Abstract
We report on the technical feasibility, dosimetric aspects, and daily image-guidance capability with megavoltage CT (MVCT) of stereotactic body radiotherapy (SBRT) using helical tomotherapy for medically inoperable T1/2 N0 M0 non-small cell lung cancer. Nine patients underwent treatment planning with 4D-CT in a double vacuum based immobilization system to minimize tumor motion and to define a lesion-specific 4D-motion envelope. Patients received 60 Gy in 5 fractions within 10 days to a PTV defined by a motion envelope plus a 6 mm expansion for microscopic extension and setup error using tomotherapy, with daily pretreatment MVCT image guidance. The primary endpoint was technical feasibility. Secondary endpoints were defining the acute and sub-acute toxicities and tumor response. Forty three of 45 fractions were successfully delivered, with an average delivery time of 22 minutes. MVCT provided excellent tumor visualization for daily image guidance. No significant tumor regression was observed on MVCT in any patient during therapy. Median mean normalized total doses were: tumor 117 Gy10; residual lung 9 Gy3. Maximum fraction-size equivalent dose values were: esophagus 5 Gy39; cord 7 Gy36. No patient experienced ≥ grade 2 pulmonary toxicity. 3 complete, 4 partial and 2 stable responses were observed, with <3 months median follow-up. The mean tumor regression is 72%. SBRT using tomotherapy proved to be feasible, safe and free of major technical limitations or acute toxicities. Daily pretreatment MVCT imaging allows for precise daily tumor targeting with the patient in the actual treatment position, and therefore provides for precise image guidance.
Treatment of medically inoperable non-small cell carcinoma of the lung continues to present a challenge, with conventional treatments resulting in overall survival significantly lower than in stage-matched surgical series. Stereotactic body radiotherapy (SBRT) is one method of increasing the dose delivered to the tumor while minimizing the dose to surrounding normal tissue. This technique has been well described in the literature, and preliminary clinical trials suggest promising results Citation[1–6]. These trials have all examined linear accelerator (LINAC) based SBRT, mostly without the use of IMRT, often without lesion-specific motion compensation, and also frequently without margin expansion for microscopic disease coverage; further, the daily IGRT process has often been cumbersome, requiring moving the patient to a simulator or a CT in a different location. We decided to perform a feasibility study of helical tomotherapy delivery for this complex situation, with the specific goal of integrating these steps into a smooth, seamless process, which could then be applied, to future larger efficacy-driven trials. The specific objectives that we wanted to achieve included: a) lesion specific motion envelope characterization using 4D-CT and incorporation of this in treatment planning; b) minimization of motion using a reproducible positioning and immobilization system; c) daily pre-treatment image guidance, using high quality rapid imaging without introducing further sources of patient dislocation; d) efficient and automated pretreatment patient position recalibration based on target matching, rather than bony anatomy matching; e) the delivery of highly conformal dose distribution with avoidance and minimization of dose to lungs, heart, major bronchii, brachial plexus, spinal cord and esophagus; f) utilization of a short duration multi-fraction schedule with extreme dose-per-fraction escalation; and g) successful and efficient delivery of several treatments to demonstrate feasibility.
Methods and materials
Patients
A pilot group of nine patients with early stage (T1-2 N0 M0) medically inoperable non-small cell lung cancer were treated with definitive radiotherapy using SBRT delivered by tomotherapy at the University of Wisconsin between November, 2004 and April, 2006. Patient characteristics, including histology, initial tumor size, and tumor location, are detailed in . To be eligible for this treatment, the primary tumor could not be within the zone of the proximal bronchial tree, which was defined as a volume 2 cm in all directions around the carina, primary, and secondary bronchi. Three patients lacked definitive pathologic confirmation of malignancy. This was due to non-diagnostic biopsy attempts in two patients, and contraindication to the biopsy procedure because of medical comorbidities in one patient. Each of these three patients demonstrated a lung nodule with increased FDG uptake on PET scan. All of the patients were evaluated in a multi-disciplinary lung clinic, which afforded input from cardiothoracic surgery, radiation oncology and medical oncology in terms of a definitive plan of care. All patients were deemed poor surgical candidates on the basis of either age or co-morbidities.
Table I. Patient demographics.
Treatment planning
For all patients, two CT-scans were acquired a regular thin slice treatment planning CT-scan and a 4D-CT-scan. Both scans were acquired using a custom made double vacuum based immobilization system into which an abdominal pressure pillow has been incorporated to minimize respiratory excursion of the target Citation[7], Citation[8]. The regular thin slice treatment planning CT is acquired for treatment planning and pretreatment localization on the Tomotherapy treatment unit and the 4D-CT is acquired to define the motion-defined envelope (MDE) of the GTV. The generation of the 4D-motion-defined envelope of the GTV is described in detail Citation[8]. A 6 mm margin is then added to the MDE to account for possible microscopic extensions and setup error to form the PTV. The rationale for this is derived from pathologic data demonstrating that 95% of microscopic tumor extension occurs within 6 to 8 mm depending on tumor histology Citation[9]. The spinal cord, esophagus, ipsilateral brachial plexus, heart, and trachea were contoured as avoidance structures, with maximum dose constraints assigned as follows: Spinal cord < 18 Gy36, esophagus < 27 Gy39, ipsilateral brachial plexus < 24 Gy38, heart < 30 Gy310, trachea and ipsilateral bronchus < 30 Gy310. These are notated as fraction-size equivalent doses (FED), a concept advanced by Tomé and Fenwick Citation[10]. The FED concept allows NTCP models fitted to data from one fractionation schedule to be used to study any other fractionation schedule. Used in this way, the FED concept is a logical extension of the biologically equivalent dose (BED) concept. In the BED concept doses delivered using any fractionation schedule are converted to their biologically equivalent levels delivered at an ultra low dose rate, the only parameter needed for this conversion being the α/β-ratio for the biological endpoint studied. In the FED concept, one reconverts BED values back to biologically equi-effective doses delivered at some standard dose-per-fraction, fs, for which the modeled normal tissue complication data were derived. FED is calculated as follows:The treatment plan was optimized on the TomoTherapy Treatment Planning System (Tomotherapy, Inc. Middleton, WI) using an iterative planning process. The treatment plans were reviewed, and mean normalized total doses (NTD) were obtained for both the primary tumor and the total lung volume outside the PTV (residual lung) Citation[11]. The plan was optimized so that the ratio of the prescription isodose volume (PIV) to the volume of residual healthy lung remained below the modeled toxicity threshold as published by us previously Citation[12] and hence the residual mean lung NTD was less than 18.5 Gy3 for all cases. The maximum FED for the avoidance structures was also obtained for each treatment plan. The prescription isodose surface was then selected such that 99% of the PTV received the prescription dose of 60 Gy in 5 fractions (the prescription isodose surface as a percentage of maximum dose is given in ), and the equivalent uniform dose (EUD) to the PTV was greater than or equal to the prescription dose Citation[13].
Table II. Dosimetric values and radiographic results.
Treatment delivery
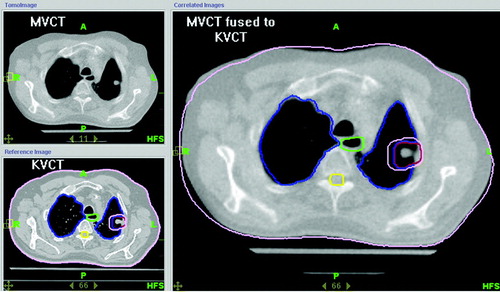
The patients in this study were prescribed a dose of 60 Gy delivered in 5 fractions of 12 Gy each, and received their treatments within 2 weeks on the TomoTherapy HiArt System®. Prior to the delivery of each fraction, patients were placed in their custom made double vacuum whole body mold and immobilized by applying a 80 mbar vacuum. Breathing was restricted using an abdominal pressure pillow. In order to confirm the correct internal target position, a pretreatment MVCT-scan was obtained and fused to the treatment planning CT using the auto-fusion software. Note that Tomotherapy is a slow CT-scanner, since one full gantry rotation takes 10 s in MVCT imaging mode. Therefore, the motion pattern of the target is encoded into the pre-treatment MVCT-scan yielding a motion encoded treatment target. The image fusion was reviewed prior to treatment, and adjusted such that the motion encoded treatment target was centered within the PTV. Any positional modifications based upon the kVCT-MVCT fusion were made prior to daily treatment. shows the result of a pre-treatment kVCT-MVCT fusion for target localization. All treatments were delivered within 10 calendar days of initiation.
Data review
A detailed review of the patients’ treatment plans, and clinical and radiographic information was performed to evaluate the feasibility objectives outlined above. Clinical data acquired consisted of the patients’ initial stage, histology, and tumor location. The treatment visits and subsequent clinical follow-up visits were reviewed to assess for any acute toxicities. Toxicities are reported according to the NCI Common Toxicity Criteria, version 2. Pre-treatment volumes were determined from the 4D-CT treatment planning scans. Intra-treatment volumes were assessed on the daily MVCT images. Post-treatment volumes were obtained by uploading the individual follow-up CT-scans into the Pinnacle Treatment Planning System, and contouring the radiographically identifiable abnormality, after image-fusion with the original planning CT. Response to therapy was determined as follows:
Complete response (CR)
No radiographic evidence of disease.
Partial response (PR)
Decrease in tumor volume by greater than 65%.
Stable disease
Either a decrease in volume by 65% or less, or an increase in volume less than 40%.
Progressive disease
Increase in volume of 40% or more Citation[14].
Median clinical and radiographic follow-up was 2.1 months (range 1.8–13.3 months). Recommendations were made to each patient to return to clinic 1 month after treatment, and subsequently every 3 months for the first 2 years after treatment for physical examination and CT-scan of the chest.
Results
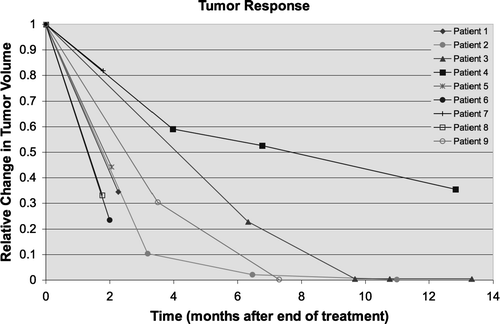
All patients successfully underwent 4D-CT acquisition, allowing for specific, lesion-individualized motion envelope generation Citation[8]. The volume of the motion envelope varied from 2.5 to 39.3 cm3. Tumor motion was minimized with the use of a double vacuum whole body mold and an abdominal pressure pillow, with the measured motion for these patients (defined as the root mean square distance between the center of mass of the GTV in the maximum inspiration and maximum expiration phase) ranging from 1 to 4.9 mm. Generation of IMRT plans using the pre-specified planning objectives was successfully achieved in all patients. The normal tissue FED doses for the nine patients, mean tumor NTD, mean residual healthy lung NTD, as well as the above mentioned pre-treatment volumetric and motion parameters are detailed in . MVCT images obtained prior to treatment successfully and reproducibly visualized the tumor for each treatment, allowing tumor-specific daily set-up corrections. The set-up corrections for the 43 fractions are enumerated in . Forty three of the 45 planned treatments were successfully delivered, with two patients each receiving a single treatment using a LINAC based IMRT backup plan. This occurred because of maintenance issues on the TomoTherapy unit. There were, however, no unanticipated treatment delays or schedule prolongation. The average treatment duration was 22 min 9 s. Radiographic volumetric change was assessed during treatment using MVCT and no objective tumor response was identified during the course of treatment Citation[15]. All of the patients have had at least one post treatment CT-scan to assess tumor response. Five of the patients have had only a single scan, with a median follow-up time of 2 months. The remaining four patients have been followed for a median of 11.9 month and all of them have multiple evaluable scans. Post-treatment radiographic imaging of the four patients with long term follow-up demonstrated a partial response in one patient and a complete response in three patients. The partial response was evident at 12.8 months. In the patients with complete responses, this was observed after 7.3, 9.7 and 11 months, respectively. The five patients with short follow-up demonstrated three partial responses and two stable diseases. Mean tumor regression for all patients was 72% (range 18–100%). Time-volume curves for each patient are shown in . One patient experienced a recurrence in the contralateral lung, in an area where wedge resection of a carcinoma had been previously performed. The treatments were extremely well tolerated by all of the patients, with no report of grade 2 or higher acute toxicity. Review of the follow-up imaging demonstrated evidence of grade 1 radiation pneumonitis (defined as radiographic changes in the absence of clinical symptoms) in all of the available scans. Late toxicities are not assessable in the five patients with short (i.e. less than 3 months) follow-up. However, those patients with longer follow-up remain free of clinically meaningful radiation related toxicities, with no reported late toxicity of grade 2 or higher, using the NCI Common Toxicity Criteria version 2. One patient died of co-morbid pulmonary illness, which was not deemed attributable to either lung cancer or the treatment, within 9 weeks after completion of the treatment, and was clinically correlated, with acute infectious exacerbation of COPD, leading to cardiac death.
Table III. Average and standard deviation of positional shifts and total beam-on times.
Discussion
Conventionally delivered radiotherapy for patients with medically inoperable NSCLC utilizes non-IMRT techniques with somewhat large ports, to compensate for motion and geometric set-up uncertainty, and the total dose is limited by normal tissue toxicity. Doses typically range from 60–70 Gy, over 6–7 weeks. Efforts at dose escalation have met with mixed results. Martel and colleagues presented an intriguing set of clinical data from a Phase 1 dose-escalation trial estimating the dose required for 50% tumor control (TCD50) at 30 months to be approximately 84 Gy (delivered in 42 fractions of 2 Gy each) Citation[16]. Delivery of these high doses using conventional treatment planning comes at the price of increased toxicity Citation[17], Citation[18]. Utilizing a highly conformal treatment planning technique such as IMRT or tomotherapy has two aims; increasing the dose to the tumor, and minimizing the dose to surrounding normal tissue by decreasing the volume of normal tissue receiving the prescribed dose as far as possible Citation[12]. However, improvements in tumor localization and set-up reproducibility have to be integral components of these techniques, and become increasingly important as treatment fields decrease in size. Adequate tumor localization is ensured with Tomotherapy using pretreatment MVCT imaging in the actual treatment position and correlating this pretreatment MCVT scan to the treatment planning kVCT using image annealing techniques, focusing on soft-tissue, i.e. target alignment, as opposed to bony alignment.
Clinical efforts to deliver high doses of radiation to lung tumors using highly conformal techniques are described in the literature Citation[1–6]. These trials typically incorporate a stereotactic body frame to minimize tumor motion and ensure more reliable patient set-up. They have all successfully delivered high doses of radiation using extreme hypofractionation. The treatments are generally very well tolerated, and produce excellent radiographic response rates. These series all describe LINAC-based treatments. One potential limitation of LINAC-based treatments is the use of external stereotactic coordinate systems, skin markers, and/or internal markers as a surrogate for tumor position in conjunction with port films for tumor targeting. Visualization of the actual tumor, especially relatively small ones, is difficult, if not impossible, using these methods. Wulf and colleagues sought to improve on this by utilizing “CT-on-rails” technology in their series Citation[6]. Patients were placed in the treatment position, and underwent a pre-treatment CT-scan by means of a moving gantry. This allowed them to localize the tumor on each treatment day, and make any necessary adjustments to ensure appropriate tumor coverage within the treatment field. Tomotherapy represents the next logical step in this progression. Patients are set-up in the treatment position, and undergo a pretreatment MVCT in the treatment position on the treatment machine. Fusing this pretreatment MVCT to the treatment planning kVCT then allows for precise daily tumor localization.
This series represents the first reported cases of medically inoperable early stage lung cancer patient treated with hypofractionated SBRT delivered via helical tomotherapy, with a specific focus on each step, and validating feasibility. The clinical response rates, while encouraging, remain of uncertain significance given the extremely small sample size. The evaluation of post-treatment tumor volumes is complicated by the inevitable development of fibrosis in the treated area. This is an expected consequence in normal lung tissue following high-dose, hypofractionated radiotherapy, and is likely to yield an underestimate of response based upon the contouring of fibrotic lung as opposed to residual tumor.
Conclusion
Delivery of IG-SBRT via helical tomotherapy using extreme hypofractionation in patients with early stage medically inoperable non-small cell lung cancer is feasible and well tolerated. Use of 4D-CT allows for a more precise delineation of tumor motion during the respiratory cycle, which in turn allows us to confidently treat patients with smaller margins applied to the motion derived envelope. The daily MVCT-scans allow for precise daily tumor targeting, and represent a substantial advantage over conventional portal imaging. Further studies are warranted to evaluate the efficacy of this treatment modality.
This research was funded by PO1 CA88960—01-05
References
- Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003; 124: 1946–55
- Uematsu M, Shioda A, Suda A, Fukui T, Ozeki Y, Hama Y, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: A 5-year experience. Int J Radiat Oncol Biol Phys 2001; 51: 666–70
- McGarry RC, Papiez L, Williams M, Whitford T, Timmerman RD. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: Phase I study. Int J Radiat Oncol Biol Phys 2005; 63: 1010–5
- Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005; 63: 1427–31
- Onimaru R, Shirato H, Shimizu S, Kitamura K, Xu B, Fukumoto S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys 2003; 56: 126–35
- Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: A noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004; 60: 186–96
- Keall P. 4-dimensional computed tomography imaging and treatment planning. Semin Radiat Oncol 2004; 14: 81–90
- Zhang T, Orton NP, Tomé WA. On the automated definition of mobile target volumes from 4D-CT images for stereotactic body radiotherapy. Med Phys 2005; 32: 3493–502
- Giraud P, Antoine M, Larrouy A, Milleron B, Callard P, De Rycke Y, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys 2000; 48: 1015–24
- Tomé WA, Fenwick JD. Letter to the Editor: Analysis of radiation-induced liver disease using the Lyman NTCP model: In regard to Dawson et al. IJROBP 2002;53:810–21. Int J Radiat Oncol Biol Phys 2004;58:1318–9.
- Fowler JF, Tomé WA, Fenwick JD, Mehta MP. A challenge to conventional radiotherapy. Int J Radiat Oncol Biol Phys 2004; 60: 1241–56
- Tomé WA, Fenwick JD, Mehta MP. How can tumor effect and normal tissue effect be balanced in Stereotactic Body Radiotherapy. Radiosurgery 2006; 6: 87–98
- Tomé WA, Fowler JF. On cold spots in tumor subvolumes. Med Phys 2002; 29: 1590–8
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16
- Siker ML, Tomé WA, Mehta MP. Tumor volume changes on serial imaging with megavoltage CT for Non-Small Lung cancer during Intensity Modulated Radiotherapy: How reliable, consistent and meaningful is the effect? Int J Radiat Oncol Biol Phys 2006 (in press).
- Martel MK, Ten Haken RK, Hazuka MB, Kessler ML, Strawderman M, Turrisi AT, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999; 24: 31–7
- Rosenzweig KE, Fox JL, Yorke E, Amols H, Jackson A, Rusch V, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer 2005; 103: 2118–27
- Miller KL, Shafman TD, Anscher MS, Zhou SM, Clough RW, Garst JL, et al. Bronchial stenosis: An underreported complication of high-dose external beam radiotherapy for lung cancer?. Int J Radiat Oncol Biol Phys 2005; 61: 64–9