To the Editor
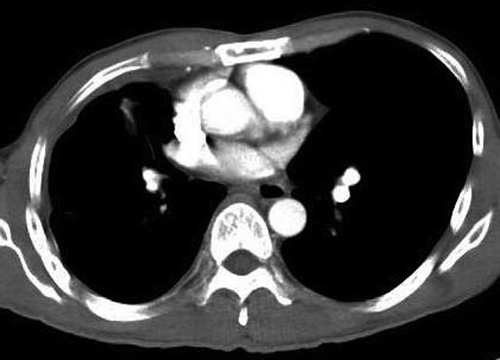
A 47 year old male non-smoker was diagnosed with advanced adenocarcinoma of the right lung in February 2005. He presented with shortness of breath and was found to have a right pleural effusion. He underwent a thoracocentesis and pleural biopsy. Both pleural fluid cytology and pleural biopsy confirmed the presence of malignant cells with cytoplasmic vacuoles indicating adenocarcinoma. Serum carcinoembryonic antigen (CEA) level was 129 µg/L. Computed tomography (CT) scan of the thorax showed a right middle lobe mass with right pleural effusion (). He underwent esophagogastroduodenoscopy and colonoscopy which excluded a primary tumor in the gastrointestinal tract. The disease progressed after first line treatment with gemcitabine and carboplatin. Serum CEA level rose to 312 µg/L.
Figure 1. Computed Tomography of the thorax showing a right middle lobe mass with a moderate right pleural effusion (at diagnosis).
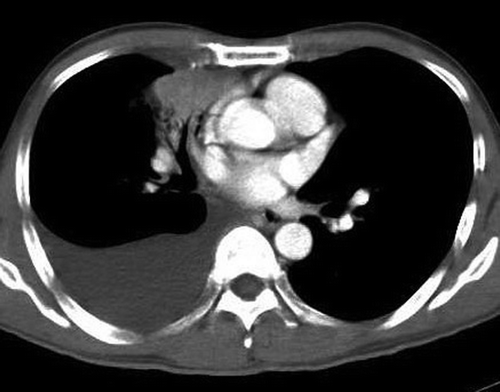
One month after progression from chemotherapy, he was started on erlotinib (TarcevaTM) 150 mg/day monotherapy. He developed fever, cough and increasing breathlessness twelve days after taking erlotinib. Chest x-ray showed a moderate right pleural effusion, which had increased compared to the pre-treatment scan. Erlotinib was stopped and antibiotics started. Four days later, his symptoms did not improve and a CT scan of the thorax showed that the right pleural effusion had increased in size and caused a shift in the mediastinum (). There was no evidence of interstitial pneumonitis in the left lung. Serum CEA level had dropped to 84.4 µg/L. He underwent thoracoscopic drainage of the massive right pleural effusion, followed by talc pleurodesis. Pleural fluid collected during drainage showed a mixed inflammatory yield of lymphocytes and neutrophils without evidence of malignant cells. Two pleural biopsies revealed only fibrinopurulent exudates with no malignant cells. Bacteriology of urine, pleural fluid and blood samples did not yield any bacterial growth. Erlotinib was re-started one week after stopping. His latest serum CEA level was 15.9 µg/L and the recent CT scan evaluation showed reduction in size of the primary tumor (). The patient had partial response and is still currently on erlotinib treatment.
Figure 2. Computed Tomography of the thorax showing a large right pleural effusion with shift of the mediastinum to the left (14 days after treatment with erlotinib).
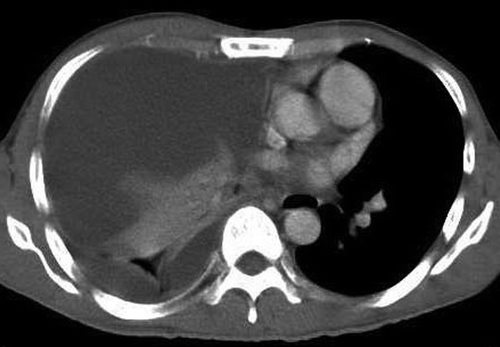
Figure 3. Computed Tomography of the thorax showing remnant of the right middle lobe mass and a right pleural effusion (4 months after treatment with erlotinib).
A significant proportion of patients with lung cancer have malignant pleural effusions. Increased pleural effusion often portends progression of disease. At times, it may be a result of concomitant pneumonia and only occasionally, it is due to adverse effects of treatment with chemotherapeutic agents, of which docetaxel is the main culprit Citation[1]. Progressive development of peripheral edema and non-malignant effusions were described with docetaxel and studies have suggested that it is a result of microtubule disruption causing abnormal capillary permeability with transudation of protein into the interstitium Citation[2–4]. Administration of corticosteroid prophylaxis has reduced the severity and onset of this cumbersome complication which can be moderately relieved with diuretics. However, increasing non-malignant pleural effusions as a result of treatment with an oral epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor has not been reported.
Here, we report a case of markedly increased pleural effusion in a patient who was responding to erlotinib, an oral EGFR tyrosine kinase inhibitor. An infective cause was excluded, as all blood and pleural fluid cultures were negative for bacteria. Clinically, our patient was also non-toxic. The other differential at presentation of increased pleural effusion was whether he had progressive disease. In view of the fact that he only received erlotinib 150 mg daily for 12 days, it would have been presumptive to assume that he had disease progression within such a short period of time. The significant drop in his serum CEA level was further evidence to prove that our patient was responding to erlotinib. The response was also subsequently confirmed on the CT scan evaluation of the primary tumor.
The symptoms started 12 days after the initiation of erlotinib. In the review by Kataoka et al. on interstitial lung disease (ILD) related to gefitinib, the authors found that the median time to onset of ILD was 15 days Citation[5]. An inflammatory response to treatment has been postulated as part of the mechanisms of ILD. We postulated that the mechanism underlying the increased pleural effusion in our patient is also related to an inflammatory response. This is supported by the following: 1) pleural fluid was filled with inflammatory cells, 2) pleural biopsy showed fibrinopurulent material, and 3) no clinical evidence of sepsis or an identified infectious agent. To support our hypothesis that there is significant inflammatory response, we measured the levels of cytokines in the pleural fluid. Interleukin-12 (IL-12) was not detected while interleukin-8 (IL-8) was present at a very high titre, 2 ng/mL, and interleukin-10 (IL-10) was detected at a level of 700 pg/mL. IL-8 is a proinflammatory molecule that has been shown to promote neutrophil chemotaxis as well as recruitment of lymphocytes in human disease Citation[6]. Pace et al. compared IL-8 concentrations in pleural effusions of patients with congestive heart failure, tuberculosis and cancer Citation[7]. They found that the level of IL-8 is the highest in pleural effusions of patients with cancer and suggested that both pleural structural and cancer cells can produce this cytokine. The IL-8 concentration in the pleural fluid of our patient is much higher than the 12 patients with cancer in the study by Pace et al. It is plausible that treatment with erlotinib resulted in an excellent response such that there was massive release of IL-8 from lysis of the cancer cells, resulting in an inflammatory cascade with increased pleural effusion. It is known that the subtype of T-helper (Th) cell response, Th1, may be deficient in patients with cancer which led to the development of stimulation of Th1 response in cancer immunotherapy Citation[8]. This is consistent with the finding in our patient that IL-12, a mediator of Th1 response, is undetectable while IL-10, an inhibitor of cytokine production by Th1 cells, was present at moderate amounts Citation[9].
There is evidence to suggest that an immune response against lung cancer may exist, as suggested by improved survival of patients who develop empyema and the isolation of tumor-infiltrating lymphocytes in lung cancer Citation[10], Citation[11]. This inflammatory response with increased pleural effusion in our patient may be part of an immune reaction towards fighting the cancer cells or simply just an immune-mediated response towards the by-products of tumor lysis as a result of treatment.
Fortunately, unlike ILD, this phenomenon of increased pleural effusion was not life threatening in our patient and re-challenging with the same drug did not result in similar events. We propose that a course of corticosteroids may have been helpful in the control of increasing pleural effusion in such patients, as it is likely to be an inflammatory phenomenon. Although this event is rare, we did encounter another patient with NSCLC treated with gefitinib who demonstrated an increased pleural effusion while other metastatic sites showed response. Unfortunately, she passed away from a myocardial infarct shortly after thoracoscopic drainage and further details could not be obtained.
Although this phenomenon is uncommon, it is important to recognize that not all increased pleural effusion is a manifestation of disease progression when patients are on treatment with small molecule EGFR tyrosine kinase inhibitors. Such patients should not be denied the continued usage of an oral EGFR tyrosine kinase inhibitor that could result in meaningful prolonged survival.
References
- Gelmon K. The taxoids: Paclitaxel and docetaxel. Lancet 1994; 344: 1267–72
- Schrijvers D, Wanders J, Dirix L, Prove A, Vonck I, van Odsterom A, et al. Coping with the toxicities of docetaxel (Taxotere). Ann Oncol 1993; 4: 610–1
- Semb KA, Aamdal S, Øian P. Capillary protein leak syndrome appears to explain fluid retention in cancer patients who receive docetaxel treatment. J Clin Oncol 1998; 16: 3426–32
- Brønstad A, Berg A, Reed RK. Effects of the taxanes paclitaxel and docetaxel on edema formation and interstitial fluid pressure. Am J Physiol Heart Circ Physiol 2004; 287: H963–H968
- Kataoka, K, Taniguchi, H, Hasegawa, Y, Kondoh, Y, Knonura, T, Nishiyama, O, et al. Interstitial lung disease associated with gefitinib. Respir Med 2005;29, (Epub).
- Anthony VB, Godbey SW, Kunkel SL, Hott JW, Hartman DL, Burdick MD, et al. Recruitment of inflammatory cells to the pleural space: Chemotactic cytokines, IL-8, and monocyte chemotactic peptide-1 in human pleural fluids. J Immunol 1993; 151: 7216–33
- Pace E, Gjomarkaj M, Melis M, Profita M, Spatafora M, Vignola AM, et al. Interleukin-8 induces lymphocyte chemotaxis into the pleural space: Role of pleural macrophages. Am J Respir Crit Care Med 1999; 159: 1592–9
- Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 2005; 54: 721–8
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 1991; 146: 3444–51
- Ruckdeschel JC, Codish SD, Stranahan A, McKneally MF. Postoperative empyema improves survival in lung cancer: Documentation and analysis of a natural experiment. N Engl J Med 1972; 287: 1013–7
- Wei YQ, Hang ZB. In situ observation of lymphocyte-tumor cell interaction in human lung carcinoma. Immunol Invest 1989; 18: 1095–105