Abstract
We investigated whether a short course in communication skills for physicians would improve the quality of informed consent in a randomized clinical adjuvant trial on breast cancer. In this prospective, case-controlled intervention study, physicians and research nurses who introduced the cancer treatment trial to patients at three of the participating hospitals first attended a one-day communication skills course. The quality of informed consent was then evaluated by addressing a standardized questionnaire, QuIC, to trial patients at the three intervention hospitals and at control hospitals. Response rate was 90.0% (n =288). Of the patients treated by the intervention group, 73% were very satisfied with the information received compared with 56% of those of the control group (p = 0.003). The patients of the intervention group considered the time given for making their decision sufficient more often than those of the controls (98% vs. 90%, p=0.004). The patients of the intervention group recalled more often than those of the controls that the physician had also offered other therapeutic options than the trial treatment (91% vs. 97%, p=0.032). They also understood the main aim of the study better than the patients of the controls (89% vs. 78%, p=0.030). In conclusion, a short communication skills course for the trial physicians and nurses improved the quality of informed consent and patient satisfaction in the trial.
Conducting randomized clinical trials demands strict adherence to ethical rules that are intended to protect the subjects who are participating in the study. There is international agreement about the basic elements of information to be given in connection with a clinical trial Citation[1]. When deciding to participate the patient signs a form of informed consent; however, it has become apparent that consent is not always as informed as it should be Citation[2], Citation[3]. The patient may have failed to understand relevant aspects of the trial despite reading and signing the consent form and discussing the trial with the doctor or other trial personnel Citation[4–14].
Many clinical trials that evaluate systemic cancer treatments enrol patients shortly after primary surgery. A physician informing a cancer patient about the possibility of participating in such a trial of adjuvant therapy faces a demanding task. The patient has recently fallen ill with a serious disease and needs emotional support as well as information about prognosis and treatment. Many patients find it difficult to retain information about a clinical study in such a vulnerable situation.
Various methods have been used to improve patient information, such as having a research nurse ensure that the patient has understood the information, improving the readability of written information, using questionnaires to evaluate the attitudes of the patients toward clinical trials and their need for information before meeting with the doctor, and developing an educational training package for physicians about the disclosure of patient information to patients Citation[8], Citation[11], Citation[15–17].
Training in communication has been shown to improve the communication skills of physicians in their usual consultations with patients Citation[18–20]. Here we describe results of an intervention study designed to investigate whether a short course in communication skills for physicians and research nurses improves the quality of informed consent and the satisfaction of patients who enrolled in a randomized clinical trial of adjuvant treatment for breast cancer.
Methods
The present study was done within a trial of adjuvant treatment in breast cancer, known as FINHER Citation[21]. Both studies were approved by an ethical committee.
In the FINHER trial breast cancer patients 65 years of age or younger were randomized into two chemotherapy arms. Patients with tumours that had amplification of the HER2 oncogene were furthermore randomized to receive the monoclonal antibody, trastuzumab, or to the control group. In terms of the patient information, these treatments with their rather complicated information are a challenging situation like many clinical trials today.
The study participants were given both oral and written information, and they signed an informed consent prior to randomization. The written information included a description of the main patient selection criteria, the study treatments, expected adverse events, safety examinations performed during the study, alternative treatment options, the randomization procedure, investigator contact information, data protection, and statement of the medical authorities having a legal right to inspect the study data.
Patient information study
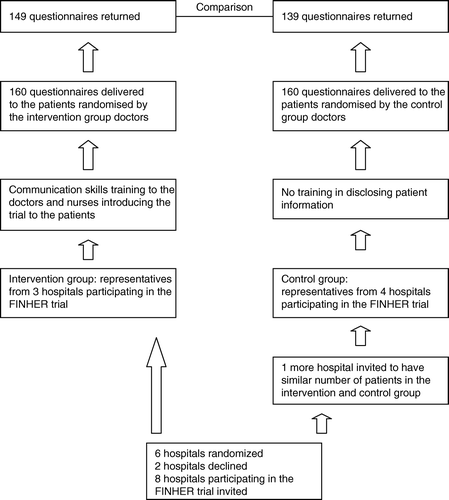
The structure of the patient information study is shown in . All the main hospitals that had recruited patients to the FINHER trial were invited and agreed to participate in the present study. Half of the hospitals were assigned randomly to the intervention group while the rest formed the control group. An additional hospital was randomly invited to the control group so that the total number of patients recruited to the FINHER trial was similar for the intervention and control groups. The physician, who introduced the trial to the patients, and the research nurse, from each of the intervention group hospitals were invited to participate in a one-day training course in communication. These doctors and nurses also took care of the patients after the randomization of the patients in the trial.
The intervention group included representatives of the oncology departments of Helsinki and Turku University Central Hospitals and the Central Hospital of Central Finland. The control group came from Tampere and Oulu University Hospitals and the Central Hospitals of South Karelia and Satakunta.
Communication skills training
The intervention group participated in communications skills training lasting for one evening and one morning. The facilitators were teachers experienced in training physicians in communications skills, an oncologist and an oncologist-psychotherapist (PH). The training began with a three-hour evening lecture on theory covering the psychological reaction to somatic disease, interviewing techniques and the patient's needs when receiving information about a clinical trial. Research data on the uneven quality of informed consent were presented. The next morning the participants applied the theory in role-play as described by Maguire and Faulkner Citation[22]. The physicians rehearsed how to communicate information about the FINHER study with the nurses acting as patients. The participants were given feedback for their performance. They also had the opportunity to share their earlier experiences in communicating information about the FINHER study.
The participants received the article “Information in the context of clinical trial” and the checklist “What the patient should know when signing consent” Citation[4], Citation[23]. Members of the control group were not trained in disclosing patient information, but they were aware that their performance would be evaluated.
Both the intervention and the control group physicians were sent a brief background questionnaire covering demographic characteristics, earlier experience in conducting clinical trials or participation in communication skills training. The physicians and nurses of the intervention group also received a form for reporting what they had learned in the training and whether some aspects would have warranted more attention. They were also asked to give feedback to the facilitators.
Questionnaires
Three and half months after the randomization a research nurse gave each patient (n = 320) a questionnaire addressing the quality of information given about the trial and the communication skills of the physicians who had introduced the trial to them. The questionnaires were distributed to the patients entering the trial between September 2001 and December 2003. They contained the Quality of Informed Consent (QuIC) questionnaire translated into Finnish Citation[24]. QuIC is a standardized questionnaire for evaluating the quality of informed consent. Part A assesses objectively the patient's understanding of the clinical trial (20 questions). Part B evaluates how the patient herself rates her understanding (14 questions). The questionnaire addresses all aspects of informed consent as defined by international regulations. It also covers general misconceptions about clinical trials. We complemented the QuIC with 15 questions from a questionnaire that we had used in a previous study Citation[4]. These questions covered demographic characteristics, details about the decision to participate and the patient's experience of the clinical trial. The questionnaires were completed anonymously and returned to the researchers in prepaid envelopes.
Analysis of the results and statistical methods
The analysis was performed 30 months after the training when 90.0% (n = 288) of all questionnaires had been returned: 93.1% (n = 149) of the intervention and 86.9% (n = 139) of the control group. Differences in distributions between the two groups were analysed using the χ2 test and t-test. P < 0.05 was considered the limit for statistical significance. All p-values were 2-sided.
Results
The four physicians in the control group were older (ages 47, 51, 52 and 55) compared to the three physicians in the intervention group (ages 33, 42, and 49). Further, all control group physicians had earlier experience in conducting clinical trial, and two of them also of communication skills training. The corresponding numbers in the intervention group were two and one. The patients of the intervention and control groups did not differ significantly for demographic characteristics ().
Table I. Demographic characteristics of patients randomized by physicians of the intervention and control groups.
The patients’ satisfaction with the discussion about the clinical trial
The majority of patients in both groups were very satisfied with the discussion about the clinical trial, with higher numbers in the intervention group (73%) than the control group (56%) expressing such satisfaction ().
Table II. Statistically significant differences in the patients' views and awareness of information given in the intervention and control groups.
Almost all patients, i.e. 92% in the intervention and 86% of control group (p=0.28), reported that they had received enough information for making their decision. Patients in the intervention group considered the time given for making their decision sufficient, while 10% of those in the control group thought it was too short. Patients in both groups believed that they had made their decision independently (94% vs. 89%).
Sixty-four percent in the intervention group and 59% in the control group found that their experience of the treatment had corresponded to the prior information. In the intervention and control groups 16% and 20% had expected the side effects to be worse, and 18% and 20% less severe as compared with the information received (p=0.74), respectively. Compared with the patients in the control group (91%), more patients in the intervention group (97%, p=0.03) responded that the physician had offered other alternatives to the trial treatment ().
Understanding the information about the clinical trial
The majority of the patients (95% vs. 92%, p=0.85) felt that they had understood all aspects of the trial well or rather well. However, questions about the details of the trial revealed that patients in both groups had misconceptions (). Patients in the intervention group understood the main aim of the study, the comparison of two treatments, better than those in the control group (p=0.03). About 20% of the patients thought that the aim was to find the highest possible dose for the new drug, although the study purpose was to compare chemotherapy regimens and not doses. About 25% of the intervention group and about 30% of the control group thought that the treatments were standard for their type of cancer. Almost 50% of the patients thought that the trial treatment had been shown most effective for their cancer type and that participation did not involve incremental risks or adverse effects compared with standard treatments. Of the intervention group 29% considered that participation would benefit them medically; the corresponding figure for the control group was 36% (p=0.28). Half of the patients did not know that the medical records of those participating in clinical trials are available to medical authorities.
Table III. Participants’ views on the information given. Selected questions on the quality of informed consent which revealed misconceptions (QuIC, part A) Citation[24].
Almost all patients (96%) knew that treatments were allocated randomly, and there was no difference between the groups.
Physicians’ experience of the training
The physicians and nurses who participated in the course on communication skills gave feedback anonymously. All had found the training very useful. Four of six suggested that the training could have continued for longer or included another session of role-play. The participants commented positively on the small size of the group, the secure and calm atmosphere and the good preparation of cases for role-play.
Discussion and conclusion
Discussion
Along with the development of new therapies, clinical trials are becoming increasingly complex. Earlier studies suggest that patients have misconceptions about many important aspects of the trials Citation[4–11]. Since training in communication skills has been shown to improve physicians’ ability to interact with their patients, we hypothesized that training focused on disclosing information about clinical trials would improve the quality of informed consent Citation[18–20].
In the present study the patients of the intervention group were significantly more satisfied with interaction with their physician than those of the control group. In the study of Fleissig, where patient satisfaction was measured in the context of a clinical trial, investigators attempted to improve interaction by asking the patients to fill in a form addressing their need for information and attitudes toward clinical trials Citation[17]. Providing such information to the physicians may not have been sufficient to modify their behaviour and there was no difference in patient satisfaction. Communication skills training with live role-play that enables instant feedback and exchange of ideas appears to be more efficient. In a study by Jenkins with interactive rehearsal, the skills of the participants were analysed by videotaping their performance before and after the training with an actor portraying a patient Citation[17]; this intervention did lead to improved competence when communicating about randomised clinical trials.
Patients of our intervention group were better aware of the main objective of the study than those of the control group. They considered the time given for decision making sufficient significantly more often and remembered that physicians had presented treatment alternatives more often than those of the control group; however, misconceptions about patient safety and the probability of improved efficacy were found in both groups. “Therapeutic misconception” which helps the patient to adjust to his illness and treatment has been described Citation[25], Citation[26]. Half of the patients in our study believed that the trial drugs provided the proven best treatment. In a study by Joffe the corresponding figure was 29% Citation[12]. In practice, none of the adjuvant therapies used in our study was standard care in Finland at the time, although they included effective drugs for treatment of breast cancer. Similarly, many patients in Joffe's study (70%) failed to realize that the treatment was non-standard. The fact that patients made their positive interpretation concerning the trial treatment was probably due to the information presented in the written patient material. This information emphasized that all drugs used in the trial were very effective. Almost half of the patients thought that participation did not involve incremental risks or adverse effects. In Joffe's study this figure was 63%. One third of patients believed that participation would bring them personal medical benefit. The hope of personal gain has been found to motivate participation in other studies Citation[13], Citation[15], and often the patients have not comprehended that the main aim of clinical trials is to benefit future patients. According to some studies, patients who participate in clinical study have better outcome than those who do not participate, but there are insufficient data to conclude that enrolment in clinical trials leads to improved outcomes in patients with cancer Citation[27].
Half of the patients did not know that participation might reduce the confidentiality of medical records. A similar result has been obtained earlier Citation[15]. Although stated in the consent form, it is likely that researchers do not emphasise this, as it is not necessarily considered as important as other issues related to the trial.
The number of doctors in the present study was small because the number of doctors who enrolled patients to the national clinical trial was limited. One doctor was responsible for informing the patient in each participating hospital. This is a weakness of the present study, which can not be compensated with the high number of patients. The small number of doctors created differences in the background factors between the intervention and control group. The doctors in the control group were older and all had experience in informing patients of the possibility to participate in a clinical trial. Two of them had received some education in communication skills compared to one in the intervention group. This might actually lead to the differences in our study being underestimates. However, with so few doctors, the opposite might also be possible. The younger doctors in the intervention group might have had personal aptness in communication skills and this had lead to better results in the intervention group without training. However, the experience of the facilitators of the communication training did not indicate this possibility.
The patients of the intervention and control groups did not differ statistically significantly in their demographic characteristics. However, there were slightly more university graduates in the intervention group, which might have affected so that the information was better understood in this group Citation[4].
The responses to many of the questions did not differ significantly between the intervention and control groups. This might be due in part to the fact that the control group physicians were aware that their performance would be evaluated. For example, almost all patients understood the meaning of randomization compared to studies by Simes and Aaronson in which the intervention group was better aware of allotment than the control group Citation[8], Citation[11]. Most significant differences were received in the appreciation of the information. It is likely that presenting our earlier research results on this topic had already improved the standard of information given in the context of clinical trials in Finland. On the other hand, on the basis of the misunderstandings of the information, which are shown in , there is still room for further improvement in the patients’ actual understanding.
Would paying more attention to difficult issues further improve the quality of informed consent and would an extra session of communication skills training have any effect are the subjects of further study. Razavi et al. found that a short follow-up session after communication skills training improved the communication of physicians and subsequent patient satisfaction significantly, although this study was not undertaken in the context of a clinical trial Citation[20]. In our study, communication skills training was brief and it is possible that additional role-play practice would have further improved the results. An English and an Australian group have produced education training packages for improving the communication skills of physicians in the context of clinical trials Citation[17], Citation[28]. The first one has already been shown to improve the participants’ skills compared with baseline performance.
Compared with other measures to improve the quality of informed consent, training physicians in communications skills offers some advantages. Training may make the physician generally more interested in disclosure of patient information. Research results also suggest that patients are more satisfied with information about the study and participate more often in clinical trials if the physician has good communication skills and the information is given in a patient-centred manner Citation[29]. Furthermore, training offers physicians an opportunity to share problems that they have encountered and relieve the emotional burden caused by their work. Physicians conducting research should be supported in their demanding task.
Conclusion and practice implications
The patients’ actual understanding of the clinical trial information is not optimal. A short communication skills course for physicians engaged in a clinical trial about disclosing patient information improved the quality of informed consent and patient satisfaction. This kind of training should be further improved and included in the clinical trial planning process.
We are grateful to the patients and physicians who participated in this study making it possible and to the Finnish Breast Cancer Group and Pharmacia for financially sponsoring the communication courses for the intervention group doctors and nurses. The authors also thank senior researcher Pilvikki Absetz from the National Public Health Institute who helped to collect the data.
We have not received commercial funding for this study apart from a grant from Pharmacia for organizing the communication courses for the intervention group doctors and nurses. The authors do not have any conflicts of interest relevant to the subject of the manuscript.
References
- International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva: CIOMS; 2002.
- Edwards SJL, Lilford RJ, Thornton J, Hewison J. Informed consent for clinical trials: In search of the “best” method. Soc Sci Med 1998; 47: 1825–40
- Meisel A, Roth LH. What we do and do not know about informed consent?. JAMA 1981; 246: 2473–7
- Hietanen P, Aro A.R, Holli K, Absetz P. Information in the context of a clinical trial. Eur J Cancer 2000; 36: 2096–104
- Daugherty C, Ratain MJ, Grochowski E, Stocking C, Kodish E, Mick R, et al. Perceptions of cancer patients and their physicians involved in phase I cancer trials. J Clin Oncol 1995; 13: 1062–72
- Schaeffer MH, Kranz DS, Wichman A, Masur H, Reed E, Vinicky JK. The impact of disease severity on the informed consent process in clinical research. Am J Med 1996; 100: 261–8
- Lynoe N, Sandlund M, Dahlqvist G, Jacobsson L. Informed consent: Study of quality of information given to participants in a clinical trial. BMJ 1991; 303: 610–3
- Simes RJ, Tattersall MH, Coates AS, Raghavan D, Solomon HJ, Smartt H. Randomised comparison of procedures for obtaining informed consent of procedures for obtaining informed consent in clinical trials of treatment of cancer. Br Med J (Clin Res Ed) 1986; 293: 1065–8
- Penman DT, Holland JC, Bahna GF, Morrow G, Schmale AH, Derogatis LR, et al. Informed consent for investigational chemotherapy: Patients’ and physicians’ perceptions. J Clin Oncol 1984; 2: 849–55
- White DR, Muss HB, Michielutte R, Cooper MR, Jackson DV, Richards F 2nd, et al. Informed consent: Patient information forms in chemotherapy trials. Am J Clin Oncol 1984; 7: 183–90
- Aaronson NK, Visserpol E, Leenhouts GHMW, Muller MJ, van der Schot ACM, van Dam FSAM, et al. Telephone-based nursing and intervention improves the effectiveness of the informed consent process in cancer clinical trial. J Clin Oncol 1996; 14: 984–96
- Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: A cross-sectional survey. Lancet 2001; 358: 1772–7
- Pentz RD, Flamm AL, Sugarman J, Cohen MZ, Daniel Ayers G, Herbst RS, et al. Study of the media's potential influence on prospective research participants’ understanding of and motivations for participation in a high-profile phase I trial. J Clin Oncol 2002; 20: 3785–91
- Cox K. Informed consent and decision-making: Patients’ experiences of the process of recruitment to phases I and II anti-cancer drug trials. Patient Educ Couns 2002; 461: 31–8
- Coyne CA, Xu R, Raich P, Polmer K, Dignan M, Wenzel LB, et al. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: A study of the Eastern Cooperative Oncology Group. J Clin Oncol 2003; 21: 836–42
- Fleissig A, Jenkins V, Fallowfield L. Results of an intervention study to improve communication about randomised clinical trials of cancer therapy. Eur J Cancer 2001; 37: 322–31
- Jenkins V, Fallofield L, Solis-Trapala I, Langridge C, Farewell V. Discussing randomised clinical trials of cancer therapy: Evaluation of a Cancer Research UK training programme. BMJ 2005; 330: 400–3
- Fallowfield L, Jenkins V, Farewell V, Saul J, Duffy A, Evesi R. Efficacy of a Cancer Research UK communication skills training model for oncologists: A randomised controlled trial. Lancet 2002; 23: 650–6
- Fallowfield L, Jenkins V, Farewell V, Solis-Trapala I. Enduring impact of communication skills training: Results of a 12-month follow-up. Br J Cancer 2003; 89: 1445–9
- Razavi D, Meckaert I, Marchal S, Libert Y, Conradt S, Boniver J, et al. How to optimize physicians’ communication skills in cancer care: Results of a randomized study assessing the usefulness of posttraining consolidation workshops. J Clin Oncol 2003; 21: 3141–9
- Joensuu H, Kellokumpu-Lehtinen P-L, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006; 354: 809–20
- Maguire P, Faulkner AJ. How to do it. Improve the counselling skills of doctors and nurses in cancer care. BMJ 1988; 297: 847–9
- Good clinical trial practice, NLN Publication No 28, Nordiska Läkemedelsnämnden. Uppsala: Nordic Council on Medicines; 1989.
- Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: A new measure of understanding among research subjects. J Natl Cancer Inst 2001; 93: 139–47
- Advisory Committee on Human Radiation Experiments. Final report of the Advisory Committee on Human Radiation Experiments. New York (NY): Oxford University Press; 1996.
- Appelbaum PS, Roth LH, Lidz CW, Benson P, Winslade W. False hopes and best data: Consent to research and the therapeutic misconception. Hastings Cent Rep 1987; 17: 20–4
- Peppercorn J, Weeks J, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structural review. Lancet 2004; 363: 263–70
- Brown RF, Butow PN, Butt DG, Moore AR, Tattersall MH. Developing ethical strategies to assist oncologists in seeking informed consent to cancer clinical trials. Soc Sci Med 2004; 58: 379–90
- Grant CH 3rd, Cissna KN, Rosenfeld LB. Patients’ perceptions of physicians communication and outcomes of the accrual to trial process. Health Commun 2000; 12: 23–39