Abstract
In forthcoming multicentre studies on stereotactic body radiotherapy (SBRT) compliance with volume and dose prescriptions will be mandatory to avoid unnecessary heterogeneity bias. To evaluate compliance in a multicentre setting we used two cases from an ongoing phase II study of SBRT of T1-T2N0M0 inoperable NSCLC in a dummy run oriented on volumes and doses. Six Scandinavian centres participated. Each centre received CT-scans covering the whole lung volumes of two patients with instructions to follow the study protocol when outlining tumour and target volumes, prescribing doses and creating dose plans. Volumes and doses of the 12 dose plans were evaluated according to the study protocol. For the two patients the GTV volume range was 24 to 39 cm3 and 26 to 41 cm3, respectively. The PTV volume range was 90 to 116 cm3, and 112 to 155 cm3, respectively. For all plans the margin between CTV and PTV in all directions followed in detail the protocol. The prescribed dose was for all centres 45 Gy/3 fractions (isocentre dose about 66 Gy). The mean GTV doses ranged from 63 to 67 Gy and from 63 to 68 Gy, respectively. The minimum doses for GTV were between 50–64 Gy and between 55–65 Gy, respectively. The dose distribution was conformed to PTV for 10 of 12 plans and 2 of 12 plans from one centre had sub-optimal dose distribution. Most of the volume and dose parameters for the participating centres showed fully acceptable compliance with the study protocol.
Stereotactic body radiation therapy (SBRT) is expected to have a precise target definition and an accurate radiotherapy delivery. This technology makes it possible to treat small tumours with a reduced irradiated volume with a very high dose per fraction with fewer side effects than conventionally fractionated external therapy Citation[1]. A few differences from the conventional radiation therapy may be pointed out. The margin between gross tumour volume (GTV) and planning target volume (PTV) is smaller. Three to 4 fractions with doses of 10–20 Gy are given, compared to 2 Gy/fraction in conventional therapy. The dose is prescribed to the periphery of PTV, which leads to a 20–50% higher dose to the central parts of PTV. The number of beams is usually higher Citation[2], Citation[3].
The precise nature of dose delivery and geometric exactness in the set-up procedure makes it important to understand uniformity e.g. between different treatment centres. The present investigation was performed with the Scandinavian phase II multicentre SBRT study of T1-T2 N0M0 medical inoperable non-small cell lung cancer (NSCLC). In the study 60 patients have been included from August 2003 until August 2005. The aim of the study was to investigate local high dose SBRT in controlling early stage NSCLC and understanding the toxicity level of treatment.
It is well known that deviations from a study protocol of the multicentre type can have adverse effect on the result of the study. A dummy run was therefore performed in December 2004 within the Scandinavian SBRT study group with six active participating centres from Denmark, Norway and Sweden. CT slices with some clinical information about two patients with NSCLC were sent to the different centres. Radiation oncologists were asked to delineate GTV, CTV and PTV on the CT slices and some organs at risk (OAR). Treatment plans were then created and optimised by the physics teams. The material was then returned to Sahlgrenska University Hospital that performed the evaluation.
The aim of this dummy run was to evaluate compliance with the clinical study protocol in outlining of volumes and dose planning of the test patients at the different participating centres. Dummy run is one part of a quality assurance process for multicentre studies Citation[4]. The procedure and evaluation of dummy runs are well established for clinical multicentre studies and has been tested within the EORTC radiotherapy group Citation[5], Citation[6].
Materials and methods
The study protocol requires that the clinical target volume (CTV) is defined as GTV with anticipated tumour growth at the border of the tumour. The PTV is the CTV with a margin of 5 mm in the transversal plane and 10 mm in the longitudinal direction. Patients with a tumour size of less than 5 cm were included in the study. The dose is prescribed to the periphery of the PTV, 45 Gy in 3 fractions, which gives an approximately 50% higher dose in the centre of PTV. The study protocol suggests a treatment plan with 5–7 static coplanar or non-coplanar beams, which should be spread in the largest possible solid angle. The definition of volumes and doses follow the ICRU recommendation Citation[7]. Multi-leaf collimators create the shape of the beam. The fields are smaller than in conventional radiotherapy and the edges of the beams are often close to the periphery of PTV. The dose distribution was calculated in all transversal CT-slices through PTV and organs at risk. Also, dose volume histograms (DVH) of GTV, CTV, PTV and lung minus GTV should be calculated. The different volumes, minimum dose, mean dose, median dose and maximum dose of target volumes and organs at risk were recorded in the case report forms (CRF).
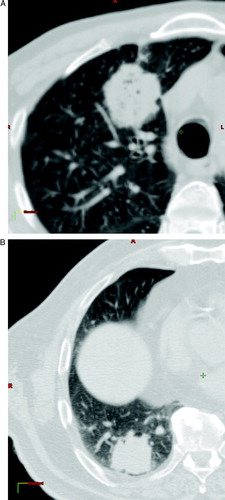
For the dummy run, the participating six centres received anatomical and clinical data of two patients with medical inoperable lung tumours of type T2N0M0. Data consisted of CT-scans in DICOM3 formats over the volume of interest and a short description of the patients. The CT slices had a thickness of 3 mm and were separated 3 mm with a margin of about 5 cm around GTV and 10 mm outside this volume. shows CT slices in the central part of the GTV of the two patients. Patient 1 had a tumour in the upper left lobe at the ventral part of the lung, close to sternum and ribs. Patient 2 had a tumour in the lower left lob close to diaphragm and ribs.
Figure 1. Transversal CT slices in the central part of the tumours. a) Patient 1 with a tumour in the upper left lobe at the ventral part of the lung, close to sternum and ribs. b) Patient 2 with a tumour in the lower left lob close to diaphragm and ribs.
The task was to outline body contour, lungs, GTV, CTV and PTV according to the protocol of the study. A treatment plan was created and the dose distribution was calculated in all CT slices. The investigators from the participating institutions filled out a CRF with information about the plan, such as volumes and doses of GTV, CTV and PTV. The CT slices, structure set, plan, dose distribution and DVHs were exported in the DICOM-RT format to the evaluating centre.
At the evaluating centre, Sahlgrenska University Hospital, the data from the plans were compiled. As a complement to the information given by the investigators, data was extracted from the DVH. The treated volume was defined as the volume which was covered with the 45 Gy (100%) isodose and the irradiated volume was defined as the volume covered with 22.5 Gy (50%) isodose according to ICRU. The relative PTV volume covered by the 45 Gy (100%) isodose, PTV V-100, was also determined. The conformity index is the ratio of 45 Gy isodose volume over the PTV. The joint PTV and GTV were calculated with the Matlab-based programme CERR Citation[8]. The software also made it possible to visualise volumes outlined at different centres on the same CT-material. The margins between CTV and PTV were evaluated in superior, inferior directions and in a central transversal plane of PTV. The margins of the transversal plane are the mean of four values along the major axes.
In order to evaluate the agreement of the outlined PTV, a measurement of coincidence of two volumes were used. As earlier described in Citation[9] a parameter αAB defined aswhere
is the volume of the intersection of A and B and
is the volume of the union of A and B. If the volumes are exactly the same, the value of αAB will be zero, if they are completely disjoined the value will be one.
Results
All six centres performed the dummy run and delivered extensive datasets. Eleven of 12 plans were complete. One centre had not outlined some of the volumes and the number of beams could not be extracted from the plan. The results obtained for the two patients such as volumes, doses and some statistical parameters from the six centres are presented in and . The definition of volumes between the different centres was similar in central parts of PTV. However, the maximum extension of delineated PTV in superior and inferior direction varied somewhat between the different centres. The median GTV volume was 29 cm3, range 24–39 cm3, and 34 cm3, range 26–41 cm3, respectively. Median PTV volume was 110 cm3, range 90–116 cm3, and 129 cm3, range 112–155 cm3, respectively. The margins between CTV and PTV were in all directions according to protocol, 5 mm in transversal direction and 10 mm in superior and inferior directions. The agreement was also demonstrated in the joint volumes of the two patients; GTV 23 cm3 and 19 cm3, respectively, and the joint PTV 84 cm3 and 106 cm3, respectively. We calculated the coincidence index of PTV and the result can be seen in . The mean index was 0.19 for patient 1 and 0.22 for patient 2.
Table I. Volume, dose and other specific parameters for patient 1 and the six centres.
Table II. Volume, dose and other specific parameters for patient 2 and the six centres.
Table III. Coincidence index for PTV and the individual centres for the two patients.
To obtain the presented dose distribution all centres used 6 MV photon beams for both patients and five to nine beams per plan. Of all beams only a few were non-coplanar beams. All centres prescribed dose at the periphery of the PTV with 15 Gy in 3 fractions. The mean GTV doses ranged from 63 to 67 Gy, median 64 Gy, for patient 1 and from 63 to 68 Gy, median 65 Gy, for patient 2. For the two patients the minimum doses for GTV ranged from 50–64 Gy, median 59 Gy, and between 55–65 Gy, median 61 Gy, respectively. Both patients had a similar maximum and mean dose for PTV, but the minimum dose varied to a larger extent. The treated and irradiated volumes, however, varied considerably between the centres for both patients. As a consequence of this variation of conformity of dose distribution the mean dose to the ipsilateral lung varied.
Discussion and conclusion
All centres delineated the volumes and showed good agreement for the central and superior parts of the tumour, but for the inferior plane the variation of PTV was for patient 2 up to 2.4 cm between the different centres. This variation had limited impact on the conformity index; due to the small volumes delineated in the most superior and inferior part of PTV. For patient 2 this variation was larger than for patient 1, probably due to the expected large variation of GTV located close to the diaphragm. The margins between CTV and PTV followed in detail the requirements of the protocol.
This dummy run resulted in low values of the coincidence index for the PTV, which indicates a good conformity in target definition. Our coincidence index is significant better compared to results published by Valley et al. where the coincidence index had a mean of 0.56, range 0.35–0.85. The ongoing Swedish head and neck study, ARTSCAN, had a mean coincidence index of 0.3 [pers. comm.]. The definition of volumes and the different margins are described thoroughly in the study protocol. This is probably the reason behind the small and acceptable variations in delineation of the different volumes.
We found a very good agreement for both patients regarding the prescribed dose, mean and maximum dose to the GTV and PTV, but the minimum dose to PTV did not show the same congruence. For patient 1, centre 1 had a minimum dose of 11 Gy, which was low, compared to centre 6 with a minimum dose of 52 Gy. This may be due to how the centres gave priority to organs at risk, in this case the ribs and sternum. The number of beams (5 to 9) in the different plans seemed to have had no significant effect on the dose conformity or other dose parameters. In this dummy run five beams was enough to obtain a good dose plan for SBRT. This is in contrast to the specification in the RTOG protocol a phase II study similar to ours, which recommended at least seven beams Citation[10].
The largest variation found in this dummy run was the spread of the treated and irradiated volumes. One centre had large volumes outside PTV for both patients receiving 45 Gy or more (treated volume). The volume of 22.5 Gy or more (irradiated volume) was also much larger for this centre compared to the others. This sub-optimal dose distribution gave a high conformity index and also a high mean dose to the ipsilateral lung, and . A consequence will be an increased risk for complications in lung and other organs receiving high dose. Another reason to this variation in conformity index was the lack of acceptability of conformity of the dose distribution. The study protocol had no limitation regarding the conformity index but this dummy run demonstrates that the prospective protocol should have such a restriction. Other SBRT protocols have included a restriction of conformity index Citation[10].
In conclusion, six centres participating in the Scandinavian SBRT study for NSCLC stage I delivered detailed volume and dose data to the evaluating centre. The delineated volumes followed study protocol with only a small variation between the different centres. The prescribed dose, mean and maximum dose to the GTV and PTV were in good agreement, but the minimum dose to PTV did not have the same congruence. One centre differed from the others with a large conformity index and a zero optimal dose distribution.
We thank the Nordic Cancer Union for financial support.
References
- Blomgren H, Lax I, Göransson H, Kraepelien T, Nilsson B, Näslund I, et al. Radiosurgery for tumours in the body: Clinical experience using a new method. J Radiosurgery 1998; 1: 63–74
- Lax I, Blomgren H, Larsson D, et al. Extracranial stereotactic radiosurgery of localised targets. J Radiosurgery 1998; 1: 135–48
- Lax I. Target dose versus extratarget in stereotactic radiosurgery. Acta Oncol 1993; 32: 453–7
- Johansson KA, Hansson WF, Horiot JC. Workshop of the EORTC Radiotherapy group on quality assurance in cooperative trials of radiotherapy: A recommendation for EORTC Cooperative groups. Radiother Oncol 1998; 11: 201–3
- Van Tienhoven G, van Bree NAM, Mijnher BJ, Bartelink H. Quality assurance of the EORTC trial 22881/10882. Assessment of the role of booster dose in breast conserving therapy: Dummy run. Radiother Oncol 1991; 22: 290–8
- Dusserre A, Garavaglia G, Giraud JY, Bolla M. Quality assurance of the EORTC radiotherapy trial 22863 for prostatic cancer: The dummy run. Radiother Oncol 1995; 36: 229–34
- International Commission on Radiation Units and Measurement, ICRU, Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50), Report 62, Washington, DC, 1999.
- Washington University in ST Louis. Computational Environment for Radiotherapy Research, CERR, 2003. http://radium.wustl.edu/CERR/about.php.
- Valley JF, Bernier J, Tecier PA, et al. Quality assurance of the EORTC radiotherapy trial 22931 for head and neck Carcinomas: The dummy run. Radiother Oncol 1998; 47: 37–44
- Radiation Therapy Oncology group, RTOG A phase II trial of stereotactic body radiation therapy in the treatment of patients with medically inoperable stage I/II none small cell lung cancer. 2004. http://www.rtog.org/members/protocols/0236/0236.pdf.
