Abstract
The aim of this study was to evaluate the treatment outcomes of stereotactic body radiation therapy for treating primary or metastatic thoracic tumors using a stereotactic body frame. Between January 1998 and February 2004, 101 lesions from 91 patients with thoracic tumors were prospectively reviewed. A dose of 10–12 Gy per fraction was given three to four times over consecutive days to a total dose of 30–48 Gy (median 40 Gy). The overall response rate was 82%, with 20 (22%) complete responses and 55 (60%) partial responses. The one- and two-year local progression free survival rates were 90% and 81%, respectively. The patients who received 48 Gy showed a better local tumor control than those who received less than 48 Gy (Fisher exact test; p = 0.004). No pulmonary complications greater than a RTOG toxicity criteria grade 2 were observed. The experience of stereotactic body frame based radiation therapy appears to be a safe and promising treatment modality for the local management of primary or metastatic lung tumors. The optimal total dose, fractionation schedule and treatment volume need to be determined after a further follow-up of these results.
Stereotactic radiosurgery (SRS) is a high precision radiation therapy that has been widely used to treat intracranial metastatic tumors Citation[1–3]. The local tumor control rate was very high with several thousand patients being treated using this technique. A prerequisite for successful treatment is an exact localization of the lesion using a stereotactic head frame, which is attached firmly to the skull by pins. In contrast to intracranial lesions, it is difficult to use this technique for extracranial tumors owing to the set-up uncertainty and the patient's movement. Recently, a stereotactic body frame was designed at the Karolinska Hospital Citation[4] and this technique was expanded to treat the extracranial tumors in the thorax and abdomen Citation[5]. Since then, many authors have used extracranial stereotactic radiotherapy for treating primary or metastatic lung tumors with various doses and fractionation schedules and have reported its safety and efficacy for local tumor control Citation[6–11]. Some investigators have also reported promising results for inoperable stage I non-small cell lung cancer, which suggests that stereotactic radiotherapy might be an alternative to a surgical resection in early stage lung cancer patients with an advanced age, poor pulmonary function, and an acute or systemic illness Citation[6], Citation[7], Citation[11].
We have employed extracranial fractionated stereotactic body radiation therapy using a stereotactic body frame at the Asan Medical Center to treat various tumors particularly in the lung since January 1998 and have described the treatment results previously Citation[12], Citation[13]. This paper reports our further experience using this technique for managing primary or metastatic lung tumors.
Methods and materials
Patients population
Between January 1998 and February 2004, 101 lesions in 91 patients with primary or metastatic thoracic tumors were treated using a fractionated stereotactic body radiation therapy technique at the Department of Radiation Oncology, Asan Medical Center. The eligibility criteria for the primary lung cancer patients were a histologically confirmed diagnosis of non-small cell lung cancer, a stage I (T1-2N0M0) tumor or a recurrent pulmonary nodule after a curative treatment, a relatively small tumor size (< 5 cm in diameter), a medical contraindication to surgery or the refusal of surgery by the patient, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. The eligibility criteria for metastatic lung cancer were a tumor size <5 cm in diameter, 1–3 pulmonary nodules, an ECOG performance status of 0 – 2, and a stable primary tumor. Thirty-eight patients with primary lung cancers and 53 with metastatic lung cancers were included and analyzed. Of the 38 patients with the primary lung cancers, 21 were treated with a definitive aim (medically inoperable, n = 16; refusal of surgery, n = 5) and the remaining 17 were treated with a salvage aim after diagnosing the recurrence of the tumor. Of the 21 patients with the primary non-small cell lung cancers, 13 patients were diagnosed with T1N0M0 and eight patients were diagnosed with T2N0M0 stage by the American Joint Committee on Cancer (AJCC) TNM staging system (squamous cell carcinoma, n = 11; adenocarcinoma, n = 6; non-small cell lung cancer not specified, n = 4). In the 53 patients with metastatic lung cancers, the primary site was lung cancer in 14, a primary liver tumor in 12, colorectal cancer in ten, esophageal cancer in four, tracheal cancer in four, head and neck cancer in two, uterine cervical cancer in two, gastric cancer in one, anal cancer in one, pleural mesothelioma in one, breast cancer in one, and a soft tissue sarcoma of the thigh in one. Prior to treatment, all the patients had a complete medical history and a physical examination, full blood counts and biochemical profile, cardiovascular system and pulmonary function test, high-resolution thorax computed tomography (CT) scans, and/or a whole body bone scan. In 13 of 21 primary lung cancer patients, a 2-[F-18] fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET) scan was taken in order to exclude those patients with a mediastinal lymph node and hematogenous distant metastasis. Informed consent was obtained from all patients, and summarizes the patient characteristics.
Table I. Patient characteristics of the primary or metastatic thoracic tumors
Treatment planning and treatment procedures
The basic concept of extracranial stereotactic body radiation therapy at our hospital was based on the method reported by Lax and Blomgren Citation[4], Citation[5] and the detailed radiosurgery procedure information was described elsewhere Citation[12], Citation[13]. All the patients were fixed with a vacuum pillow using a stereotactic body frame (Stereotactic Body Frame®, Elekta, Sweden). Six point set-up markings were marked on the anterior or posterior chest wall using a chest marker and two reference lines were drawn on the lower leg using a leg laser marker. The respiratory and cardiac movement was then observed using simulator fluoroscopy. The respiratory movement was restricted using a diaphragm controller when the tumor movement was > 5 mm. Recently, in order to further restrict the tumor movement, an active breathing control (ABC) apparatus developed at the William Beaumont Hospital was used particularly in those patients whose tumors were located in the lower lobe Citation[14], Citation[15]. The clinical target volume (CTV) was defined on the lung-window setting CT axial image by encompassing the small spiculate area around the gross tumor volume. The planning target volume (PTV) was defined from the CTV by a 5 mm safety margin in the axial plane and an approximately 10 mm margin in the longitudinal direction in order to compensate for respiratory motion. Treatment planning was carried out using a 3-D planning system (Render Plan, Elekta, Sweden) with coplanar and/or non-coplanar radiation beams.
Initially, the total dose of this study was 30 Gy, which was given in 3 fractions on consecutive days. After confirming the safety of the initial protocol, the total dose was increased to 4 fractions of 10 Gy from May 2000, and the total dose was further increased to 48 Gy in 4 fractions on consecutive days from June 2003 to increase the probability of tumor control.
Evaluation of the treatment results
Follow-up examinations were carried out in all patients. After completing treatment, the serial chest CT scans taken one month and every three months thereafter were used to evaluate the result and/or 18FDG-PET scans were taken one month after the radiotherapy. The survival time was determined from the first day of stereotactic treatment and the local progression free survival and overall survival were calculated using the Kaplan-Meier method Citation[16]. The significance of total doses in respect to local control was assessed by Fisher's exact test and the dose-response (local control) relationship was evaluated by logistic regression analysis. The acute and late toxicity were defined according to the RTOG toxicity criteria. The treatment response was defined as follows: a complete response was defined as the complete disappearance of all known tumors. A partial response was defined as a reduction in tumor size of at least 50%. Stable disease was defined as no change or a < 50% reduction and progressive disease was defined as an increase in the tumor volume. The local control (progression free) was assessed as being either a complete or partial response or stable disease.
Results
Treatment planning
In all cases, a well-trained dosimetrist made the treatment planning and at least two radiation oncologists and medical physicist confirmed the planning results. With three to eight (median 5) coplanar and/or non-coplanar beams, each target volume was covered successfully. The planning target volumes ranged from 4.3 to 213 cc (average 43.9 cc). The median prescribed point was the 90% isodose line (range 80–95%), which covered most of the clinical target volume in each case (average 95.2%, range 74–100%). The maximum CTV dose was 100–121% (median 107%) and the minimum CTV dose was 48–95% (median 83%). Therefore, the mean dose to CTV given as biologically equivalent dose (BED) ranged from 58.6 to 118.8 Gy10. The dose volume histograms for the normal organs were analyzed and acceptable doses were delivered to all the critical organs. The volume irradiated with more than 20 Gy in the ipsilateral lung was calculated and was found to range from 1.8 to 28.6% (average 8.9%), and the volume irradiated with > 20 Gy in the whole lung was 0.8–12.8% (average 4.3%). The reproducibility of patient position within the body frame, which was confirmed by CT-simulation and verification films in every treatment, was <5 mm in all directions.
Overall treatment outcome
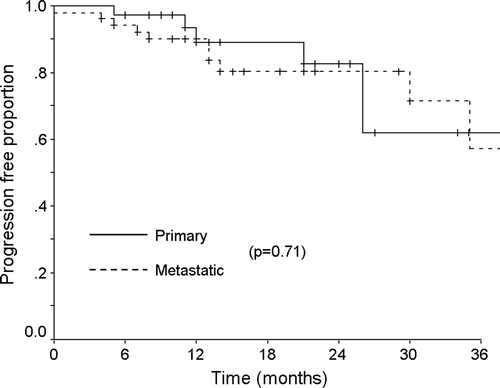
The overall response rate was 82%, with 20 (22%) complete responses and 55 (60%) partial responses at one month after completing treatment (). The median follow-up period was 14 months (range 4–56 months). During this period, 16 cases (18%) developed local tumor progression and the median interval between the date of treatment and–tumor progression was 12 months (range 1–35 months). The one- and two-year local progression free survival rates were 90% and 81%, respectively (). Among the 20 patients who received 30 Gy in 3 fractions, six patients (30%) experienced a local progression between 1 and 35 months after treatment (median 8 months). Ten of the 44 patients (23%) who were treated with a total dose of 40 Gy in 4 fractions also experienced tumor progression between 5 and 30 months after radiosurgery (median 13 months). However, no patient treated at a dose of 48 Gy in 4 fractions (BED 105.6 Gy10), had a local progression even though the follow-up period was relatively short (median 10 months). Although there was somewhat difference in the follow-up period, those patients who received 48 Gy achieved a better local control than those who received less than 48 Gy and this result was statistically significant that was analyzed by Fisher's exact test (p = 0.004). The dose-response (local control) curve using mean dose to CTV (BED, α/β ratio: 10) evaluated by logistic regression showed that there was a good correlation between mean CTV dose and local control (p = 0.017) (). This study also analyzed the local tumor control according to the treatment aim, and found no difference in the local progression free survival between primary and metastatic tumors (). There was no significant relation between a tumor volume and a local control (logistic regression, p = 0.985). All the patients tolerated the fractionated stereotactic radiation therapy quite well without an occurrence of major treatment-related toxicity. No pulmonary complications greater than the RTOG toxicity criteria grade 2 were observed and the other treatment-related acute complications were negligible.
Figure 1. Local progression-free survival rate of the patients with primary or metastatic thoracic tumors. The one- and two-year local progression free survival rates were 90% and 81%, respectively.
Figure 2. Local tumor control probability showed a good correlation with mean dose to CTV (BED10, Gy) by logistic regression analysis (p = 0.017).
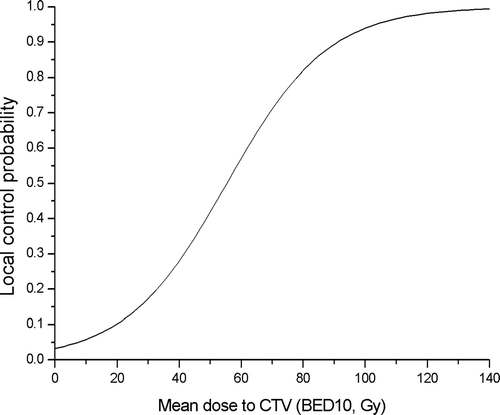
Table II. Initial response of the stereotactic radiotherapy
Treatment result of stage I primary lung cancer
As mentioned previously, 21 patients with a primary non-small cell lung cancer were treated using this technique and the detailed patient characteristics are described in . Seven patients died during the follow-up period of 5–50 months (median 13 months). Of these, one patient died from local tumor progression, one from a myocardial infarction, one from acute renal failure, two from pneumonia almost two years after treatment and two from other aging processes without pulmonary symptoms. At the time of the analysis, three patients who received a dose of 40 Gy developed local progression at 12, 14, and 24 months, respectively. One of these three patients developed a pleural metastasis after local progression. However, no further local recurrence or distant metastasis was noted in the rest of the 18 patients. shows the overall and local progression free survival curves. The one- and two-year overall survival rate of the patients were 89% and 51%, respectively and the one- and two-year local progression free survival rates were 93% and 81%, respectively.
Figure 4. The overall and local progression-free survival rates of the patients with stage I non-small cell lung cancer.
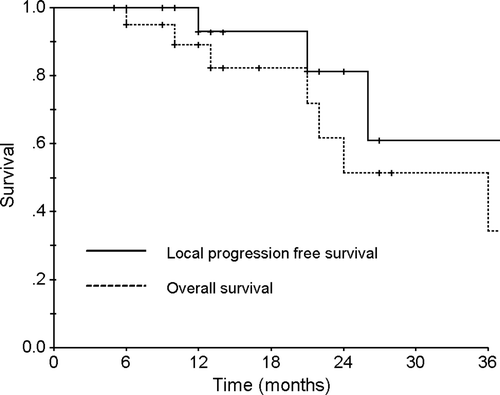
Table III. Patient characteristics of the stage I non-small cell lung cancer
Discussion
The results of this study suggest that stereotactic body radiation therapy is a high precision radiation therapy and a safe modality for treating extracranial intrathoracic tumors. Although this treatment is somewhat inaccurate compared with radiosurgery for intracranial tumors, the set-up error of the patient is acceptable to deliver a focal high dose radiation without significant complications. The set-up error (the reproducibility of patient position within the body frame) confirmed by the CT-simulator and verification films in every treatment was within 5 mm in all directions. Nagata et al. also reported that the average set-up error using this stereotactic body frame was 3.2 mm Citation[6]. Considering these results, the safety margin of the planning target volume was acceptable for adequately covering the gross tumor volume. There is a more accurate method for eliminating the daily differences in the target center or set-up error. Uematsu et al. reported the fusion of CT and linac (FOCAL) system for achieving the direct positioning of the target with a CT scanner and immediate radiotherapy using a linear accelerator Citation[11], Citation[17]. Onishi et al. also introduced a similar treatment unit to provide precise targeting of the tumor Citation[18]. These systems shared a couch for CT scanning and treatment delivery, the patients did not need to move on the couch during verification and treatment. Therefore, the accuracy of the treatment set-up between the linac isocenter and the CT image center was less than 0.5 mm. Recently various methods were introduced to reduce the set-up uncertainty using image guidance Citation[19]. If these image-guiding methods could be combined with a stereotactic body frame, a more accurate treatment result could be achieved and the safety margin could be further reduced to cover the gross tumor volume.
Another important factor for both reproducing and maintaining the tumor position is the control of respiratory motion. According to the treatment protocol, the patients were treated without a respiratory control device if the longitudinal tumor movement was < 5 mm. When the respiratory movement was more than this range, a diaphragm controller was used, which is a flat board that presses the abdomen, to restrict the diaphragm motion and reduce the irradiated normal lung volume. However, this technique had also a limitation in controlling the tumor movement, which is > 15 to 20 mm in the longitudinal direction. Therefore, an ABC apparatus has been used for minimizing this type of movement since October 2003 particularly if the respiratory movement was > 10 mm Citation[14], Citation[15]. Until now, more than ten patients have been treated using stereotactic body radiation therapy with a breathing control technique and the results will be reported elsewhere.
The reported total dose and fractionation schedule in the literature shows large variations. In recent reports, the fraction size varied from 6 to 26 Gy and the overall treatment time was also diverse from one day to approximately two weeks Citation[6–9], Citation[11], Citation[18]. Although the local control rate in the literature was relatively high, ranging from 83% to 94%, the local-relapse free survival at two years varied from 61% to 100%. Therefore, the optimal fraction size and total dose still remains to be determined. In order to address this question, a linear-quadratic (LQ) model Citation[20] was used to compare our different fractionation and total dose, and to calculate the BED of each treatment. The starting dose of this protocol was 30 Gy in 3 fractions (BED 60 Gy10). Thirty percent of these patients experienced a local tumor progression. Subsequent cohorts of patients received 40 Gy in 4 fractions (BED 80 Gy10), and ten patients (23%) also experienced local progression. However, no patient treated at the dose of 48 Gy in 4 fractions (BED 105.6 Gy10) had a local progression even though the follow-up period was relatively short. Nagata et al. already recommended that a BED > 100 Gy might be effective for the stereotactic irradiation of a solitary lung cancer Citation[6] and Timmerman et al. reported that a local recurrence was observed most commonly at the lower doses (median 36 Gy in 3 fractions) but no patient had a local recurrence treated at dose of more than 54 Gy in 3 fractions in their phase I study Citation[9]. A large retrospective Japanese multicenter analysis in 245 stage I lung cancer patients also reported better local control for BED ≥ 100 Gy compared with BED < 100 Gy (8.1% vs. 26.4%, p < 0.01) Citation[21]. Representative examples of dose regimens performed in this study that provided approximate BEDs >100 Gy were 48 Gy/4 fractions or 50 Gy/5 fractions. In our results, the range of the mean CTV dose of 48 Gy/4 fractions regimen was 92.7∼118.8 Gy equivalent dose and the dose-response (local control) curve using mean dose to CTV showed that above the 90%-probability of local control could be achieved with 48 Gy/4 fractions dose regimen. From the analysis of our data and previous reports, it might be recommended cautiously that higher radiation doses (more than BED 100 Gy10) would be better for achieving local tumor control than the lower dose levels. However, the LQ model may not be sensitive for the larger doses used in the stereotactic treatments and for the small fraction numbers due to the uncertainty in the α/β ratio. Therefore, the use of this model in stereotactic radiation therapy should be considered carefully in order to avoid unexpected adverse effects Citation[22], Citation[23].
During the follow-up period, sixteen cases (18%) developed local tumor progression and the median interval between the date of treatment and tumor progression was 12 months (range 1–35 months). Of these, eight patients (50%) experienced local failure within 12 months after completing treatment, and four patients (25%) developed local failure within 12 to 24 months after treatment. However, the remaining four (25%) had local failure at 26, 30, and 35 months after treatment. The tumor volumes of these patients ranged from 23.0 to 213 cc (average 53.6 cc) and these were nearly the same as the average tumor volume of the whole population. There was also no significant difference in the local tumor progression between the primary and metastatic tumors (primary, n = 6; metastasis, n = 10). Therefore, there might be other factors that determine the possibility of a local recurrence after treatment. From this analysis, it should be noted that half of the patient experienced local progression one year after completing treatment, and almost all within three years of completing treatment. Therefore, patients who receive this type of treatment should be followed up carefully over a long period.
The most difficult point on the evaluation of the treatment results was the discrimination between the residual tumor and the radiation-induced pulmonary changes. Aoki et al. reported that the pulmonary changes in the CT images developed two – six months after stereotactic radiation therapy Citation[24]. Generally, the regression of a tumor as well as this change occurred simultaneously. Accordingly, care must be taken to assess the treatment response and evaluate of the serial changes in the CT images. This study evaluated the initial tumor response with chest CT and/or 18FDG-PET scan and defined the tumor progression as an increase in the lesion size by the serial CT images. Consequently, there might be a chance to overstate the treatment results. However, not all the results could be confirmed by a histological examination. Therefore, more accurate modalities are needed for evaluating the tumor response and tumor recurrence. 18FDG-PET scans are occasionally used as an alternative method for evaluating the treatment response, and it would be a promising modality for diagnosing the disease condition. When using 18FDG-PET scans after radiotherapy, it should be noted that radiation can incite macrophage-mediated inflammation, which may be metabolically active on PET imaging, and these changes usually peak within six to 12 weeks after completing therapy Citation[25], Citation[26]. Therefore, it is important to determine the optimal scanning time after radiotherapy. Having the follow-up 18FDG-PET scan one month after the completion of radiotherapy was not too rapid for evaluating the response to radiation in patients with a squamous cell carcinoma of the head and neck Citation[27]. If this result can be used to evaluate the response of a lung tumor to stereotactic radiation therapy, it is possible that the initial response can be diagnosed before the appearance of pulmonary changes. However, it is still unclear as to whether 18FDG-PET can be a useful tool for a serial follow-up after the appearance of a pulmonary injury.
Stereotactic radiotherapy may be an alternative to a surgical resection in the early stage, particularly stage I, lung cancer patients with an advanced age, poor pulmonary function and acute or systemic illness. Moreover, some investigators have also revealed promising results for inoperable stage I non-small cell lung cancer Citation[6], Citation[7], Citation[11], Citation[18]. We also treated 21 patients with stage I NSCLCa who could not tolerate or refused major surgery. Three patients experienced local progression within two years of treatment. Although the follow-up period was insufficient for estimating the efficacy, it may be a beneficial treatment for early stage lung cancer.
In summary, the experience of the stereotactic body frame based radiation therapy appears to be a safe and promising treatment modality for the local management of a primary or metastatic lung tumor. Although there is some controversy about the optimal total dose and fractionation schedule, it is believed that higher a radiation dose (more than BED 100 Gy10) might be needed to achieve better local tumor control. Additional study related to the optimal evaluation tools for the response will also be needed to differentiate a local tumor progression from a radiation-induced pulmonary injury.
This study was supported by grants from the Ministry of Science and Technology, Republic of Korea (BAERI) and a grant of the National Cancer Control R&D Program 2003, the Ministry of Health and Welfare, Republic of Korea. It was presented at the 46th Annual Meeting of the ASTRO, 3-7 October, 2004, Atlanta, GA and also at the 4th S. Takahashi Memorial International Workshop on 3-Dimensional Conformal Radiotherapy, 10–12 December, 2004, Nagoya, Japan. The authors would like to thank John Wong, Ph.D. for supporting the ABC apparatus.
References
- Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys 1994; 28: 797–802
- Alexander E. 3rd, Moriarty TM, Davis RB, et al. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst 1995; 87: 34–40
- Kim KH, Cho MJ, Kim DW, et al. Clinical experience in conformal stereotactic radiotherapy of irregularly shaped intracranial tumors. Cancer Res Treat 2003; 35: 69–74
- Lax I, Blomgren H, Naslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 1994; 33: 677–83
- Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995; 34: 861–70
- Nagata Y, Negoro Y, Aoki T, et al. Clinical outcomes of 3D conformal hypofractionated single high-dose radiotherapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2002; 52: 1041–6
- Hof H, Herfarth KK, Munter M, et al. Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 2003; 56: 335–41
- Wulf J, Hadinger U, Oppitz U, et al. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol 2001; 177: 645–55
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003; 124: 1946–55
- Nakagawa K, Aoki Y, Tago M, et al. Megavoltage CT-assisted stereotactic radiosurgery for thoracic tumors: Original research in the treatment of thoracic neoplasms. Int J Radiat Oncol Biol Phys 2000; 48: 449–57
- Uematsu M, Shioda A, Suda A, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: A 5-year experience. Int J Radiat Oncol Biol Phys 2001; 51: 666–70
- Ahn SD, Yi BY, Choi EK, et al. Preliminary results of stereotactic radiosurgery using stereotactic body frame (Korean). J Korean Soc Ther Radiol 2000; 18: 251–6
- Lee SW, Choi EK, Park HJ, et al. Stereotactic body frame based fractionated radiosurgery on consecutive days for primary or metastatic tumors in the lung. Lung Cancer 2003; 40: 309–15
- Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys 1999; 44: 911–9
- Wilson EM, Williams FJ, Lyn BE, et al. Validation of active breathing control in patients with non-small-cell lung cancer to be treated with CHARTWEL. Int J Radiat Oncol Biol Phys 2003; 57: 864–74
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958; 53: 457–81
- Uematsu M, Shioda A, Tahara K, et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: A preliminary experience. Cancer 1998; 82: 1062–70
- Onishi H, Kuriyama K, Komiyama T, et al. Clinical outcomes of stereotactic radiotherapy for stage I non-small cell lung cancer using a novel irradiation technique: Patient self-controlled breath-hold and beam switching using a combination of linear accelerator and CT scanner. Lung Cancer 2004; 45: 45–55
- Mackie TR, Kapatoes J, Ruchala K, et al. Image guidance for precise conformal radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56: 89–105
- Barendsen GW. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys 1982; 8: 1981–97
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004; 101: 1623–31
- Liu L, Bassano DA, Prasad SC, et al. The linear-quadratic model and fractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 2003; 57: 827–32
- Sasai K, Nagata Y, Hiraoka M. Is the linear-quadratic formula available in stereotactic radiosurgery or hypofractionated radiotherapy? The problem of the uncertainty of the α/β ratio in the LQ formula. Int J Radiat Oncol Biol Phys 2000; 47: 1157–8
- Aoki T, Nagata Y, Negoro Y, et al. Evaluation of lung injury after three-dimensional conformal stereotactic radiation therapy for solitary lung tumors: CT appearance. Radiology 2004; 230: 101–8
- Frank A, Lefkowitz D, Jaeger S, et al. Decision logic for retreatment of asymptomatic lung cancer recurrence based on positron emission tomography findings. Int J Radiat Oncol Biol Phys 1995; 32: 1495–512
- Rohren EM, Lowe VJ. Update in PET imaging of nonsmall cell lung cancer. Semin Nucl Med 2004; 34: 134–53
- Nam SY, Lee S, Im KC, et al. Early evaluation of the response to radiotherapy of patients with squamous cell carcinoma of the head and neck using 18FDG-PET. Oral Oncol 2005; 41: 390–5