Abstract
The role of receptor activator of nuclear factor-κB ligand (RANKL)/osteoprotegerin (OPG) system, and osteopontin (OPN) was studied in patients with solid tumors metastatic to the bone in relation to the type of malignancy and the neoplastic burden to the skeleton. Levels of soluble RANKL (sRANKL), OPG and OPN were assessed in 61 patients with breast, lung and prostate cancer with newly-diagnosed metastasis to the bone, in parallel with bone resorption [C-telopeptide of type-I collagen (CTX), tartrate-resistant acid phosphatase-5b (TRACP-5b)] and bone formation markers [bone-alkaline phosphatase (bALP), osteocalcin (OC), and C-terminal propeptide of collagen type-I (CICP)]. Patients had elevated serum levels of sRANKL, OPG, OPN, TRACP-5b, and bALP, and reduced OC levels compared to controls. OPG correlated with the extent of metastatic bone burden. Patients with breast and lung cancer shared increased levels of sRANKL, OPG, and OPN whereas prostate cancer patients had elevated values of OPG and bALP only. These results suggest that patients with solid tumors metastatic to the bone have severe disruption of the sRANKL/OPG axis. Breast and lung cancer seem to exert their osteolytic action through upregulation of the sRANKL/OPG system and OPN, whereas prostate cancer seems to provoke profound elevation of OPG levels only, thus leading to increased osteoblastic activity.
The skeleton represents the most common site of tumor metastases, with at least 25% of cancer patients having bone metastases at autopsy examination Citation[1]. Most frequently, they occur with prostate, breast, lung and renal cell carcinomas (88, 73, 32 and 25% respectively) whereas patients with multiple myeloma present almost universally with bone lesions Citation[2]. Skeletal complications from bone metastases cause significant morbidity and present a major challenge in disease management Citation[3], Citation[4]. In the normal skeleton there is a tightly coordinated process of balanced osteoclast-mediated bone resorption and osteoblast-mediated bone formation that counteract and contribute to the constant remodeling of bone tissue Citation[5]. In metastatic bone disease, disruption of this balance can lead to increased bone turnover resulting in excessive osteolytic or osteoblastic activity and consequent skeletal disease.
Biochemical markers of bone remodeling, which reflect the formation and/or resorption of bone have been implemented in clinical trials in an effort to clarify tumor and bone microenvironment interactions into this dynamic process. Metastatic bone disease has been correlated with increase in “traditional” bone resorption markers, such as urinary Calcium (uCa), urinary N-terminal cross-linked telopeptides of type I collagen (NTX) and serum C-terminal cross-linked telopeptides of type I collagen (CTX), but also with more recent ones, like tartrate-resistant acid phosphatase type isoform 5b (TRACP 5b). The latter is produced exclusively by osteoclasts and has been found significantly elevated in osseous metastases reflecting skeletal tumor burden Citation[6]. On the other hand, bone formation markers, such as C-terminal propeptide of procollagen type I (CICP), N-terminal propeptide of procollagen type I (PINP) and bone-specific alkaline phosphatase (bALP) have not been consistently correlated with a specific tumor pattern and are currently considered to reflect enhanced osteoblastic activity as a secondary reaction to excessive bone resorption Citation[7]. Osteocalcin (OC) is another marker believed to reflect osteoblast activity Citation[8], while osteopontin (OPN), a multifunctional protein that has been implicated in a broad array of pathological processes involving mainly the lung, has been recently recognized as an osteoclast stimulator Citation[9].
Lately, it has been clarified that the final mediator of osteoclastogenesis is the receptor activator of nuclear factor-κB ligand (RANKL), which binds and activates its receptor RANK on the surface of osteoclasts Citation[10]. RANKL is a member of the tumor necrosis factor-superfamily and is expressed as a type II transmembrane glycoprotein or as a soluble ligand by osteoblasts, bone marrow stroma, and perhaps myeloma or other tumor cells Citation[11]. The binding of RANKL to its receptor provokes osteoclast differentiation and maturation and inhibits osteoclast apoptosis, thus leading to increased bone resorption Citation[12–14]. Osteoprotegerin (OPG) is the “decoy” receptor for RANKL and has been shown to prevent bone destruction by blocking the binding of RANKL with its receptor and thus it inhibits the osteoclast differentiation and activation Citation[15], Citation[16]. Preclinical data have documented that transgenic mice that over-express OPG develop severe osteopetrosis Citation[17] and targeted deletion of OPG leads to severe osteoporosis Citation[18]. OPG mRNA has been found ubiquitously in most body tissues, prostate cancer and predominantly osteoblastic cells Citation[19].
Hence, the system RANKL/RANK/OPG seems to play a substantial role in the above-mentioned balance of osteoblastic and osteoclastic activity induced by tumor cells. The importance of this system has been initially documented in multiple myeloma, where the RANKL/OPG ratio has been proposed as an independent prognostic factor for the disease Citation[20], but the substantial role of this system has been expanded in a subset of solid tumors also, including lung, breast and prostate cancer, as well as non-neoplastic skeletal disorders, such as Paget's disease and rheumatoid arthritis Citation[21].
Despite the considerable amount of data on markers of bone turnover and their significance in monitoring bone remodeling, the precise value of these indexes in regard to diagnosis, therapeutic response and prognosis of skeletal metastases has not been extensively studied in controlled trials. The aim of this exploratory cohort study was to clarify the role of the above mentioned molecules in relation to the type of malignancy, the pattern of bone turnover impairment and the neoplastic burden to the skeleton in patients with solid tumors and newly-diagnosed metastasis to the bone.
Patients and methods
Patients
The study enrolled adult patients with histologically or cytologically confirmed breast, lung or prostate cancer with at least one newly diagnosed osteolytic or osteoblastic lesion confirmed by diagnostic imaging (99Tc-bone scan). The inclusion criteria also included: (i) Eastern Cooperative Oncology Group (ECOG) performance status less than or equal to 2; however, patients with an ECOG performance status of 3 were allowed to participate when their activity was restricted because of the presence of bone lesions; (ii) total bilirubin <3 mg/dl; and (iii) serum creatinine <2 mg/dl to avoid the effects of secondary hyperparathyroidism on bone remodeling balance.
The exclusion criteria of this study were: (i) radiation therapy within 6 months before enrolment; (ii) diagnosis of postmenopausal osteoporosis or receipt of oestrogen supplementation therapy at the time of enrolment; (iii) administration of calcitonin, vitamin D or calcium supplements within three months from enrolment; (iv) prior therapy with bisphosphonates; (v) women in pregnancy or lactation.
The control group consisted of healthy, gender- and age-matched volunteers with no history of osteoporosis, malignancy or other metabolic disease affecting the skeleton. Postmenopausal female controls were excluded if they had been receiving hormonal supplementation therapy or steroid analogs for any reason due to the profound effects of hormonal intervention onto bone metabolic process. The study was conducted with the approval of the local ethical committee in keeping with the guidelines of the Declaration of Helsinki.
Study design
Measurement of bone remodeling markers
Blood was drawn at the time of diagnosis of the skeletal metastases and before initiation of biphosphonate therapy. After vein puncture serum was separated within four hours and stored at −80°C until the day of measurement. An enzyme-linked immunosorbent assay (ELISA) was used for the detection of serum sRANKL (Biomedica Medizinprodukte, No. BI-20422H, Gesellschaft GmbH & Co KG, Wien, Austria), OPG (Biomedica Medizinprodukte, Gesellschaft GmbH & Co KG, Wien, Austria), OPN (IBL GmbH D, Hamburg, Gemany), TRACP-5b (BoneTRAP®, SBA, Oulu, Finland), CTX (Serum CrossLaps®, Nordic Bioscience Diagnostics A/S, Herlev, Denmark), bALP (Metra® BAP, Quidel Corporation, San Diego, CA, USA), OC (N/MID® Osteocalcin, Nordic Bioscience Diagnostics A/S, Herlev, Denmark), and CICP (Metra® CICP, Quidel Corporation, San Diego, CA, USA), according to manufacturers instructions, as previously described Citation[22]. All samples from the same patient were measured on the same ELISA plate, while the assays were performed blindly.
Assessment of skeletal tumor burden
The presence of skeletal metastastes in each patient was illustrated using 99Tc-bone scan and was confirmed using supplementary diagnostic imaging (X-ray or CT-scan) in cases of ambivalent diagnosis The skeletal tumor burden in each patient was quantified using the EOD (extent of disease) scale system Citation[6] modified for the skeleton (EOsD) as follows: EOsD I for patients having few skeletal lesions (1–5), EOsD II for patients with moderate skeletal tumour burden (6–10 lesions) and EOsD III for patients having either extensive skeletal metastases (>10 lesions or diffuse cancer-related osteopenia or osteopetrosis) or had experienced pathological fracture, spinal cord compression or severe osteolysis requiring local radiotherapy to prevent fracture.
Statistical Analysis
Mean and standard deviations for each of the nine bone parameters for patients and controls were reported. Differences between patients and controls were evaluated using analysis of variance (ANOVA) and Kruskal-Wallis test. When a significant association was found, post hoc bonferroni comparisons were used and mean differences along with the corresponding 95% Confidence Intervals (CI with 95% limits of credibility) were reported. Correlation between EOsD and markers of bone remodeling was defined using paired student's t-test.
Results
Patients
Between March 2004 and August 2005, sixty-one patients entered the study (28 men, 33 women, median age: 67 years, range: 35–82). Forty healthy individuals (18 men, 22 women, median age: 65.5 years, range: 32–78 years) participated in the control group. The majority of patients had an ECOG status of 0 or 1 (74%) and 90% had already received prior chemotherapy for their malignancy (range: 1–4 cycles). Twenty patients (33%) had received locoregional radiotherapy for their disease. Thirty patients had breast cancer (median age: 63 years, range: 35–76 years), 14 had non-small cell lung cancer (NSCLC) (11 men and 3 women, median age 64 years, range 40–78) and 17 patients had prostate cancer (median age 72.5 years, range 56–82). In regard to skeletal tumour burden, 14 patients belonged to EOsD = I stage and 13 patients to EOsD = II, whereas 34 patients presented with extended skeletal lesions (EOsD = III) at the time of diagnosis of bone metastases. Patient characteristics are shown on . Mean values for each marker along with 95% confidence intervals for both patient and control groups and relevant p-values are illustrated in . Patients had elevated serum levels of osteoclastic activity markers, such as sRANKL (p = 0.004), TRACP-5b (p = 0.022) and OPN (p < 0.0001) but not CTX (p = 0.49) compared to controls. Osteoblastic activity markers were also significantly elevated, including OPG (p < 0.0001), and bALP (p < 0.0001) but not CICP (p = 0.26), whereas OC levels were significantly lower (p = 0.0001) compared to controls. Notably, there was a significant positive correlation between OPG and bALP (r = 0.35, p < 0.001), as well as between sRANKL/OPG ratio and OPN (r = 0.26, p < 0.05) and OPN with TRACP-5b (r = 0.24, p < 0.05).
Table I. Patient characteristics.
Table II. Mean values for each bone marker along with 95% confidence intervals for both the patient and control groups.sRANKL: soluble receptor activator of nuclear factor-kB ligand; OPG: osteoprotegerin, OPN: osteopontin; TRACP-5b: 5b isoenzyme of tartrate resistant acid phosphatase; CTX: C-terminal cross-linked telopeptide of type I collagen; bALP: bone alkaline phosphatase, OC: osteocalcin and CICP: C-terminal propeptide of procollagen type I.
Tumor specific analysis
Patients with breast or lung cancer and skeletal metastases followed a rather similar pattern of bone remodelling: significantly increased levels of sRANKL (mean values±SD: 1.94±0.67 pmol/L, p = 0.014 and 3.2±1.83 pmol/L, p = 0.005, for breast and lung cancer, respectively) and OPG (8.3±1.91 pmol/L, p = 0.014 and 9.97±3.12 pmol/L, p = 0.0046, respectively) compared to the control group that led to an equally elevated sRANKL/OPG ratio for this subgroup of patients (30.5±13.69×10−2, p = 0.014 and 36.6±8.64×10−2, p = 0.005 for breast and lung cancer patients, respectively) []. TRACP-5b levels were significantly elevated in the breast cancer group (2.45±0.43 U/L, p = 0.032) but not in the lung cancer group (1.87±0.28 U/L, p = 0.43). A marked elevation was disclosed in OPN levels especially in patients with lung cancer (116.88±62.76 ng/mL, p = 0.0005 and 175.52±33.47 ng/mL, p < 0.0001, for breast and lung cancer, respectively) in comparison with the control group. On the contrary, CTX levels remained rather unchanged in both the breast and lung cancer group (0.4±0.1 ng/mL, p = 0.1 and 0.46±0.04 ng/mL, p = 0.23, respectively). Interestingly, OC levels appeared to be reduced in breast cancer patients compared to the control group and rather stable in lung cancer patients (10.95±2.87 ng/mL, p = 0.005 and 17.15±2.54 ng/mL, p = 0.29, respectively), whereas CICP levels were marginally elevated only in the lung cancer group (8.44±3.98 ng/mL, p = 0.07). Finally, bALP levels were significantly elevated in both groups compared to the control group (50.63±14.27 U/L, p < 0.0001 and 40.48±10.22 U/L, p = 0.015 for breast and lung cancer patients respectively).
Figure 1. Comparative distribution of bone marker levels for each of the patient subgroup (lung, breast, prostate) and the control group. Statistical relevance for each of the patient subgroup is indicated by p-value. Each box illustrates the median value, the quartiles and the extreme values within each category. sRANKL: soluble receptor activator of nuclear factor-kB ligand; OPG: osteoprotegerin, OPN: osteopontin; TRACP-5b: 5b isoenzyme of tartrate resistant acid phosphatase; CTX: C-terminal cross-linked telopeptide of type I collagen; bALP: bone alkaline phosphatase, OC: osteocalcin and CICP: C-terminal propeptide of procollagen type I.
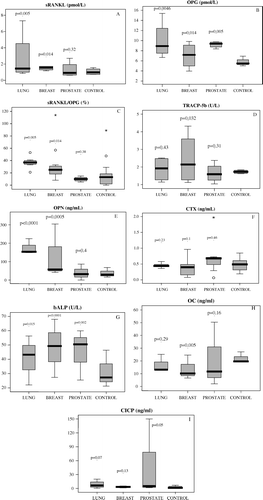
On the contrary, a significant rise in the levels of OPG (9.33±0.46 pmol/L, p = 0.005), which was not accompanied by a comparable increase in sRANKL levels (1.24±0.3 pmol/L, p = 0.32) was observed in patients with prostate cancer; this result led to a moderate suppression of the sRANKL/OPG ratio (mean value 9.6×10−2, CI = 8.38–10.82, p = 0.38) compared to the control group. Furthermore, sRANKL/OPG ratio levels in prostate cancer patients were significantly lower than these of breast and lung cancer patients (p = 0.0005 and 0.0001 respectively). Other markers of bone formation were significantly upregulated too, including bALP (50.46±31.5 U/L, p = 0.002) and CICP (33.54±16.61 ng/mL, p = 0.05), but not OC (15.13±7.4 ng/mL, p = 0.16). On the other hand, bone resorption markers remained downregulated in levels similar to these of the control group, including TRACP-5b (1.78±0.2 U/L, p = 0.31), CTX (0.68±0.28 ng/mL, p = 0.46) and OPN (29.21±12.38 ng/mL, p = 0.4) ().
EOsD specific analysis
The specific analysis of the correlation of bone turnover markers with the extent of skeletal disease () revealed that even patients with relatively low skeletal tumor burden (EOsD I) presented with significantly higher levels of bone turnover markers compared to the control group, including OPG (p = 0.007), TRACP-5b (p = 0.01), bALP (p = 0.0003) and OPN (p < 0.0001) (). OPG, in particular, seems to be the only marker to correlate adequately with the extent of metastatic tumour burden. Patients with EOsD II had significantly higher levels of OPG than patients with EOsD I (9.82±0.96 pmol/L and 7.99±0.52 pmol/L, respectively, p = 0.04) and patients with EOsD III had significantly higher levels of OPG (11.79±3.42 pmol/L) compared to patients with EOsD I (p = 0.015) and EOsD II (p = 0.04) ().
Figure 2. Comparative distribution of bone marker values for patients with extend of skeletal disease category I (EOsD I) and for the control group. 2A: OPG, 2B: TRACP-5b, 2C: bALP, 2D: OPN and 2E: Comparative distribution of Osteoprotegerin values for patients with EOsD I, II and III. Statistical relevance is defined by the p-value for each marker. Each box illustrates the median value, the quartiles and the extreme values within each category.
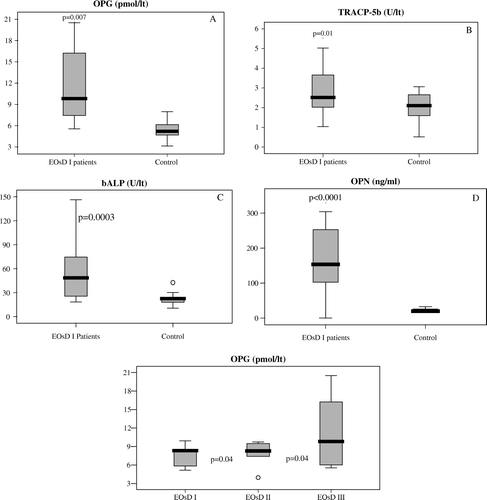
Table III. Mean values along with 95% confidence intervals for each bone marker for all the EOsD subgroups and the control group.sRANKL: soluble receptor activator of nuclear factor-kB ligand; OPG: osteoprotegerin, OPN: osteopontin; TRACP-5b: 5b isoenzyme of tartrate resistant acid phosphatase; CTX: C-terminal cross-linked telopeptide of type I collagen; bALP: bone alkaline phosphatase, OC: osteocalcin and CICP: C-terminal propeptide of procollagen type I.
Discussion
An emerging bulk of evidence emphasizes the crucial role of feedback interactions between tumor cells and bone marrow microenvironment leading to the establishment of a vicious circle which acts by upregulating the physiological mechanisms that normally favor bone resorption. Many researchers have reported that tumor cells mainly express RANKL when they adhere to the bone microenvironment Citation[12], Citation[13]. The nuclear factor kappa B, which is the one of the final transcriptional targets of the RANKL/RANK pathway, plays a key role in the induction of pro-inflammatory gene expression, leading to the synthesis of cytokines, adhesion molecules, chemokines, growth factors and enzymes Citation[23].
In the present study, when all patient groups are taken together, our results suggest that imbalance of the physiological bone remodeling process is mediated by severe disruption of the sRANKL/OPG system towards either osteolysis or bone formation resulting in subsequent changes in the levels of the bone turnover markers. Levels of OPG, in particular, could safely predict the extent of skeletal tumor burden (). Our results also suggest that in patients with breast and lung cancer there is a severe disruption in RANKL/OPG axis in favor of RANKL leading to increased osteoclast function. Subsequent rise in the levels of TRACP-5b and OPN reflect excessive bone resorption in this subset of patients, whereas increase in bALP and OPG levels possibly represents a compensatory effect of reactive bone formation, which, however, cannot counterbalance the increased bone destruction. On the contrary, OC was found to be down regulated, especially in the breast cancer subgroup, probably reflecting inefficient osteoblastic activity in these patients. Nevertheless, all the measured patient and control values of OC were within normal limits according to the manufacturer.
OPN levels were excessively elevated in lung cancer patients, confirming the close relation of this cytokine to a broad spectrum of lung diseases, such as pulmonary granuloma, fibrosis, and malignancy Citation[9]. Fedarko et al. proved that elevated levels of OPN could enable the diagnosis of lung, breast, prostate and colon cancer even without radiological evidence of osseous metastases Citation[24]. The last remark is very important since it implies a significant role of OPN in the biology of cancer even if it does not directly involve the bone. Schneider et al. reported recently that OPN mRNA expression is an independent prognostic marker in curatively resected NSCLC Citation[25].
Patients with prostate cancer metastatic to the skeleton seem to follow a rather different pattern of bone turnover with predominance of bone formation, reflected by increased levels of bALP and CICP, resulting in the well-defined osteoblastic lesions. Prostate cancer cells seem to provoke profound elevation of OPG only, resulting in moderate suppression of the sRANKL/OPG ratio with subsequent increase in bone formation markers. It is thought today that the tumor microenvironment can release high amounts of OPG to counterbalance the high RANKL concentration produced by tumor cells. OPG acts in this case as a “decoy” receptor of RANKL and must therefore be considered as a “protector” of bone Citation[19]. Recently, promising results of a phase I study using recombinant OPG in patients with multiple myeloma or patients with breast cancer-related bone metastases were reported Citation[26] and the future will show whether OPG has a therapeutic potential in this area.
Several considerations have been raised regarding the results of bone markers’ measurements. It must be noted that absolute changes in marker values are often misleading if the interpretation does not take into account the respective marker's analytical and biological variability. It has recently been reported that serum levels of CTX and OC follow a circadian rhythm as a result of diurnal variation of cortisole in both breast cancer patients and healthy controls Citation[27]. This fact could possibly explain the failure of these two markers to correlate adequately with increased osteoclastic and osteoblastic activity, respectively, in the present study. Fohr et al. suggested that a change of 30% in a bone formation marker should be considered significant, whereas for most bone resorption markers, the least significant change should be at least 60% because of their higher coefficient variation Citation[9].
A consideration of this study is the low number of patients, especially in the lung cancer subgroup (n = 14). Nevertheless, apart from bALP, all the other p-values are so highly significant (0.005 for sRANKL, 0.0046 for OPG, 0.005 for sRANKL/OPG ratio and < 0.0001 for OPN) that override the disadvantage of the low number of the patients for this subgroup. The impact of previously received or concurrent chemotherapy (CT) and whether or not hormonal supplements or targeted hormonal therapies (estrogens, anti-estrogens, anti-androgens, aromatase inhibitors and LHRH analogs) were included in the treatment regimen is also an issue of major concern, due to the profound effects of hormonal intervention onto bone metabolic process Citation[28]. Marker levels might also be expected to change during the course of the disease, either in response to the effects of antineoplastic therapy-which may obscure the original differences in marker levels between patients and controls- or due to disease progression or regression Citation[4]. In patients with breast cancer metastatic to bone, a rise in serum OC or bALP after CT has been associated with local recalcification and therefore considered as a sign of therapeutic success Citation[29]. Recent data indicate that high baseline levels of OC could be predictive of better progression-free survival in patients with hormone-refractory prostate cancer Citation[30]. Increased uNTX levels have been recently shown to correlate negatively with clinical outcome and with 2-fold increase in the risk for skeletal complications and disease progression Citation[31].
In conclusion, skeletal morbidity remains a major problem in cancer patients and many aspects of the pathophysiology of malignant bone disease have not yet been fully clarified. Although the central role of the sRANKL/OPG system in both physiological and pathological bone remodeling process has been elucidated, it remains unlikely that a single marker has sufficient diagnostic or prognostic value in malignant bone disease. Further larger-scale studies to assess the clinical utility of the combination of these markers with other laboratory tests (e.g. tumor markers) or imaging techniques are therefore required. Furthermore, important clinical questions regarding the use of these markers in monitoring bisphosphonate therapy or their prognostic value in predicting response to antineoplastic chemotherapy remain to be addressed.
This study was supported by an unrestricted grant from the Hellenic Society of Medical Oncology (HSMO).
References
- Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–93
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001; 411: 375–9
- Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Clin Orthop Relat Res 2003; 415(Suppl): S138–47
- Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer and other solid tumours. J Natl Cancer Inst 2005; 97: 59–69
- Coleman RE. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001; 27: 165–76
- Koizumi M, Takahashi S, Ogata E. Comparison of serum bone resorption markers in the diagnosis of skeletal metastasis. Anticancer Res 2003; 23: 4095–9
- Coleman RE. The clinical use of bone resorption markers in patients with malignant bone disease. Cancer 2002; 94: 2521–3
- Fohr B, Dunstan C, Seibel M. Markers of bone remodeling in metastatic bone disease. J Clin Endocrinol Metab 2003; 88: 5059–75
- O’ Regan A. The role of osteopontin in lung disease. Cytokine Growth Factor Rev 2003; 14: 479–88
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 1999; 96: 3540–5
- Politou M, Terpos E, Anagnostopoulos A, Szydlo R, Laffau M, Layton M, et al. Role of receptor activator of nuclear factor-kappa B ligand (RANKL),osteprotegerin and macrophage protein 1-alpha (MIP-1α) in monoclonal gammopathy of undetermined significance (MGUS). Br J Haematol 2004; 126: 686–9
- Nagai M, Kyakumoto S, Sato N. Cancer cells responsible for humoral hypercalcaemia express mRNA encoding a secreted form of ODF/TRANCE that induces osteoclast formation. Biochem Biophys Res Commun 2000; 269: 532–6
- Huang L, Cheng YY, Chow LT, Lee KM, Zheng MH. Tumor cells produce receptor activator of NF-κB ligand (RANKL) in skeletal metastases. J Clin Pathol 2002; 55: 877–8
- Milligan SA, Nopajaroonsri C. Inhibition of NF-(B with proteasome inhibitors enhances apoptosis in human lung adenocarcinoma cells in vitro. Anticancer Res. 2001; 21: 39–44
- Hofbauer LC, Neubauer A, Heufelder AE. Receptor activator of nuclear factor kappa B ligand and osteprotegerin: Potential implications for the pathogenesis and treatment of malignant bone diseases. Cancer 2001; 92: 460–70
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997; 89: 309–19
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPG is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999; 397: 315–23
- Bucay N, Sarosi I, Dunstan CR, Morony S, Zarpley J, Capparelli C, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998; 12: 1260–8
- Jung K, Lein M, von Hosslin K, Brux B, Schnorr D, Loening SA, et al. Osteoprotegerin in serum as a novel marker of bone metastatic spread in prostate cancer. Clin Chem 2001; 47: 2061–3
- Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, et al. Soluble receptor activator of nuclear factor kappa B ligand (RANKL)/osteoprotegerin (OPG) ratio predicts survival in multiple myeloma. Proposal for a novel prognostic index. Blood 2003; 102: 1064–9
- Goltzman D, Karaplis AC, Kremer R, Rabbani SA. Molecular basis of the spectrum of skeletal complications of neoplasia. Cancer 2000; 88: 2903–8
- Terpos E, Mihou D, Szydlo R, Tsimirika K, Karkantaris C, Politou M, et al. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodelling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia 2005; 19: 1969–76
- Terpos E, Dimopoulos MA. Myeloma bone disease: Pathophysiology and management. Ann Oncol 2005; 16: 1223–31
- Fedarko NS, Jain A, Karadag A, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate and lung cancer. Clin Cancer Res 2001; 7: 4060–6
- Schneider S, Yochim J, Brabender J, Uchida K, Danenberg KD, Metzger R, et al. Osteopontin but not osteonectin messenger RNA expression is a prognostic marker in curatively resected non-small cell lung cancer. Clin Cancer Res 2004; 10: 1588–96
- Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct in patients with multiple myeloma or breast-cancer related bone metastases. Cancer 2003; 97: 887–92
- Generali DG, Tedoldi S, Tampellini M. Circadian rhythm of bone turnover markers in breast cancer patients with bone metastases and in control subjects. ASCO annual meeting 2005; 737(Suppl): 116–7
- Lonning P, Geisler J, Krag LE, Erikstein B, Bremmes Y, Hagen AI, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol 2005; 23: 5126–37
- Piovesan A, Berruti A, Torta M, Connone R, Sperone P, Panero A, et al. Comparison of assay of total and bone-specific alkaline phosphatase in the assessment of osteoblast activity in patients with metastatic bone disease. Calcif Tissue Int 1997; 61: 362–9
- Lara PN, Longmate J, Stadler W. Markers of bone metabolism predict survival in hormone refractory prostate cancer (HRPC): Results from a randomized California Cancer Consortium & Univ. of Chicago trial. ASCO annual meeting ;(Suppl) 2005; 4569: 423–4
- Coleman R, Major P, Lipton A, Brown JE, Lee KA, Smith M, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the biphosphonate zoledronic acid. J Clin Oncol 2005; 23: 4925–35