with fluorouracil, the microvessel network and capillary sprouts were altered in many ways: No. MS, No. BP and No. SP were significantly reduced, while the longest Le. MS, those of the 90–100 percentile, lengthened significantly, as did the longest Le. SP, those of the 90–100 percentile.
At angiosuppressive doses, cyclophosphamide and paclitaxel significantly reduced No. SP (), in agreement with the effect in terms of VA (data not shown) and TMVL (). At a proangiogenic dose, cisplatin increased No. SP significantly, while fluorouracil increased No. SP insignificantly (), in line with the effect in terms of VA (data not shown) and TMVL (). Moreover, cisplatin, fluorouracil and paclitaxel significantly shortened the shortest Le. SP, those of the 0–10 percentile, and insignificantly reduced the median Le. SP (). In contrast, cyclophosphamide insignificantly lengthened the shortest Le. SP and significantly lengthened the median Le. SP. The effects on sprouting were thus agent specific.
Table II. Effect on capillary sprouting in VEGF-A-mediated angiogenesis following metronomic treatment with the antiangiogenic cytotoxics cyclophosphamide and paclitaxel and the proangiogenic cytotoxics cisplatin and fluorouracil for seven consecutive days before sacrifice. Number of capillary sprouts per unit tissue volume, No. SP; and length of the individual capillary sprouts, Le. SP.All Le. SPs were pooled and ranked according to size in each treatment group. For statistical comparisons of the treatment groups, we focused on the shortest and the longest sprouts, i.e. those of the 0–10 and 90–100 percentiles. Lengthening of Le. SP, [+]. Shortening of Le. SP, [−]. P-values are for comparison with vehicle controls. Each treatment group comprised 10–12 animals. The number of sprouts (No. SP) varied between 768 and 1,369 per treatment group. Mean±SEM. Cyclophosphamide and paclitaxel significantly reduced the No. SP. Cisplatin, on the other hand, increased the No. SP significantly, while fluorouracil insignificantly increased the No. SP. Moreover, cisplatin, fluorouracil and paclitaxel significantly shortened the shortest Le. SP, those of the 0–10 percentile, and insignificantly reduced the median Le. SP. By contrast, cyclophosphamide insignificantly lengthened the shortest Le. SP and significantly lengthened the median Le. SP. Clearly, sprouting was affected agent-specifically.
Effect of NAC infusion on angiogenesis
Continuous NAC (192 mg/kg/day) monotherapy for seven days insignificantly reduced body weight by 3% compared with the rapidly growing saline vehicle controls, insignificantly suppressed angiogenesis in terms of VA (by 4%), MVL (by 8%) and TMVL (by 14%, p = 0.48). NAC reduced the number of microvessel segments per unit tissue volume (No. MS; mean±SEM 191.5±24.1; p ≤ 0.025), reduced the number of branching points per unit tissue volume (No. BP; mean±SEM 170.2±19.5; p ≤ 0.03) compared with the No. MS of 274.0±30.7 and the No. BP of 235.0±24.1 in the vehicle control group, however. Moreover, the shortest Le. MS, those of the 0–10 percentile, were significantly (p ≤ 0.0001) shortened following the NAC monotherapy as compared with the saline controls (data not shown).
Overall modulation of angiogenesis following metronomic and bolus chemotherapy
Irrespective of whether they were administered metronomically or as a bolus dose, cisplatin, cyclophosphamide and paclitaxel exerted parallel and approximately similar effects on overall angiogenesis in terms of TMVL (). In contrast, metronomic treatment with doxorubicin at two of the three tested doses increased TMVL insignificantly, while the bolus treatment at all three tested doses significantly reduced TMVL in statistical terms (). With either treatment schedule, paclitaxel generally exerted the most prominent antiangiogenic effect, while cisplatin generally exerted the most prominent proangiogenic effect. Fluorouracil bolus doses between 10 and 50 mg/kg did not significantly affect angiogenesis Citation[16].
Effects on capillary sprouting following metronomic and bolus treatment with Cyclophosphamide and Paclitaxel
Cyclophosphamide
The metronomic treatment significantly reduced the No. SP and lengthened the shortest Le. SP, those of the 0–10 percentile (). The bolus treatment (1) significantly lengthened the shortest Le. SP, those of the 0–10 percentile, and (2) significantly shortened the longest Le. SP, those of the 90–100 percentile ().
Table III. Metronomic and bolus treatment: Effect on capillary sprouting in VEGF-A-mediated angiogenesis by paclitaxel and cyclophosphamide. The number of sprouts per unit tissue volume, No. SP; the length of individual sprouts, Le. SP, in terms of the shortest Le. SP, i.e. the 0–10 percentile, the longest Le. SP, i.e. the 90–100 percentile; and the median Le. SP.The bolus data are from [16]. The vehicles were the same in the bolus and the metronomic scheduling. Paclitaxel was given metronomically at 16 mg/kg/w for seven consecutive days and at 10 mg/kg when given as a single intravenous bolus injection seven days before sacrifice. Cyclophosphamide was given metronomically at 100 mg/kg/w for seven consecutive days and at 60 mg/kg when given as a single intravenous bolus injection seven days before sacrifice. P-values are given for differences with vehicle controls. Statistically insignificant difference compared with vehicle controls, n.s. Lengthening of Le. SP, [+]. Shortening of Le. SP, [−]. Each treatment group comprised 9 or 12 animals. The number of sprouts (No. SP) per treatment group varied between 768 and 1,088. The metronomic and bolus treatments induced approximately similar effects on the No. SP and the Le. SP with the exception of cyclophosphamide. Cyclophosphamide metronomic treatment significantly reduced the No. SP, while the cyclophosphamide bolus treatment insignificantly increased the No. SP.
Paclitaxel
The metronomic scheduling significantly reduced the No. SP and shortened the longest Le. SP, those of the 90–100 percentile (). The bolus administration significantly shortened both the shortest and the longest Le. SP, those of the 0–10 and the 90–100 percentiles (). Notably, the effects of cyclophosphamide and paclitaxel bolus treatment on sprout lengths were highly significant in statistical terms, as recorded seven days after treatment.
Comment
Considering that the median life span of rats that are commonly used in experiments is in the order of 2.5 to 3 years, 1 week corresponds to ∼1.6 to 2 months in humans. Even though this reasoning is simplistic, one week's treatment may not be exceptionally short.
Because experiments with cisplatin and fluorouracil produced a varying overall result, we looked in retrospect for any difference in the rats delivered by the breeder during the extended period during which the experiments were performed, i.e. virtually all year round. We noted that the proangiogenic effect of cisplatin occurred in all the experiments (body weight increase 60.2–67.2 g), apart from the one in which the controls displayed the smallest increase in physiologic body weight (53.6 g) during the period of chemotherapy. The proangiogenic effect of fluorouracil was observed in the one experiment of the five experiments performed in which the vehicle controls displayed the greatest increase in body weight (70.1 g) during the seven-day chemotherapy; the body weight increased between 53.6 and 60.2 in the other experiments. A rapid body-weight gain therefore correlated somehow with a proangiogenic effect of the metronomic treatment with cisplatin and fluorouracil. The difference in the body-weight gain rate of the controls between experiments may suggest that, despite being healthy and normal in all conventional respects and being fed identical food, the animals were not, after all, in an identical state in all the experiments. The body-weight gain during the chemotherapy treatment was uncorrelated to the age or body weight of the animals at the start of the experiments.
Discussion
From a clinical point of view, metronomic therapy carries a far greater anticancer potential than conventional chemotherapy, as discussed in the Introduction. In terms of a number of the variables measured within the expanding microvessel network in the VEGF-A-mediated angiogenic response, all the agents except doxorubicin displayed a distinctly dose-dependent, drug-specific action following metronomic chemotherapy. Distinctly agent-specific and dose-dependent effects have previously been reported following bolus chemotherapy in the model used here Citation[14], Citation[16]. The data are compatible with the notion that most individual cytotoxic agents, regardless of scheduling, chiefly target a specific as yet unidentified intrinsic component of the complex VEGF-A-mediated angiogenic cascade, as the effect of metronomic and bolus treatment on overall angiogenesis ran in parallel for most agents (). The lack of angiogenesis-modulating effect by metronomic doxorubicin treatment (), in contrast to the antiangiogenic influence of doxorubicin bolus treatment, may perhaps indicate that the metronomic treatment duration was too short. Prolonged metronomic treatment is required to clarify this question.
Tumors are angiogenesis dependent Citation[3], Citation[4] with only minor exceptions Citation[36]. The main target of metronomic treatment is the angiogenically activated endothelial cell within the tumor vasculature. One major impediment to the study of the antiangiogenic effects of chemotherapeutics and treatments is the fact that there is no reliable assay to quantify the antiangiogenic effect in tumors Citation[11], Citation[12]. Indirect estimation of the antiangiogenic effect per se of chemotherapy in tumors can, however, be made using various assays including the dorsal skin fold and cranial window assays allowing intravital microscopy, as well as other microscopic models Citation[15], Citation[37]. In an effort to circumvent this impediment, we used the tumor-free, normally vascularized rat mesentery as a surrogate tissue for the true quantitative assessment of the angiogenesis-modulating influences of chemotherapy per se. Like all models, the angiogenesis assay used here has certain limitations when it comes to the study of the angiogenesis-modulating effect of chemotherapy in tumors, as discussed elsewhere Citation[14].
Despite the fact that angiogenesis in tumors often leads to a highly disorganized vasculature Citation[38], endothelial cells in tumor blood vessels use the same signaling pathways as endothelial cells in tumor-free tissue, although there may be some distinctions between tumor and non-tumor endothelium at the mRNA level Citation[13], Citation[39]. It is obvious that the present model of visceral angiogenesis in tumor-free tissue might display many differences compared with tumor angiogenesis that is subjected not only to the local imbalance between pro- and antiangiogenic endogenous factors but also to hypoxia and substrate deprivation. The present assay has, however, been shown closely to reflect the actual growth-retarding and indirectly assessed antiangiogenic effects of metronomic chemotherapy in a syngeneic rat prostate cancer model Citation[15], which is noteworthy. To imitate the clinical situation, outbred animals were used instead of inbred or genetically manipulated animals. This fact may partly explain the often greater intra- and inter-experimental variations observed, as genetic loci appear to control individual angiogenic potential, including VEGF-A-induced angiogenesis Citation[40], Citation[41]. In previous series of experiments not involving cytotoxics, we have not observed such a marked degree of variation in the outcome between successive experiments as that following cisplatin and fluorouracil treatment in the present study.
The data presented here appear to constitute the first demonstration of low-dose metronomic or continuous chemotherapy (with cisplatin and fluorouracil) being able to stimulate angiogenesis in vivo. It is important to emphasize that these data do not refute the existence of endothelial-cell proapoptotic, cytotoxic and antiangiogenic effects of continuous low-dose therapy with a number of cytotoxics in mice Citation[5], Citation[7], Citation[8], Citation[10], as was also found in the present study in terms of cyclophosphamide and paclitaxel. In the present study, a proangiogenic effect by low-dose continuous treatment occurred frequently in the case of cisplatin and infrequently in the case of fluorouracil. The proangiogenic effect was dose dependent in a non-linear fashion, suggesting a finely tuned dose-effect situation.
Many chemotherapeutic agents, including cisplatin, are potent pro-oxidative stressors Citation[42], Citation[43]. Notably, the infusion of NAC, a potent antioxidant, which, when administered as monotherapy did not significantly affect overall angiogenesis. When co-treated with cisplatin NAC completely negated the proangiogenic effect of cisplatin and significantly suppressed angiogenesis in animals co-treated with fluorouracil. The data support the possibility that subtle changes in the quantity of reactive oxygen species, ROS, following chemotherapy can stimulate VEGF-A-mediated angiogenesis under certain conditions. Interestingly, NAC acts antiangiogenically in mice in the s.c. Matrigel assay Citation[44] and promotes the production of the endogenous antiangiogenic peptide angiostatin in a human orthotopic xenograph breast cancer model in athymic mice Citation[45]. In the present study, NAC co-administered with fluorouracil significantly affected certain variables related to microvessel pattern formation and capillary sprouting (). Clearly, the role of NAC in chemotherapy-mediated angiogenesis modulation may be complex. Our additional finding that vehicles with an antioxidant character, i.e. mannitol and cremophor, were conductive in the antiangiogenic outcome of the metronomic cyclophosphamide and paclitaxel scheduling () suggests that ROS may play a yet unexplored and important role in low-dose chemotherapy-induced angiogenesis modulation.
Signal studies have identified a cascade comprising Ras–Raf–MEK1–ERK1/2 as the main pathway mediating oxidative stress-stimulated VEGF-A transcription Citation[46]. The autocrine expression of VEGF-A has been detected in endothelial cells under oxidative stress Citation[47]. Moreover, the expression of many additional genes related to angiogenesis, including those of FGF, PDGF, the VEGF-A receptors R1 and R2 and Ang-1 and Ang-2, is likely regulated or influenced by redox signaling Citation[48]. Our finding of occasional proangiogenic effects by metronomic chemotherapy appears to be in line with the findings by Klement et al. Citation[8] in tumor-bearing mice. They state “in some cases the various low-dose metronomic chemotherapy protocols that were studied stimulated tumor growth for reasons that were unclear”. Notably, it was recently reported for the first time that low-dose irradiation can exert ROS-related proangiogenic effects in an experimental tumor Citation[49].
Circulating endothelial progenitors (CEPs) may play important roles in angiogenesis. The CEPs are mobilized in the bone marrow and homes to sites of neovascularization, thereby enhancing angiogenesis Citation[50]. The low-dose metronomic administration of cyclophosphamide is associated with a reduction in CEPs, which, if anything, tends hardly to stimulate angiogenesis, while a single high dose of cyclophosphamide causes the vigorous mobilization of CEPs Citation[51], which tends to promote angiogenesis. It therefore appears less likely that the proangiogenic effect of the metronomically administered cisplatin and fluorouracil, as observed in the present study, is explained by the increased mobilization of CEPs.
It is noteworthy that the effect of paclitaxel and cyclophosphamide bolus treatment on capillary sprouting at the edge of the expanding network was statistically significant, as observed one week post-treatment (). Since the half-life of paclitaxel and cyclophosphamide in rats is reportedly 7.6±3.6 hrs (mean±SD) and 0.5–1 hrs (mean) respectively Citation[52], Citation[53], the data indicate a remarkably delayed effect on capillary sprouting by these agents, in agreement with our previous findings Citation[16]. This may reflect a secondary effect of the cytotoxics possibly due to induction of an endogenous inhibitor of angiogenesis such as thrombospondin Citation[2], Citation[51].
It is clear from the present data that the formulation of the vehicle may strongly influence the antiangiogenetic response by paclitaxel, cyclophosphamide, cisplatin and fluorouracil. Moreover, using clinically relevant concentrations, the vehicles, i.e. polysorbate 80 and Cremophor EL, used for docetaxel and paclitaxel could demonstrate an independent antiangiogenetic effect in vitroCitation[54] as assessed in an endothelial cell proliferation assay and the rat aortic ring assay. Furthermore, these detergents decreased the antiangiogentic response compared to DMSO (a strong antioxidant) when included in the formulation of docetaxel and paclitaxel, resulting in the need for higher concentrations of these cytotoxics in order to obtain a detectable antiangiogentic effect in vitro. This might be somewhat similar to our present finding that paclitaxel and cyclophosphamide displayed significant antiangiogentic effect when diluted in cremophor EL:ethanol and mannitol, respectively, but not when diluted in saline. Notably, in our system, there is no significant difference in the angiogenic response to VEGF-A following one week's s.c. infusion of cremophor EL: ethanol and saline as monotherapy. Therefore, from the present data, an emerging possibility of enhancing the antiangiogenetic response to metronomic antiangiogenetic chemotherapy by adding an oxygen radical scavenging compound has risen.
In the clinical situation, the presently several emerging per oral formulations of cytotoxic drugs probably best mimic our use of continuous infusion. As such, the present data raise a concern for the use of, for instance, per oral 5-fluorouracil prodrugs, although technically a metronomic treatment and possibly antitumoral in effect, it is not necessarily an antiangiogenetic therapy. However, all conversions of preclinical data to the clinical situation are subjected to many pitfalls, and thus need to be tested independently. Our data on doxorubicin, would appear to suggest that the antiangiogenic effects are best achieved when low i.v. doses are given at frequent intervals rather than using a constant low-dose exposure. Also, from , we can note approximately similar dose-related effects for metronomic and bolus scheduling of cyclophospahmide and paclitaxel, indicating that these drugs have antiangiogentic effects by either mode of administration, which could hypothetically be utilized best in the clinically most tolerable fashion. One can, moreover, anticipate that patients receiving low-dose metronomic monochemotherapy with specific agents, such as fluorouracil and cisplatin, might, under certain conditions that need elucidation, occasionally display increased tumor angiogenesis.
Each tumor or tumor model may be unique in its response to any particular type of chemotherapy. This is because of genomic heterogeneity among the tumor cells and the cross-talk that takes place between the tumor cells and the non-tumor immune cells including mast cells, fibroblasts, the endothelial cells, and the extracellular matrix within the tumor tissue Citation[18], Citation[55], Citation[56]. The influence of chemotherapy on tumor angiogenesis may, moreover, be site dependent Citation[55], Citation[57], Citation[58]. Visceral organs such as the lungs, liver, bone and brain are frequent sites of metastasis. The fact that our test tissue is visceral, natively vascularized and unexposed to surgical intervention (thereby avoiding wound healing-related angiogenesis) should make it highly biologically relevant, in addition to the fact that the variables recorded are objective and quantitative, a prerequisite for precise dose-effect studies. We believe that the present observations of drug-specific angiogenesis modulation by metronomic treatment, which for some agents was significantly influenced by the presence of exogenous antioxidants, will help in the design of future chemotherapy protocols.
The Swedish Cancer Foundation supported this study (grant 020503) together with the King Gustav V Jubilee Clinic Cancer Research Foundation. There is no conflict of interest to report.
References
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. New Engl J Med 2002; 346: 92–8
- Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nature Rev Cancer 2004; 4: 423–36
- Folkman J. Angiogenesis in cancer, vascular, rheumatic and other diseases. Nat Med 1995; 1: 27–31
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70
- Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O'Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 2000; 60: 1878–86
- Hanahan D, Bergers G, Bergsland E. Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 2000; 105: 1045–7
- Klement G, Baruchel S, Rak, Man S, Clarke K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 2000; 105: R15–R24
- Klement G, Huang P, Mayer B, Green SK, Man S, Bohlen P, et al. Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGF-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res 2002; 8: 221–32
- Hahnfeldt P, Folkman J, Hlatky L. Minimizing long-term tumor burden: The logic for metronomic chemotherapeutic dosing and its antiangiogenic basis. J Theor Biol 2003; 220: 545–54
- Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol 2003; 13: 159–67
- Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: Microvessel density, what it does and doesn't tell us. J Natl Cancer Inst 2002; 94: 883–90
- Kerbel RS, Klement G, Prichard KI, Kamen B. Continuous low-dose anti-angiogenic/metronomic chemotherapy: From the research laboratory into the oncology clinic. Ann Oncol 2002; 13: 12–5
- StCroix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science 2000; 289: 1197–1202
- Lennernäs B, Albertsson P, Lennernäs H, Norrby K. Chemotherapy and antiangiogenesis: Drug-specific, dose-related effects. Acta Oncol 2003; 42: 294–303
- Lennernäs B, Albertsson P, Damber J-E, Norrby K. Antiangiogenic effect of metronomic paclitaxel treatment in prostate cancer and non-tumor tissue in the same animals: A quantitative study. APMIS 2004; 112: 201–9
- Albertsson P, Lennernäs B, Norrby K. Chemotherapy and antiangiogenesis: Drug-specific effects on microvessel sprouting. APMIS 2003; 111: 995–1003
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–57
- Affara NI, Robertson FM. Vascular endothelial growth factor as a survival factor in tumor-associated angiogenesis. In Vivo 2004; 18: 525–42
- Ferrara N. The role of vascular endothelial growth factor in angiogenesis. Angiogenesis in Health and Disease. Basic mechanisms and clinical applications, GM Rubanyi. Marcel Dekker, New York 2000; 47–73
- Norrby K. Basic fibroblast growth factor and de novo mammalian angiogenesis. Microvasc Res 1994; 48: 96–113
- Norrby K. Angiogenesis: New aspects relating to its initiation and control. APMIS 1997; 105: 417–37
- Norrby K. Microvascular density in terms of number and length of microvessel segments per unit tissue volume in mammalian angiogenesis. Microvasc Res 1998; 55: 43–53
- DiPaola, RS, Rubin, E, Toppmeyer, D, Eid, J, Butzbach, D, Dvorzhinski, D, et al. Gemcitabine combined with sequential paclitaxel and carboplatin in patients with urothelial cancers and other advanced malignancies. Med Sci Monit 2003;9: PI, 1–PI 11.
- Glode LM, Barqawi A, Crighton F, Crawford ED, Kerbel R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer 2003; 98: 1643–8
- Gravis G, Bladou F, Salem N, Macquart-Moulin G, Serment G, Camerlo J, et al. Weekly administration of docetaxel for symptomatic metastatic hormone-refractory prostate carcinoma. Cancer 2003; 98: 1627–34
- Hellerstedt B, Pienta KJ, Redman BG, Esper P, Dunn R, Fardig J, et al. Phase II trial of oral cyclophosphamide, prednisone, and diethylstilbestrol for androgen-independent prostate carcinoma. Cancer 2003; 98: 1603–10
- Workman P, Twentyman P, Balkwill F, Balmain A, Chaplin D, Double J. United Kingdom co-ordinating committee on cancer research (UKCCCR) guidelines for welfare of animals in experimental neoplasia (2nd edition). Br J Cancer 1998; 77: 1–10
- Norrby K. Vascular endothelial growth factor and mammalian angiogenesis. Microvasc Res 1996; 51: 154–63
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: Its action with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Rad Biol Med 1989; 6: 593–7
- Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcystein actions. Cell Mol Life Sci 2003; 60: 6–20
- Robinson AP, White TM, Mason DW. MRC OX-43: A monoclonal antibody which reacts with all vascular endothelium in the rat except that of brain capillaries. Immunology 1986; 57: 231–7
- Norrby K, Mattsby-Baltzer I, Innocenti M, Tuneberg S. Orally administered bovine lactoferrin systemically inhibits VEGF165-mediated angiogenesis in the rat. Int J Cancer 2001; 91: 236–40
- Norrby K, Ridell B. Tumour-type-specific capillary endothelial cell stainability in malignant B-cell lymphomas using antibodies against CD31, CD34 and factor VIII. APMIS 2003; 111: 483–9
- Drevs J, Fakler J, Eisele S, Medinger M, Bing G, Esser N, et al. Antiangiogenic potency of various chemotherapeutic drugs for metronomic chemotherapy. Anticancer Res 2004; 24: 1759–64
- Näslund I, Norrby K. NO and de novo angiogenesis: Further evidence that NO inhibits bFGF-induced angiogenesis while not influencing VEGF165-induced angiogenesis. APMIS 2000; 108: 29–37
- Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer 2002; 38: 1564–79
- Sckell A, Leunig M. Dorsal skinfold chamber preparation in mice. Angiogenesis protocols. Methods in molecular medicine, JC Murray. Humana Press, New Jersey 2001; 95–105
- Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000; 156: 1363–80
- Cleaver O, Melton DA. Endothelial signaling during development. Nat Med 2003; 9: 661–8
- Rohan RM, Fernandez A, Udagawa T, Yuan J, D'Amato RJ. Genetic heterogeneity of angiogenesis in mice. FASEB J 2000; 14: 871–6
- Rogers MS, Rohan RM, Birsner AE, D'Amato RJ. Genetic loci control vascular endothelial growth factor-induced angiogenesis. FASEB J 2003; 17: 2112–4
- Weijl NI, Hopman GD, Wipkink-Bakker A, Lentjes EG, Berger HM, Cleton FJ, et al. Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Ann Oncol 1998; 9: 1331–7
- Mantovani G, Maccio A, Madeddu C, Mura L, Gramignano G, Lusso MR, et al. Quantitative evaluation of oxidative stress, chronic inflammatory indices and leptin in cancer patients: Correlation with stage and performance status. Int J Cancer 2002; 98: 84–91
- Cai T, Fassina G, Morini M, Aluigi MG, Masiello L, Fontanini G, et al. N-acetylcystein inhibits endothelial cell invasion and angiogenesis. Lab Invest 1999; 79: 1151–9
- Agarwal A, Munoz-Najar U, Klueh U, Shih S-C, Claffey KP. N-acetyl-cystein promotes angiostatin production and vascular collapse in an orthotopic model of breast cancer. Am J Pathol 2004; 164: 1683–96
- Schäfer G, Cramer T, Suske G, Kemmner W, Wiedenmann B, Höcker M. Oxidative stress regulated vascular endothelial growth factor-A gene transcription through Sp1- and Sp3-dependent activation of two proximal GC-rich promoter elements. J Biol Chem 2003; 278: 8190–8
- Castilla MA, Caramelo C, Gazapo RM, Martin O, Gonzalez-Pacheco FR, Tejedor A, et al. Role of vascular endothelial growth factor (VEGF) in endothelial cell protection against cytotoxic agents. Life Sci 2000; 67: 1003–13
- Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Rad Biol Med 2002; 33: 1047–60
- Sonveaux P, Brouet A, Havaux X, Gregoire V, Dessy C, Balligand JL, et al. Irradiation-induced angiogenesis through the up-regulation of the nitric oxide pathway: Implications for tumor radiotherapy. Cancer Res 2003; 63: 1012–19
- Isner JM, Kalka C, Kawamoto A, Asahara T. Bone marrow as a source of endothelial cells for natural and iatrogenic vascular repair. Ann NY Acad Sci 2001; 953: 75–84
- Bertolini F, Paul S, Mancuso P, Montestiroli S, Gobbi A, Shaked Y, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 2003; 63: 4342–6
- Coudore F, Authier N, Guillaume D, Beal D, Duroux E, Fialip J. High-performance liquid chromatographic determination of paclitaxel in rat serum: Application to a toxicokinetic study. J Chromatogr B Biomed Sci Appl 1999; 721: 317–20
- Yu LJ, Drewes P, Gustavsson K, Brain EG, Hecht JE, Waxman DJ. In vivo modulation of alternative pathways of P-450-catalyzed cyclophosphamide metabolism: Impact on pharmacokinetics and antitumor activity. J Pharmacol Exp Ther 1999; 16: 1564–9
- Ng SS, Figg WD, Sparreboom A. Taxane-mediated antiangiogenesis in vitro: Influence of formulation vehicles and binding proteins. Cancer Res 2004; 64: 821–4
- Ahmad SA, Jung YD, Liu W, Reinmuth N, Parikh A, Stoeltzing O, et al. The role of the microenvironment and intercellular cross-talk in tumor angiogenesis. Semin Cancer Biol 2002; 12: 105–12
- Norrby K. Mast cells and angiogenesis. APMIS 2002; 110: 355–71
- Fidler IJ, Ellis LM. Chemotherapeutic drugs—more really is not better. Nat Med 2000; 6: 500–2
- Kerbel RS. Tumor angiogenesis: Past, present and the near future. Carcinogen 2000; 21: 505–15