Abstract
The present study was undertaken to investigate whether the glucocorticoid receptor –α (GR-α) and –β (GR-β) mRNA may be expressed in thyroid gland. Ten normal thyroid gland and 14 follicular adenomas were studied using a real-time fluorescent quantitative RT-PCR (FQ-RT-PCR) method. The results demonstrated that there was a lower expression of GR-α mRNA (×106 GR-α cDNA copies/µg total RNA) in thyroid adenoma (1.27±0.26) than that in normal thyroid gland (3.53±1.22) (p < 0.001). The expression of GR-β mRNA was lower in all the thyroid tissues. Of note, there was a significant difference in GR-β mRNA expression (×104 GR-β cDNA copies/µg total RNA) between thyroid adenoma (80.8±13.9) and thyroid gland (1.78±0.59) (p < 0.001). The GR-α/GR-β ratios in thyroid adenoma and normal thyroid gland were 1.67±0.68 and 207.57±84.41 respectively (p < 0.001). These results revealed, for the first time, that both GR-α and GR-β mRNA expression were detectable in both thyroid gland and adenomas tissues. We therefore conclude that down-regulation of GR-α and up-regulation of GR-β mRNA expression may play an important role in the thyroid adenomas.
The actions of glucocorticoids are mediated by the glucocorticoid receptor (GR) which belongs to the steroid–thyroid–retinoid super-family of nuclear receptors Citation[1]. The human glucocorticoid receptor (hGR) is a transcriptional factor activated by glucocorticoids (GC), and regulates the expressions of various inflammatory cytokine genes by interacting with DNA bound NF-[kappa] B or activator protein 1. The hGR gene is located on chromosome five (locus 5q31) and consists of nine exons and eight introns Citation[2]. GR exists in at least two isoforms, GR-α and GR-β, originating from the same gene by alternative splicing of the GR primary transcript. GR-α is the predominant isoform of the receptor. Significant expression of GR-α was found in brain, skeletal muscle, lung, liver, kidney, and so on. The lowest GR-α expression was found in heart, colonic mucosa, and neutrophils. GR-β mRNA was detected in all inflammatory cells and tissues, except the colonic mucosa, its concentration was at least 400 times lower than the GR-α message Citation[3].
Systemic glucocorticoids treatment combined with orbital radiotherapy is an effective method of treatment of Graves' ophtahlmopathy in terms of both rapid regression of inflammatory changes in orbital soft tissues and in prevention of recurrence of the disease Citation[4]. Pain and systemic symptoms of subacute thyroiditis (SAT) relief is achieved with glucocorticoids therapy, but such therapy does not prevent early- and late-onset thyroid dysfunction Citation[5]. Glucocorticoids are probably the drug of choice for more rapidly curing type II amiodarone-induced thyrotoxicosis (AIT) Citation[6] and Riedel's thyroiditis Citation[7].
However, up to date, no author has presented an analysis of glucocorticoid receptor and its subunit in thyroid gland. In the present study, the real-time fluorescent quantitative RT-PCR (FQ-RT-PCR) method was used to investigate the expressions of glucocorticoid receptor subunits in human thyroid.
Materials and methods
Tissue preparation
We studied a total of 24 thyroid specimens that were immediately frozen in liquid nitrogen after operation and stored at −70°C for RNA extraction. The sample consisted of 14 follicular adenomas and ten normal thyroid glands adjacent to adenomas. Hematoxylin and eosin-stained sections from each case were evaluated histologically.
All thyroid specimens were obtained without any particular therapy before surgery. Informed consent was obtained from all patients. Eight male and sixteen female donors ranged in age from 18 to 65 years (mean 39.8 years).
Real-time fluorescent quantitative RT-PCR
Total RNA was prepared from either thyroid adenoma or thyroid gland tissues using Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Isolated total RNA was quantified using a spectrophotometer (Amersham Biosciences Ultraspec 3100 Pro). Aliquots of total RNA (1 µg) were reverse-transcribed using random primers and Superscript II-Reverse Transcriptase (Invitrogen) according to the manufacturer's protocol. cDNA equivalent to 20 ng of total RNA was subjected to real-time PCR analysis (ABI7000; Appliedbiosystem, USA) following standard protocols. PCR Primers (Invitrogen) and Taqman probes for GR-α, GR-β were designed by Primer Express 2.0 software (Appliedbiosystem, USA). The primer and probe for GAPDH and GR-α and GR-β () were commercially synthesized by DaAn Gene Co. Ltd. of Sun Yat-sen University (China). Each reaction (25 µl) contained 2.5 µl of reaction buffer (10×), 6 mM of MgCl2, 0.2 µM of dNTP, 0.6 µM of each primer, 0.25 µl of SureStar TaqDNA Polymerase, and 2 µl of cDNA dilutions. The cycling condition consisted of one cycle at 93°C for 10 min and 40 two-segment cycles (93°C for 30 s and 55°C for 60 s). In each run a negative control (distilled water) was included. Briefly, 10-fold serial dilutions of control cDNA were amplified by the ABI7000 PCR machine (Appliedbiosystem, USA). Ct Value (initial amplification cycle) of each standard dilution was plotted against standard cDNA copy numbers. On the basis of the standard curves for each gene, the sample cDNA copy number was calculated according to the sample Ct value. Standard curves and PCR results were analyzed using ABI7000 software (Appliedbiosystem, USA).
Table I. The sequences of the primer and Taqman probe.
Mathematical analysis
Data were expressed as the mean ± SEM. Data was analyzed by one-way analysis of variance (ANOVA). P value of less than 0.05 was considered significant.
Results
Fluorescence intensity curve and standard curve
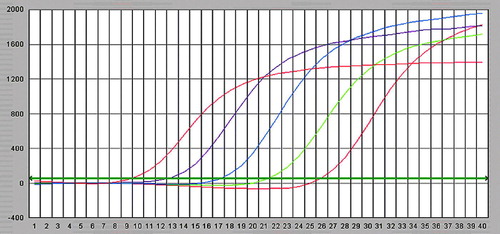
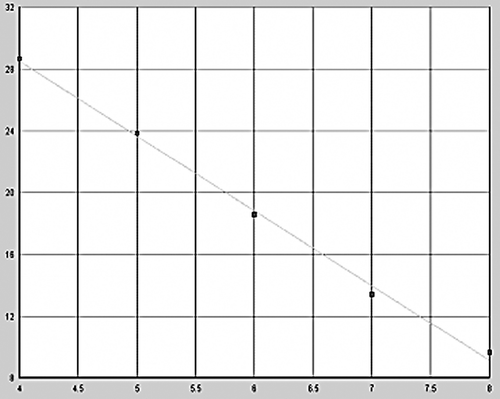
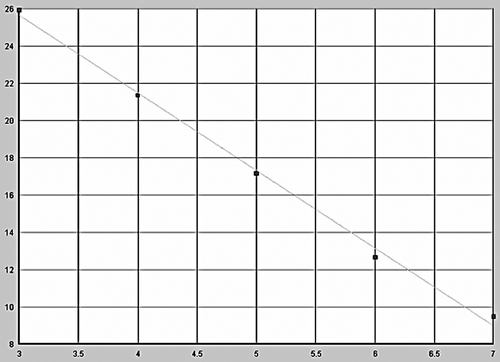
After amplification using Lightcycler fluorescent PCR system, positive samples showed a classical “S”-type curve ( and ), whereas negative controls showed an interrupted wavy line (), the correlation coefficients for standard curves of GR-α and GR-β were 0.997 and 0.996; the standard curves were produced automatically ( and ). The horizontal coordinate of the standard curve represented the logarithm of concentration of the standard and the vertical coordinate represented Ct. The regression equations of the standard curve were CtGR-α = 47.89–4.84lg [CGR-α] and CtGR-β = 38.12–4.16lg [CGR-β]
Figure 1. Fluorescent intensity curves for GR–a standard, from left to right the concentration of standard is 1×108, 1×107, 1×106 and 1×105 copies/ml.
Expression of GR-α and GR-β mRNAs
There was a lower expression of GR-α mRNA (×106 GR-α cDNA copies/µg total RNA) in thyroid adenoma (1.27±0.26) than that in normal thyroid gland (3.53±1.22) (p < 0.001). The expression of GR-β mRNA was lower in all the thyroid tissues, and significant difference was found in GR-β mRNA expression (×104 GR-β cDNA copies/µg total RNA) between thyroid adenoma (80.8±13.9) and thyroid gland (1.78±0.59) (p < 0.001). The GR-α /GR-β ratios in thyroid adenoma and normal thyroid gland were 1.67±0.68 and 207.57±84.41 respectively (p < 0.001).
Discussion
The present study showed that both GR-α and GR-β mRNA expression were detectable in the thyroid gland and adenoma tissues. The expression of GR-α mRNA was much higher and is about 208 times than that of GR-β mRNA in thyroid glands. Furthermore, we showed that GR-α mRNA expression decreased and GR-β mRNA expression increased remarkably in thyroid adenomas than that in thyroid glands, GR-α/GR-β ratio in thyroid adenoma was about two-fold.
Recent studies have characterized the expression of GR-α and GR-β in several human tissues at both the gene and the protein levels Citation[8–13]. An over expression of GR-β in pathological conditions, together with a GR-β -hsp90 unstable binding, might increase the dimerization of GR-β with GR-α and therefore inhibit GR-α activity. Increased expression of GR-β has been reported in patients with glucocorticoid-insensitive asthma Citation[13–15], ulcerative colitis Citation[16], chronic lymphocytic leukemia Citation[17], and nasal polyposis Citation[18]. Perhaps over expression of the GR-β protein contributes to the glucocorticoid insensitivity. By preventing GR-α from binding GREs, forming transcriptionally inactive GR-α/GR-β heterodimers, and/or titrating a co-activator needed by GR-α for full transcriptional activity, increased levels of GR-β would prevent GR-α from activating target genes. Exogenous glucocorticoids play an essential role in the acute and chronic therapy of many inflammatory and immune thyroid diseases because of their immunosuppressive and anti-inflammatory actions. High expression of GR-β mRNA and low ratio of GR-α/GR-β may be an important reason that thyroid adenomas are not generally treated with glucocorticoids.
The mechanisms associated with the differentially down-regulation expression of GR-α and up-regulation expressions of GR-β in thyroid adenomas are currently unknown. The regulatory mechanisms controlling steroid hormone actions are beginning to be understood at a cellular and molecular level. The potential for interaction or cross talk between the different classes of hormones, especially the ability of one hormone to regulate the abundance of the receptors for a second hormone, is being increasingly appreciated Citation[19]. The expression of estrogen receptor (ER) in human thyroid gland cells was reported Citation[20–22]. As mediators of glucocorticoid and estrogenic hormones, the GR and ER play a critical role in a diverse array of physiological processes, including metabolism, immunity, cell growth and proliferation, reproduction, and development. Both GR and ER exert important actions in tissues other than their primary target tissues. In tissues that express both receptors, glucocorticoids often oppose the actions of estrogens. Glucocorticoids exert anti-proliferative effects, whereas estrogens promote cell growth and proliferation in the mammary gland. In contrast, glucocorticoids induce bone resorption, whereas estrogens inhibit this action. Although glucocorticoids and estrogens act within the same cellular context in these biological processes, little is known about the cross talk between the GR and ER signaling pathways. As ER, GR-β antagonizes GR activity, we postulated GR-β was increasing obviously may play an important role in the thyroid adenomas.
In summary, our results revealed that both GR-α and GR-β mRNA transcripts are expressed in thyroid gland and adenoma. To our knowledge, this is the first report about the expressions of GR subunits in thyroid gland and adenoma. It seems that down-regulation of GR-α mRNA expression and up-regulation of GR-β mRNA expression may play an important role in the thyroid adenomas. The expression of protein levels of GR subunits as well as its potential significance in thyroid gland and adenoma needs further and profound investigation.
References
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G., Umesono K, et al. The nuclear receptor superfamily: The second decade. Cell 1995; 83: 835–9
- Derijk RH, Schaaf M, de Kloet ER. Glucocorticoid receptor variants: Clinical implications. J Steroid Biochem Mol Biol 2002; 81: 103–22
- Pujols L, Mullol J, Roca-Ferrer J, Torrego A, Xaubet A, Cidlowski JA, et al. Expression of glucocorticoid receptor alpha- and β-isoforms in human cells and tissues. Am J Physiol 2002; 283: 1324–31
- Kauppinen-Makelin R, Karma A, Leinonen E, Loyttyniemi E, Salonen O, Sane T, et al. High dose intravenous methylprednisolone pulse therapy versus oral prednisone for thyroid-associated ophthalmopathy. Acta Ophthalmol Scand 2002; 80(3)316–21
- Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab 2003; 88(5)2100–5
- Lo JC, Loh KC, Rubin AL, Cha I, Greenspan FS. Riedel's thyroiditis presenting with hypothyroidism and hypoparathyroidism: Dramatic response to glucocorticoid and thyroxine therapy. Clin Endocrinol (Oxf) 1998; 48(6)815–8
- Bogazzi F, Bartalena L, Cosci C, Brogioni S, Dell'Unto E, Grasso L, et al. Treatment of type II amiodarone-induced thyrotoxicosis by either iopanoic acid or glucocorticoids: A prospective, randomized study. J Clin Endocrinol Metab 2003; 88(5)1999–2002
- Oakley RH, Webster JC, Jewell CM, Sar M, Cidlowski JA. Immunocytochemical analysis of the glucocorticoid receptor [alpha] isoform (GR[alpha]) using GR[alpha]-specific antibody. Steroids 1999; 64: 742–51
- Beger C, Gerdes K, Lauten M, Tissing WJ, Fernandez-Munoz I, Schrappe M, et al. Expression and structural analysis of glucocorticoid receptor isoform gamma in human leukaemia cells using an isoform-specific real-time polymerase chain reaction approach. Br J Haematol 2003; 122: 245–52
- Boullu-Ciocca S, Paulmyer-Lacroix O, Fina F, Ouafik L, Alessi MC, Oliver C, et al. Expression of the mRNAs coding for the glucocorticoid receptor isoforms in obesity. Obes Res 2003; 11: 925–9
- DeRijk RH, Schaaf M, Stam FJ, de Jong IE, Swaab DF, Ravid R, et al. Very low levels of the glucocorticoid receptor β isoform in the human hippocampus as shown by Taqman RT-PCR and immunocytochemistry. Brain Res Mol Brain Res 2003; 116: 17–26
- Pujols L, Mullol J, Perez M, Roca-Ferrer J, Juan M, Xaubet A, et al. Expression of the human glucocorticoid receptor alpha and β isoforms in human respiratory epithelial cells and their regulation by dexamethasone. Am J Respir Cell Mol Biol 2001; 24: 49–57
- Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, et al. Increased glucocorticoid receptor β in airway cells of glucocorticoid-insensitive asthma. Am J Respir Crit Care Med 1999; 159: 1600–4
- Leung DYM, Hamid Q, Vottero A, Szefler SJ, Surs W, Minshall E, et al. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor β. J Exp Med 1997; 186: 1567–74
- Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor β-isoform. J Allergy Clin Immunol 2000; 105: 943–50
- Honda M, Orii F, Ayabe T, Imai S, Ashida T, Obara T, et al. Expression of glucocorticoid receptor β in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology 2000; 118: 859–66
- Shahidi H, Vottero A, Stratakis CA, Taymans SE, Karl M, Longui CA, et al. Imbalanced expression of the glucocorticoid receptor isoforms in cultured lymphocytes from a patient with systemic glucocorticoid resistance and chronic lymphocytic leukemia. Biochem Biophys Res Commun 1999; 254: 559–65
- Hamilos DL, Leung DYM, Muro S, Kahn AM, Hamilos SS, Thawley SE, et al. GRβ expression in nasal polyps inflammatory cells and its relationship to the anti-inflammatory effects of intranasal fluticasone. J Allergy Clin Immunol 2001; 108: 59–68
- Traynor A. Recent advances in hormonal therapy for cancer. Current Opinion Oncol 1995; 7: 572–81
- Egawa C, Miyoshi Y, Iwao K. Quantitative analysis of estrogen receptor-alpha and -beta messenger RNA expression in normal and malignant thyroid tissues by real-time polymerase chain reaction. Oncology 2001; 61(4)293–8
- Hiasa Y, Nishioka H, Kitahori Y. Immunohistochemical analysis of estrogen receptors in 313 paraffin section cases of human thyroid tissue. Oncology 1993; 50(2)132–6
- Kawabata W, Suzuki T, Moriya T. Estrogen receptors (alpha and beta) and 17beta-hydroxysteroid dehydrogenase type 1 and 2 in thyroid disorders: Possible in situ estrogen synthesis and actions. Mod Pathol 2003; 16(5)437–44