To the Editor
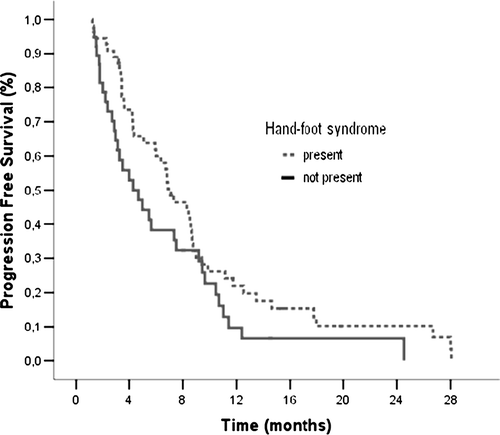
Hand-foot syndrome (HFS) is the most commonly reported adverse effect of capecitabine in metastatic breast cancer (MBC) patients, ultimately requiring either dose reduction or extension of interval between cycles or discontinuation of treatment. A negative effect on survival has not been reported in patients in whom doses were decreased due to HFS, and similarly, HFS has no known effect on the prognosis Citation[1–5]. We conducted a retrospective study to determine any effects of HFS on the course of disease in patients with MBC who were given capecitabine between December 2001 and December 2005. A total of 94 women were included in the final analysis. The median age was 50.5 years (range 32–83), and 93% of the patients had previously received anthracycline and taxane containing chemotherapy protocols. Seventy-seven patients had been given 5-fluorouracil. The median period from the time of diagnosis with breast cancer and capecitabine administration was 4.2 years (range 0.6–21.9). Most patients (42%) had Stage III disease at diagnosis, and while 51 patients had received ≤2 different chemotherapy regimens, 43 patients had >2 regimens administered. Capecitabine, given every three weeks at a dose of 2500 mg/m2/day for 14 days, was utilized medianly as a 3rd line chemotherapy regimen (range 1–9), with a median of six cycles (range 2–24). The median number of capecitabine cycles was 6 (range 2–24) in HFS group and 5 (range 2–22) in other group (p = 0.014). The overall response rate was 51% (4% complete response and 47% partial response), while progressive and stabile disease rates were 21% and 28%, respectively. The median overall survival was 17.5 months (95% CI: 11.7–23.3) and the median progression free survival (PFS) was 6.7 months (95% CI: 4.9–8.6). Fifty-five of the 94 patients (59%) developed HFS, while adverse reactions requiring dose reduction occurred in 31% of patients. In those who developed HFS, 42% had grade 3 toxicity. Their median PFS was 7.1 months (95% CI: 5.3–8.9), when compared to the 4.3 months (95% CI: 2.2–6.4) in those who did not have HFS (p = 0.058) (). The difference in overall survival between the two groups, however, was insignificant (). Patients with grade 1–2 HFS had a median PFS of 6.4 months (95% CI: 4.8–8.0), while the median PFS for those with grade 3 HFS was 8.4 months (95% CI: 5.7–11.1, p = 0.107). The median OS in the grade 3 group was also significantly higher when compared to those with grade 1–2 HFS (29.4 vs 16.0 months, p = 0.023). HFS occurred more often in progesterone positive patients and in those who had not previously received 5-FU. There was no significant difference between the two groups with regards to menopausal status, age, stage of the disease, presence of estrogen receptor, c-erb B2, site and number of metastasis, and chemotherapy or hormone therapy administered before capecitabine. These results suggest that HFS may be a valuable tool that could help us to evaluate and monitor the efficacy of capecitabine. However, our understanding of HFS etiology remains limited, and more studies are required before we can draw any conclusions or make recommendations regarding its value in the clinical use of capecitabine [1].
Table I. Patient characteristics
References
- Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 1999; 17: 485–93
- Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast cancer patients. Cancer 2001; 92: 1759–68
- Fumoleau P, Largillier R, Clippe C, Dieras V, Orfeuvre H, Lesimple T, et al. Multicentre, Phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer 2004; 40: 536–42
- Reichardt P, Von Minckwitz G, Thuss-Patience PC, Jonat W, Kolbl H, Janicke F, et al. Multicenter phase II study of oral capecitabine (Xeloda®) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol 2003; 14: 1227–33
- Wist EA, Sommer HH, Ostenstad B, Risberg T, Bremnes Y, Mjaaland I. Oral capecitabine in anthracycline- and taxane-pretreated advanced/metastatic breast cancer. Acta Oncol 2004; 43: 186–9