Abstract
The proliferative capacity of a tumor as measured by Ki-67 nuclear antigen is one of the most powerful indicators of tumor behavior. Ki-67 is considered a useful tool in determining the aggressiveness of malignant neoplasms. p53 tumor suppressor gene mutations have been linked with the development and progression of a number of various cancer types. p53 tumor suppressor protein and the volume corrected index of Ki-67 corresponding to Ki-67 /mm2 of tumor tissue (VCI Ki-67) in salivary gland tumors were evaluated by immunohistochemistry from paraffin embedded sections in a series of 212 patients. The follow-up time in this nationwide full population-based study was up to five years. The association of clinicopathological features and the results of present study with survival were examined. In multivariate analysis high VCI Ki-67 was associated with worse survival of SGC patients (p = 0.0114). Supplementary information was brought by age (p = 0.0002), lymph node status (p = 0.0014), gender (p = 0.0017) and stage (p = 0.0191). p53 expression did not have additional value in prediction of survival (p = 0.1433) compared to the commonly clinical used parameters. In this material consisting of various salivary gland carcinomas VCI Ki-67 was a good prognostic factor for survival.
Salivary gland cancer (SGC) is a relatively rare malignancy. The annual incidence range from 4 to 65 new patients per 1 000 000 person-years Citation[1]. According to the Finnish Cancer Registry the age-adjusted incidence rates during the years 1957 – 2003 ranged from 5 to 10 per 1 000 000 person-years Citation[2]. In Finland the relative five-year overall survival rates for acinic cell carcinoma, mucoepidermoid carcinoma and adenoid cystic carcinoma were 96%, 79% and 74%, respectively Citation[3]. In that material stage I, male gender and age were the most powerful predictors of patient outcome.
Ki-67 immunoreactivity has been reported to be a prognostic factor in numerous human cancers and also in some salivary gland carcinomas Citation[4–9], but its value as a prognostic factor in this histologically unhomogenous group of carcinomas has so far not been fully elucidated. The most common genetic change in human cancer is p53-mutation Citation[10]. Alterations of this tumor suppressor gene seems to be involved directly or indirectly, in the majority of human malignancies Citation[11].
The purpose of this study was to estimate whether the Ki-67 and p53 expressions are correlated with the clinical outcome in a nation wide SGC material including also rare histological types. The volume corrected mitotic index method was used when determining the VCI Ki-67 value Citation[12].
Material and methods
The material of the present study is derived from the cases of SGC diagnosed and reported to the Finnish Cancer Registry (FCR) during the period 1991 – 1996. The clinical data and the follow-up information were collected from the patient records of the five University Hospitals in Finland, which are responsible for the treatment of SGC. Survival data were obtained from The Population Registry Centre of Finland. The tumors were reclassified (P. K. and I. L.) according to uniform criteria of the WHO classification of 1991 Citation[13] and the TNM classification was done following the guidelines of the International Union Against Cancer UICC TNM classification Citation[14]. The result of this full population-based nationwide study of SGC (n = 237) has been previously published Citation[3]. Due to the lack of original paraffin block material, 210 specimens were available for Ki-67 and 212 for p53 immunohistochemical examination in the present study.
Immunohistochemistry
Immunohistochemistry was performed on deparaffinized, 5 µm sections after antigen retrieval using microwave oven heating. A MIB-1 monoclonal Ki-67 antibody and murine monoclonal antibody, DO-7 antihuman p53 protein, specific for a formalin-resistant epitope of the N-terminus of the human protein reacting with both wild and mutant types of the p53 protein (Tech Mate 500+, Dako, Copenhagen, Denmark) were used. For immunostaining, the avidin-biotin-peroxidase complex method was used. In brief, after deparaffinization and inactivation of endogenous peroxidase activity and blocking of cross reactivity with commercially HP Block reagent. The sections were incubated for one hour at room temperature with the primary antibody diluted at 1:300 for p53 and 1:100 for Ki-67. Localization of the primary antibody was achieved by subsequent incubation of biotinylated antiprimary antibody with an avidin-biotin complex conjugated to horseradish peroxidase, and diaminobenzidine. The slides were washed three times with phosphate buffered saline after each incubation and counterstained with haematoxylin. Negative controls were performed by substituting the primary antibody with non-immune mouse serum.
The criteria concordance of staining pattern for p53 and Ki-67 were assessed using a consulting microscope (Leitz, Dialux 22, Viereich Germany) by H. L. and P. K. The final evaluation was done by H. L. who was unaware of patients′ clinical status. Discordant cases were discussed, and a consensus was reached. For p53 the specimen was first assessed from several low-power fields and the area containing most positive staining of cancer cells in the field was chosen and the stained percent of positive cells was determined. The densest area for Ki-67 positive cancer cells was chosen. The volume corrected Ki-67 positive cells/square mm tumor tissue (VCI Ki-67) were counted using the volume corrected mitotic index method Citation[12]. In this study five fields were counted, at a magnification of 400, which corresponded to about 1 mm2 of neoplastic tissue in the section.
The cutoff value was selected using the cross-tabulation method in survival data of Ki-67 and p53 separately. The results of adenoid cystic carcinoma, acinic cell carcinoma and mucoepidermoid carcinoma were analyzed and the value 20 for Ki-67 and 20% for p53 were chosen.
Statistical methods
The follow-up time was calculated from the date of diagnosis to the date of death or the end of data collection on 31 December, 2001. Survival curves were estimated using the Kaplan–Meier method and compared with the non-parametric log-rank test. Univariate and multivariate Cox proportional hazards models were used to estimate the associations of p53, Ki-67, age, gender, location, T, N, diagnosis and stage with overall survival. Results were quantified by hazard ratios and their 95% confidence intervals (95% CI). The statistically significant explanatory variables in the univariate models were then included in the multivariate analysis. In all analyses, a two-sided p-value less than 0.05 were considered statistically significant. Statistical analyses were done using SAS statistical software (Version 9.1; SAS Institute Inc., Cary, NC).
Results
The study population of 212 patients consisted of 112 male and 100 female subjects, ranging in age at the time of diagnosis between 19 and 95 years. The mean age of patients was 61 and median 62 years. The commonest tumor location was the parotid gland (n = 138; 65%), followed by the minor salivary glands (n = 37; 18%), and the submandibular gland (n = 34; 16%). One cancer was diagnosed in the sublingual glands (0.5%). In Ki-67 assessment one oncocytic carcinoma and one mucoepidermoid carcinoma, both located in parotid gland, were not evaluated. The histological distribution is shown in .
Table I. The 5-year overall survival rates for volume corrected VCI Ki-67 expression measured with immunohistochemistry in different histological types of salivary cancer. * Statistical significance for difference between Kaplan-Meier curves, log-rank test, n = number of cases, VCI Ki-67 = volume corrected index of Ki-67, N.D.=Not done.
The VCI Ki-67 immunohistochemistry varied from 0 to 313. The mean value was 37 (median 16). Results of the three commonest histological types were as follows: In adenoid cystic carcinoma the VCI Ki-67 varied from 0 to 313 (mean 55, median 33); in mucoepidermoid carcinoma range 0 – 128 (mean 15, median 6) and in acinic cell carcinoma 0 – 121, (mean 16, median 5). The results and 5-year overall survival rates are given in . The figures for the three largest histological groups combined were for the VCI Ki-67 ≤ 20 84% (n = 83) and for VCI Ki-67 > 20 50% (n = 50). If a cutoff value of the VCI Ki-67 would be 10, the corresponding figures in acinic cell carcinoma are 96% (n = 23) and 73% (n = 15), respectively (p = 0.0503).
In the group of all SGC patients except the three largest histological types the survival was 57% (n = 28) and 29% (n = 49). In the whole SGC material the corresponding survival rates were 77% (n = 111) and 39% (n = 99), respectively (log-rank test, p< 0.0001) ().
Figure 1. The overall survival curves of the salivary gland cancer patients (n = 210) using the Kaplan-Mayer method for the volume corrected Ki-67 expression measured with immunohistochemistry: VCI Ki-67 ≤ 20 (n = 111); and VCI Ki-67 > 20 (n = 99).
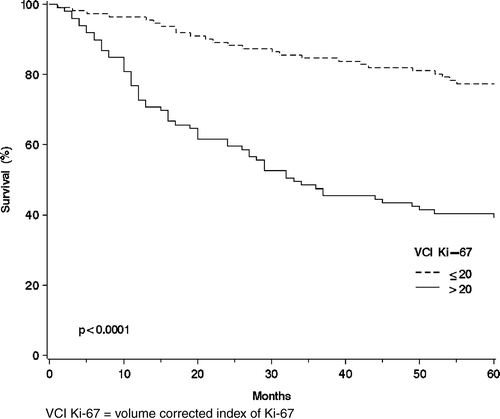
Additional examination was made of histological types representing high Ki-67. The VCI Ki-67 level over 50 was chosen as a cut off point demonstrating high proliferation. Such intensities were found in adenoid cystic carcinoma, as a matter of fact higher than in any other histological group. The VCI Ki-67 was over 50 in 20 patients whereas in mucoepidermoid carcinoma and acinic cell carcinoma that figure was 2 and 3, respectively. The 20 adenoid cystic carcinoma patients presenting with high mitotic activities had an unfavorable 5-year overall survival (35%). All ten patients with stage IV disease in this group died during the 5-year follow-up. In the whole material a high VCI Ki-67 > 50 was found in 53 SGC patients and 16 (30%) of them were well and alive in the end of study.
Twenty-two low-grade mucoepidermoid carcinoma patients were included. The VCI Ki-67 ≤ 20 was found in 20 patients and the 5-year overall survival of them was 80% () Citation[13], Citation[15].
Table II. The VCI Ki-67 expression in mucoepidermoid carcinoma and the 5-year overall survival.
The results and 5-year overall survival rates for p53 examination is shown in . The 5-year overall survival rates for the three largest histological groups combined were for p53 ≤ 20% 75% (n = 63) and for p53 > 20% 69% (n = 71), respectively. In the whole SGC material these survival rates were 66% (n = 98) and 54% (n = 114), respectively (p < 0.0436) ().
Figure 2. The overall survival curves of the salivary gland cancer patients (n = 212) using the Kaplan-Mayer method for the p53 expression measured with immunohistochemistry: p53 ≤ 20 (n = 98); and p53 > 20 (n = 114).
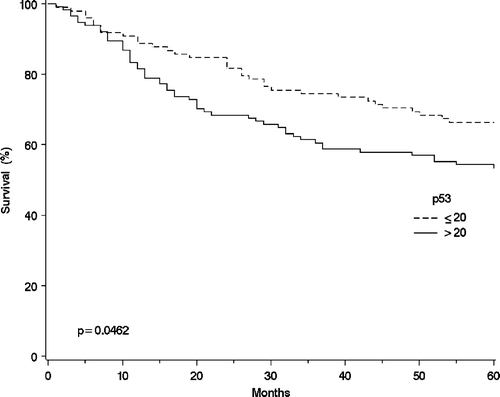
Table III. 5-year overall survival rates in correlation to the p53 expression measured with immunohistochemistry in different histological types.
In Cox′s multivariate analysis the age was the most important independent prognostic factor for five-year overall survival followed by lymph node metastasis, gender, Ki-67 positivity and stage as seen in the . The result was similar in disease specific 5-year survival and in 5-year overall survival.
Table IV. Cox's univariate and multivariate analysis of prognostic factors for 5-year overall survival in salivary gland carcinomas.
Discussion
In the present nationwide full population-based study the volume corrected index Ki-67 correlated with survival statistically highly significantly in mucoepidermoid carcinoma (p < 0.0020) and in the whole SGC-material (p < 0.0001). In acinic cell and adenoid cystic carcinoma the p values were < 0.0393 and < 0.0424, respectively.
In mucoepidermoid carcinoma VCI Ki-67 negativity (≤20) was correlated with a higher 5-year overall survival and VCI Ki-67 positivity (>20) with an unfavorable prognosis. In the cases with low-grade tumors the immunohistochemistry was mainly negative. The grading of mucoepidermoid carcinoma has been insufficient and difficult to perform. Many grading criteria has been suggested and the latest version is introduced by Brandwein et al. Citation[16]. In that system Ki-67 index is not used as grading criteria, however our results support Ki-67 immunohistochemistry to be used as an additional standard.
In previous studies the correlation with survival has not been proven, but there has been an association with prognosis. Skalova et al. reported that Ki-67 was a significant prognostic factor correlating with a higher incidence of recurrence and distant metastasis Citation[4]. Goode et al. found high Ki-67 indices to relate to the grading system of AFIP Citation[17], whereas Kiyoshima reported Ki-67 to associate with the histological grade and the clinical course Citation[8].
In acinic cell carcinoma the prognosis was the best of the three largest histological types. The VCI Ki-67 correlated statistically significantly with overall survival as reported by Skalova et al. They had a material of 30 patients and found Ki-67 to be a significant prognostic factor Citation[5]. Hellquist et al. had a similar finding in material from 16 patients Citation[6].
In adenoid cystic carcinoma a statistical correlation was found in this study. Similarly, Nordgård et al. reported, that the Ki-67 is a powerful tool for predicting the short-term prognosis of these patients Citation[7] whereas Kiyoshima et al. found no relationship between immunopositivity of Ki-67 and the morphological growth pattern or the clinical course of the patients Citation[8].
Adenoid cystic carcinoma (ACC) is well known to have aggressive biological characteristics such as local recurrences, perineural spread and late distant metastasis. Although a five year follow-up period is sufficient for most salivary gland tumors, it is well known that adenoid cystic carcinoma patients may recur late and should therefore be followed-up for up to twenty years. Thus our results may underestimate the number of recurrences in patients with this histological diagnosis. In mucoepidermoid cancer the degree of differentiation assessed histopathologically has been used for prognostic purposes.
Histopathological diagnostics of SGC does not always give a right view of the biological behavior of the tumor. Thus in the present study the volume corrected index of Ki-67 was used to avoid inaccuracy in tumor evaluation. The VMI method is easily available, but not commonly used among general pathologists, since until now clinical data has been sparse. This technique was now successfully used and we recommend the VCI Ki-67 method for salivary gland cancer evaluation.
In the present study no statistically significant relationship between p53 immunohistochemistry and overall survival in salivary gland cancer was found. In salivary duct cancer the survival ratio for p53 negative (p53 ≤ 20%) patients was 75% (n = 4) and for positive cases (p53 > 20%) 22% (n = 9), but the low number of patients diminishes the value of this finding. Our results are in concordance with those reported by Kärjä et al. where p53 did not correlate to clinical behavior nor survival in salivary gland cancer in general Citation[18].
Some histological samples were relatively small. Therefore five fields were chosen for assessment of p53 immunohistochemistry, which describes the characteristics and behavior of a tumor in a definite way. Previous studies evaluated the percentage of p53 positivity and therefore this method was chosen for this trial. In order to reach the same accuracy in Ki-67 immunohistochemistry the volume corrected index-method was preferred for utilize.
Several studies have evaluated p53 findings in SGC. Gallo et al. 1995, found p53 to correlate with clinical aggressiveness in patients with carcinoma of the parotid gland Citation[19]. p53 has also been associated with the clinical aggressiveness by others Citation[19–23]. The clinical value of p53 overexpression as a prognostic marker remains unsettled in this material. It has been associated with both favorable and unfavorable outcome of the patients and the reasons for the varying result reported are unknown. The immunohistochemical technique of determining the p53 status has been criticized by Gallo et al.1995 Citation[19]. The immunohistochemical analysis of the p53 oncogene product expression is less specific than other methods of molecular analysis of the gene, such as single strand conformation polymorphism Citation[19].
In this material consisting of various salivary gland carcinomas combining age, lymph node metastasis, gender, VCI Ki-67 and stage gave the best correlation with survival. We conclude that the VCI Ki-67 immunohistochemistry is an important independent prognostic factor for SGC patients.
This work was supported by the Cancer Society of Southwest Finland. The authors thank the Finnish Cancer Registry, the University and Central Hospitals of Finland and Dr. L. Paljärvi for using his Excel program in counting the Ki-67 values.
References
- Vander Poorten VL. Salivary gland carcinoma-Stepping up the prognostic ladder. University of Amsterdam, Amsterdam 2002; 222
- Finnish Cancer Registry. May 12th 2005. Available from: URL: http://www.cancerregistry.fi/tilastot/.
- Luukkaa H, Klemi P, Leivo I, Koivunen P, Laranne J, Makitie A, et al. Salivary gland cancer in Finland 1991 – 96: An evaluation of 237 cases. Acta Otolaryngol 2005; 125(2)207–14
- Skalova A, Lehtonen H, von Boguslawsky K, Leivo I. Prognostic significance of cell proliferation in mucoepidermoid carcinomas of the salivary gland: Clinicopathological study using MIB 1 antibody in paraffin sections. Hum Pathol 1994; 25(9)929–35
- Skalova A, Leivo I, von Boguslawsky K, Saksela E. Cell proliferation correlates with prognosis in acinic cell carcinomas in salivary gland origin. Immunohistochemical study of 30 cases using the MIB 1 antibody in formalin-fixed paraffin sections. J Pathol 1994; 173(1)13–21
- Hellquist HB, Sundelin K, Di Bacco A, Tytor M, Manzotti M, Viale G. Tumour growth fraction and apoptosis in salivary gland acinic cell carcinomas. Prognostic implications of Ki-67 and bcl-2 expression and of in situ end labelling (TUNEL). J Pathol 1997; 181(3)323–9
- Nordgård S, Franzen G, Boysen M, Halvorsen TB. Ki-67 as a prognostic marker in adenoid cystic carcinoma assessed with the monoclonal antibody MIB1 in paraffin sections. Laryngoscope 1997; 107(4)531–6
- Kiyoshima T, Shima K, Kobayashi I, Matsuo K, Okamura K, Komatsu S, et al. Expression of p53 tumor suppressor gene in adenoid cystic and mucoepidermoid carcinomas of the salivary glands. Oral Oncol 2001; 37(3)315–22
- Xin W, Paulino AF. Prognostic factors in malignant mixed tumors of the salivary gland: Correlation of immunohistochemical markers with histologic classification. Ann Diagn Pathol 2002; 6(4)205–10
- Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature 1991; 351(6326)453–6
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000; 408(6810)307–10
- Haapasalo H, Pesonen E, Collan Y. Volume corrected mitotic index (M/V-INDEX). The standard of mitotic activity in neoplasms. Pathol Res Pract 1989; 185(5)551–4
- Seifert SG. Histological typing of salivary gland tumours. WHO International Histological Classification of Tumours2nd ed. Springer, Berlin 1991
- Sobin LH, Wittekind C. UICC TNM Classification of Malignant Tumours5th ed. John Wiley & Sons, Inc., New York 1997
- Ellis GL, Auclair PL. Atlas of tumor pathology, tumors of the salivary glands. In: series 3 f, editor. Washington, DC: Armed Forces Institute of Pathology; 1996. p. 163.
- Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: A clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001; 25(7)835–45
- Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: Clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998; 82(7)1217–24
- Kärjä VJ, Syrjänen KJ, Kurvinen AK, Syrjänen SM. Expression and mutations of p53 in salivary gland tumours. J Oral Pathol Med 1997; 26(5)217–23
- Gallo O, Franchi A, Bianchi S, Boddi V, Giannelli E, Alajmo E. p53 oncoprotein expression in parotid gland carcinoma is associated with clinical outcome. Cancer 1995; 75(8)2037–44
- Soini Y, Kamel D, Nuorva K, Lane DP, Vahakangas K, Päkkö P. Low p53 protein expression in salivary gland tumours compared with lung carcinomas. Virchows Arch A Pathol Anat Histopathol 1992; 421(5)415–20
- Doi R, Kuratate I, Okamoto E, Ryoke K, Ito H. Expression of p53 oncoprotein increases intratumoral microvessel formation in human salivary gland carcinomas. J Oral Pathol Med 1999; 28(6)259–63
- Zhu QR, White FH, Tipoe GL. p53 oncoprotein accumulation in adenoid cystic carcinoma of parotid and palatine salivary glands. Pathology 1997; 29(2)154–8
- Lim JJ, Kang S, Lee MR, Pai HK, Yoon HJ, Lee JI, et al. Expression of vascular endothelial growth factor in salivary gland carcinomas and its relation to p53, Ki-67 and prognosis. J Oral Pathol Med 2003; 32(9)552–61