Abstract
The widely accepted image of breast cancer with non-inflammatory skin involvement (T4b) is determined by the tenet that all these tumors are locally advanced (Stage IIIB). The study addresses the question whether this view is justified. Data from 453 non-metastatic breast cancer patients were collected retrospectively. Eighty-one patients had T4b disease. To assess the malignant potential of tumors independent of the feature skin involvement, a reclassification only considering tumor size was undertaken. We compared the clinical course of three study groups (A: Stage II; B: Stage IIIA; C: Stage IIIC) with control groups of 372 patients without skin involvement. In the study groups, we found a broad distribution among the stages (A:36.2%; B:33.7%; and C:27.7%) with significant differences in disease-specific survival (DSS) (A/B: p = 0.032; B/C: p = 0.048). There were no significant differences in DSS between the study and the corresponding control group. In multivariate analysis, skin involvement was not a significant predictor of DSS. Heterogeneity of the T4b category and a lack of prognostic significance expand the widely accepted image of breast cancer with non-inflammatory skin involvement. The highest T category, or Stage III, is not the appropriate classification for a considerable number of patients having this clinicopathologic entity.
According to the current edition of the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) TNM staging system Citation[1], Citation[2], non-inflammatory breast carcinomas with direct extension to the skin are classified as T4b lesions. These tumors, eliminating cases with distant metastasis, are included in Stage III (Stage IIIB: T4N0 – 2M0, Stage IIIC: any TN3M0), which is considered to be synonymous with locally advanced breast cancer (LABC). The current definition of non-inflammatory skin involvement, as well as the placement of all breast carcinomas with skin involvement in the highest T category, is a continuation of classification principles defined by Haagensen and Stout in the Columbia Clinical Classification first published in 1943 Citation[3]. Ulceration, as well as edema of limited extent, were defined as grave prognostic signs Citation[3], Citation[4].
In the current study we describe, on the one hand, the incidence of T4b breast cancer. On the other hand, we assess systematically the impact of the morphologic parameter “skin involvement” on the prognosis of non-metastatic breast carcinoma independent of disease stage. For this purpose, we compared the clinical course of study groups with non-metastatic and non-inflammatory skin involvement breast cancer with the outcome of control groups including patients with tumors of comparable disease stage but without skin involvement.
Ultimately, our study addresses the question whether the T4 category still has any justification in terms of the current TNM classification.
Materials and methods
Between January 1988 and August 1999, 119 women with newly diagnosed breast carcinoma and histologically proven non-inflammatory skin involvement accompanied by macroscopic and typically readily discernible ”classical” skin changes, such as ulceration, edema, peau d'orange, and satellite skin nodules (T4b), who had no history of contralateral breast cancer or local recurrence were evaluated and treated at the Department of Gynecology and Obstetrics of the University Hospital Basel (Basel, Switzerland), the Department of Surgery of the University Hospital Basel, and the Women's Hospital and Breast Center Rheinfelden (Rheinfelden, Germany).
Two patients who presented with additional histologically proven chest wall involvement were classified as having T4c disease and were not considered in the analysis. According to the current edition of UICC/AJCC TNM staging system, only tumors accompanied by macroscopic and typically readily discernible ”classical” skin changes should be placed in the T4b category Citation[1], Citation[2]. In the TNM Supplement Citation[5], it is stated explicitly that microscopic invasion of the dermis alone is not sufficient for placing a lesion in the T4b category; when no accompanying “classical” clinical signs are present, the T classification is based solely on tumor size (T1–3). During the study period, 63 patients with histologically proven, non-inflammatory skin involvement, showed a lack of the “classical” clinical signs and exhibited only subtle (e.g., dimpling, retraction) or no skin changes. These cases, as well as patients with Paget's disease, were not considered in the study group.
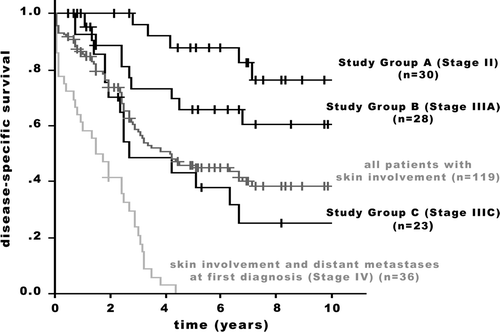
Of the 119 patients, whose tumors were classified as T4b, two were reclassified as having Stage I disease and 36 had distant metastases at presentation (Stage IV). These patients were excluded from analysis. Their data, not compared to a control group, are shown in . Furthermore, shows all patients with skin involvement and relates the different outcome of Study Groups with the entire group.
Figure 1. Disease-specific survival of 119 patients with T4b breast carcinoma. Comparison of the entire population and the Study Groups A, B, and C. Group A/B (p = 0.032); Group B/C (p = 0.048). +: censored.
The data on 81 patients (3.3% of all newly diagnosed breast carcinomas), who presented with Stage II or Stage III non-inflammatory breast cancer, histologically proven skin involvement and the “classical” clinical signs, form the basis of the current study. The analysis of the data of 372 consecutive breast cancer patients who were classified as having Stage II or Stage III disease and were treated at the Department of Gynecology and Obstetrics and the Department of Surgery of the University of Basel between January 1990 and August 1999 served as the control group. Patients with metastatic disease, history of contralateral breast cancer, local recurrence, bilaterality, multicentricity (not for Stage IIIC), and male gender were the exclusion criteria.
Histopathological analyses were performed at the Institute of Pathology of the University of Basel. All patients in this study had invasive ductal or invasive lobular carcinoma; patients with rare histologic types of invasive carcinoma with more favorable prognosis (e.g., tubular carcinoma, papillary carcinoma, medullary carcinoma) were not included. Histopathological analyses also included grading according to the Bloom-Richardson-Elston scheme and immunohistochemical staining for estrogen (ER) and progesterone receptors. Additional biologic predictive factors (e.g. over-expression of HER-2/neu, p53, EGFR), which eventually may prove to be a more reliable index of tumor aggressiveness, were assessed regularly beginning in the mid-1990s. Therefore, information on these factors was not available for all patients and was not taken into account in the final analysis. Each patient underwent a staging work-up, which included a recording of clinical history, physical examination, routine blood studies, chest x-ray, sonography of the liver, and additional diagnostic studies as needed to rule out metastatic disease. There was no standard therapeutic approach during the study period (). All patients were followed until their death or for a minimum of five years if they remained alive (). These patients were seen a maximum of three months before conclusion of the study. The study was carried out in accordance with the guidelines of the Ethics Committee of the University of Basel.
Table I. Patient and tumor characteristics.
Staging was performed for all patients in accordance with the current (6th) edition of the UICC/AJCC TNM Classification. As a second step, the T4b category was eliminated from classification in order to assess the malignant extent independent from the parameter “skin involvement”. All tumors were reclassified based on tumor size, and therefore, the category T4b was replaced with the categories T1–3. In this manner, patients who had Stage IIIB disease underwent restaging.
Based on the TNM staging, all patients were placed into one of three stage groups: Stage II (Group A: Study Group n = 30; Control Group n = 226); Stage IIIA (Group B: Study Group n = 28; Control Group n = 97); Stage IIIC (Group C: Study Group n = 23; Control Group n = 49).
Statistical methods
Using the Kaplan-Meier method, disease-specific survival (DSS) was calculated from the date of diagnosis to the date of death, or for patients who remained alive, to the date of last follow up. Non-malignancy-related deaths were censored in the statistical analyses according to the same method used for patients who were alive and disease-free. Statistical differences between groups in terms of survival curves were analyzed using the log rank test. Comparisons between nominal parameters were made with the Fisher exact test. Multivariate analysis was performed using the Cox proportional hazards model. Statistical analyses were performed with SPSS 12.0 software (SPSS Inc., Chicago, IL).
Results
Patient and tumor characteristics
Patient, tumor and treatment characteristics of the 453 patients in the study (81 patients in the study groups, 372 patients in the control groups) are summarized in and . The median patient age was considerably higher in the study groups than in the control groups (80, 73, and 68 years vs. 59, 58, and 61 years).
Table II. Treatment data and patient outcomes.
The distribution of tumor sizes within the entire group of all non-inflammatory breast carcinomas with skin involvement and without metastases at first diagnosis was: up to 2.9 cm: 28.9%; 3.0–5.0 cm: 33.7%; 5.1–10.0 cm: 21.7%; >10.0 cm: 15.7%.
The distribution of disease stages according to current UICC/AJCC criteria, after disregarding skin involvement in favor of tumor size, was for the entire group: Stage I: 1.7%, Stage II: 25.2%, Stage IIIA: 23.5%, Stage IIIC: 19.3%, and Stage IV: 30.3%. The distribution of stages within the above mentioned group, but excluding patients with metastatic disease at initial presentation, is shown in : Stage I: 2.4%, Stage II (Study Group A): 36.2%, Stage IIIA (Study Group B): 33.7%, and Stage IIIC (Study Group C): 27.7%.
In comparison to the respective control groups, patients with skin involvement had larger tumor sizes (Group A: 3.2 cm vs. 2.5 cm; Group B: 5.6 cm vs. 4.0 cm; Group C: 5.8 cm vs. 4.4 cm) and were less likely to have established indicators of tumor aggressiveness such as high grade and ER-negative status. Patients in Study Group A often presented significantly less with ER-negative tumors (p = 0.036). A higher percentage of control group patients (61.5% vs. 46.7%; p = 0.172) had high-grade carcinomas. In Study Group B, there was a trend observed toward high-grade (62.9% vs. 46.4%; p = 0.091) and ER-negativity (28.8% vs. 10.7%; p = 0.084). Significant differences were not observed regarding ER-status (p = 0.558) or grade (p = 0.762) in Study Group C:
Disease-Specific Survival (DSS)
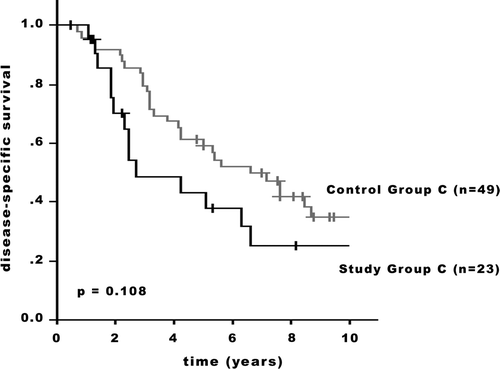
The DSS between the Study Groups () showed significant differences (Study Groups A/B: p = 0.032; Groups B/C: p = 0.048). There was no statistical significant difference in outcome between the Study and the Control groups. While both the 5-year and 10-year adjusted survival rates were superior for the patients of the Groups A and B ( and ), in the stage subset including patients with extensive regional lymph node disease (Group C), less favorable rates for the Study Group patients were observed (). In Group A, the 5-year adjusted survival rates were 88.0% in the Study Group and 82.3% in the Control Group; the 10-year rates were 75.9% and 67.2%, respectively (p = 0.636; ). In Group B, the 5-year adjusted survival rates were 65.7% in the Study Group and 64.9% in the Control Group; the 10-year rates were 60.7% and 45.3%, respectively (p = 0.779; ). In Group C, the 5-year adjusted survival rates were 44.7% in the Study Group and 61.1% in the Control Group; the 10-year rates were 25.8% and 35.7%, respectively (p = 0.108; ).
Figure 2. Disease-specific survival of patients with T4b breast carcinoma and a control group with breast cancer patients without skin involvement. Group A: Stage II (p = 0.636). +: censored.
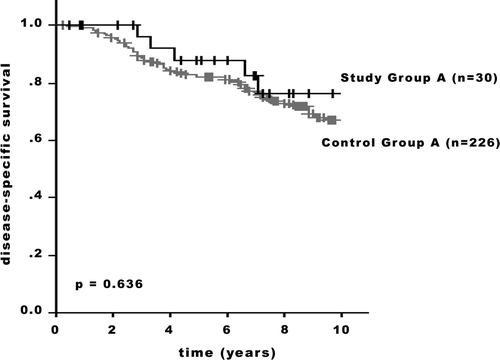
Figure 3. Disease-specific survival of patients with T4b breast carcinoma and a control group with breast cancer patients without skin involvement. Group B: Stage IIIA (p = 0.779). +: censored.
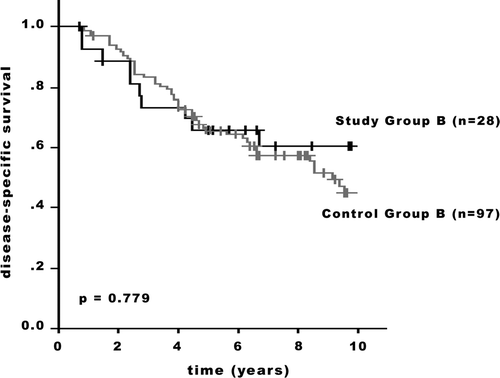
Figure 4. Disease-specific survival of patients with T4b breast carcinoma and a control group with breast cancer patients without skin involvement. Group C: Stage IIIC (p = 0.108). +: censored.
In multivariate Cox regression analysis of demographic, pathologic, immunohistochemical and treatment factors associated with DSS (), skin involvement was not a significant prognostic marker. In Group A the hazard ratio was 1.07 (95% CI, 0.44 to 2.59; p = 0.886). In Group B the hazard ratio was 1.04 (95% CI, 0.51 to 2.14; p = 0.908). In Group C the hazard ratio was 1.62 (95% CI, 0.79 to 3.23; p = 0.185). The same Cox model showed that only high grade and the lack of systemic treatment (not calculated for Group C) were significantly associated with adverse outcome.
Table III. Cox multivariate analysis for factors influencing disease-specific survival.
Discussion
The widely accepted image of breast cancer with non-inflammatory skin involvement is determined by historical views that led to classification of these tumors in categories with the most advanced locoregional extent (Steinthal's Groupings, Manchester System, Portmann Classification, Columbia Clinical Classification) Citation[6]. Haagensen and Stout Citation[3], Citation[4] reported that in period from 1915–1942 only 27 patients with ulceration of the skin were eligible for radical mastectomy. Undoubtedly during this period, the vast majority of the breast cancer patients with skin involvement made up a relatively homogenous group of “classical” cases with considerable locoregional and often already distant metastatic spread. Still in 1986, Haagensen was convinced that ulceration of the skin was a grave local sign of advanced breast carcinoma and in most of these cases the disease has become incurable Citation[7]. This historical approach was perpetuated in the TNM system, where also all forms of skin involvement led to placement in the most unfavorable T-category (T4).
The few systematic studies investigating incidence and outcome for non-inflammatory types of T4 breast cancer compared only cases within the T4 category or Stage III, respectively Citation[8–22]. This mirrors that there was no doubt about the tenet that tumors with non-inflammatory skin involvement were appropriately represented in the highest T category and considered to be LABC. This seems, however, difficult to understand because the clinical picture of this tumor entity changed in the last decades, at least in the developed countries, to the effect that more often tumors of smaller size were diagnosed. Current reports analyzing study periods in the 80s and 90s found in non-inflammatory Stage IIIB disease an incidence of 38% of tumors <5 cm Citation[16], and even 70% in non-metastatic pT4b cases Citation[17]. With an increasing number of smaller tumors, an assessment is possible as to whether the morphologic parameter “non-inflammatory skin involvement” itself is a prognostic factor independent of tumor size. We have attempted a new approach in our study and call the above-mentioned tenet into question.
There is a widespread acceptance that T4 breast cancer has a very variable biologic behavior Citation[23–25]. All these “classic” T4 patients are, however, united by the poor outcome. It is in general not yet recognized, but demonstrated in this study, that the extent of heterogeneity of breast cancer with non-inflammatory skin involvement goes far beyond the accepted image of the disease. We demonstrate a considerable heterogeneity of the entity with a broad distribution of cases among the subsets of disease stages. Between the disease stages, significant differences in DSS were found. A wide variance exists in the T4b category. On one side of the scale are cases with very advanced disease. So were 30.3% of the patients of the entire group with non-inflammatory skin involvement diagnosed with distant metastases (Stage IV) already at the initial presentation. Excluding these patients from the analysis, 27.7% of the patients (19.3% of the entire group) were classified as having extensive lymph node involvement (Stage IIIC). These cases represent the classical form of T4 breast cancer and still make up the majority of all cases with non-inflammatory skin involvement. On the other side of the scale, 36.2% of the study group patients were, after reclassification, regarded as having Stage II disease, 2.4% were even put in Stage I. Furthermore, we demonstrate that skin involvement is only an associated symptom without prognostic significance in long-term survival.
In the multivariate analysis, no independent prognostic impact was observed for the variables skin involvement, age, tumor size, and hormonal receptor status. Accordingly, our results do even suggest a less aggressive tumor entity of carcinomas with skin involvement. This is reflected in the somewhat larger tumor sizes in the study group, while tumor size was not an independent prognostic factor. The higher incidence of larger tumor sizes in the Study Groups is more likely influenced by the time-dependency of tumor growth and a delay in diagnosis. Both factors may be associated with the advanced age of the study group patients Citation[26]. Age is also an independent predictor of management. Even after controlling for comorbidity, increasing age was associated with increased risk of inadequate treatment, e.g. generally less surgical treatment, increased numbers of non-breast-conserving procedures, and omitting radiation therapy after breast-conserving surgery Citation[26–28]. In the Study Groups A and B, we found also a higher proportion of patients with less aggressive locoregional surgical management (simple mastectomy, tumor excision) and more patients that did not receive any other pre- or postoperative therapy. The fact that there were no specific guidelines for the treatment of patients with LABC during the study period may explain the multitude of treatment modalities and combinations. As most of the patients in our Study Groups were elderly and postmenopausal, this would explain why endocrine therapy was preferred to chemotherapy. A critical point of this study may therefore be that prognostic features such as age and treatment are unevenly distributed in the cohorts. This study evaluates breast cancer cases in terms of TNM Classification. Since the TNM system focuses on the extent of morphologic features and does not account for above-mentioned characteristics, a slight imbalance of these prognostic factors must be taken into consideration, which does not compromise the validity of the study results. TNM Classification is appropriately focused on stratifying patients into clinically meaningful prognostic subsets by classifying exactly the anatomical extent of the disease on the basis of clinical and pathological criteria. The TNM concept means that only clearly defined homogenous entities with similar prognostic impact may be placed together in one category/stage and placement in a higher category/stage generally corresponds to a poorer prognosis. A grouping of heterogeneous cases according to a morphologic feature without a unifying malignancy profile in one T category, as in the T4 category, goes against the principles of the concept. Our results suggest that a revision of the T4 category is necessary.
References
- Sobin, L, Wittekind C, editors. TNM classification of malignant tumors. New York: John Wiley & Sons; 2002.
- Greene F, Page D, Fleming I, et al. AJCC cancer staging manual. Springer, New York 2002
- Haagensen C, Stout A. Carcinoma of the breast: Criteria of inoperability. Ann Surg 1943; 118: 859–66
- Haagensen CD, Bodian C. A personal experience with Halsted's radical mastectomy. Ann Surg 1984; 143: 143–50
- Wittekind, C, Greene, FL, Henson, DE, et al., editors. TNM Supplement. A commentary on uniform use. 3rd ed. New York: Wiley-Liss; 2004.
- Sugg, S, Donegan, W. Staging and prognosis. In:. W Donegan, Spratt, J, editors. Cancer of the breast. 5th ed. Philadelphia: Saunders; 2002. p. 477–506.
- Haagensen, C. Clinical classification of the stage of advancement of breast carcinoma. Diseases of the Breast. 3rd ed. Philadelphia: W. B. Saunders Company; 1986. p. 851–63.
- Touboul E, Lefranc JP, Blondon J, et al. Multidisciplinary treatment approach to locally advanced non-inflammatory breast cancer using chemotherapy and radiotherapy with or without surgery. Radiother Oncol 1992; 25: 167–75
- Touboul E, Lefranc JP, Blondon J, et al. Primary chemotherapy and preoperative irradiation for patients with stage II larger than 3 cm or locally advanced non-inflammatory breast cancer. Radiother Oncol 1997; 42: 219–29
- Valagussa P, Zambetti M, Bignami P, et al. T3b-T4 breast cancer: Factors affecting results in combined modality treatments. Clin Exp Metastasis 1983; 1: 191–202
- Valagussa P, Zambetti M, Bonadonna G, et al. Prognostic factors in locally advanced noninflammatory breast cancer. Long-term results following primary chemotherapy. Breast Cancer Res Treat 1990; 15: 137–47
- Rubens RD, Armitage P, Winter PJ, et al. Prognosis in inoperable stage III carcinoma of the breast. Eur J Cancer 1977; 13: 805–11
- Rao DV, Bedwinek J, Perez C, et al. Prognostic indicators in stage III and localized stage IV breast cancer. Cancer 1982; 50: 2037–43
- Sutherland CM, Mather FJ. Long-term survival and prognostic factors in patients with regional breast cancer (skin, muscle, and/or chest wall attachment). Cancer 1985; 55: 1389–97
- Zucali R, Kenda R. Small size T4 breast cancer. Natural history and prognosis. Tumori 1981; 67: 225–30
- Yildirim E, Semerci E, Berberoglu U. The analysis of prognostic factors in stage III-B non-inflammatory breast cancer. Eur J Surg Oncol 2000; 26: 34–8
- Wieland AW, Louwman MW, Voogd AC, et al. Determinants of prognosis in breast cancer patients with tumor involvement of the skin (pT4b). Breast J 2004; 10: 123–8
- Poole, GV, Thigpen, JT, Vance, RB, Barber, WH. Management of women who present with T4 breast cancer. Am Surg 2004;70:662–6; discussion 666–7.
- Price A, Kerr GR, Rodger A. Primary radiotherapy for T4 breast cancer. Clin Oncol (R Coll Radiol) 1992; 4: 217–21
- Guth U, Moch H, Herberich L, Holzgreve W. Noninflammatory breast carcinoma with skin involvement. Cancer 2004; 100: 470–8
- El-Tamer M, Hussain S, Weedon J, et al. Prognoses of T4 breast cancer subsets. Ann Surg Oncol 2002; 9: 340–5
- Hortobagyi GN, Ames FC, Buzdar AU, et al. Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer 1988; 62: 2507–16
- Witherspoon L. Discussion on “Management of women who present with T4 breast cancer” by G. V. Poole, et al. Am Surg 2004; 70: 666–7
- Hortobagyi, G, Singletary, S, Strom, E. Locally advanced breast cancer. In:. J Harris, Lippman, M, Morrow, M, Osborne, K, editors. Diseases of the Breast. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 951–70.
- Newman LA. Management of patients with locally advanced breast cancer. Curr Oncol Rep 2004; 6: 53–61
- Kimmick, G, Hughes, K, Muss, H. Breast cancer in older women. In:. J Harris, Lippman, M, Morrow, M, Osborne, C, editors. Diseases of the Breast. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 1323–38.
- Greenfield S, Blanco DM, Elashoff RM, Ganz PA. Patterns of care related to age of breast cancer patients. Jama 1987; 257: 2766–70
- Newschaffer CJ, Penberthy L, Desch CE, et al. The effect of age and comorbidity in the treatment of elderly women with nonmetastatic breast cancer. Arch Intern Med 1996; 156: 85–90