Abstract
To identify and describe late neurological complications in a Danish testis cancer cohort treated by radiotherapy. Clinical retrospective material of 94 consecutive patients with malignant testicular tumours treated at Aarhus County Hospital from 1964 to 1973. The irradiated dose in the paraaortic field varied from 27 to 55 Gy given 5 or 6 days a week, from the back and front alternately. The biological equivalent dose of the spinal cord was calculated using the linear-quadratic model. Median follow-up was 25 years, range 7 to 33 years. Seven patients were identified with late neurological complications after irradiation. One developed symptoms 9 months after treatment, but in the six other cases we found a latency period between 10 and 20 years from radiotherapy until the initial neurological symptoms began. The clinical picture in all seven patients was dominated by muscle atrophy, flaccid paresis in the lower limbs and absence of sphincter disturbances or sensory symptoms. High spinal cord dose was related to increased risk of neurological damage. During follow-up19 patients developed another primary cancer in the radiation field; nine patients were diagnosed with severe arteriosclerosis and 13 patients with long-term gastrointestinal morbidity. Seven patients were identified with late neurological complications, and a clear dose-incidence relationship was shown. The latency period, from irradiation to the initial neurological symptoms began, ranged from 9 months to 20 years with progression of symptoms beyond 25 years. Furthermore many patients in the cohort suffered from solid tumours in the radiation field, severe arteriosclerosis and long-term gastrointestinal morbidity.
Late radiation induced damage to the spinal cord and adjacent nervous structures results in chronic progressive syndromes, which include classic radiation myelopathy, plexopathy, and neuropathy. In the lumbal part of the spinal cord an isolated motor dysfunction has been described with paralysis and loss of deep reflexes, usually described as post irradiation lower motor neuron syndrome (PLMNS) Citation[1], Citation[2]. Usually these syndromes initiate up till 2 years after radiotherapy. However, a latency period of 13 years in a single patient developing PLMNS have been described Citation[3]. Long-term neurological damage after radiotherapy has usually been analysed with an average follow-up period below 10 years Citation[4]. However there is growing evidence that complication involving nerves progress in both prevalence and severity with time after radiotherapy Citation[5], Citation[6].
Radiation is an established curative organ-conserving therapy and the success of cancer treatment has led to longer patient survival, therefore it is important to know the late effects many years after treatment.
The aim of this study was to identify and describe late neurological complications in a Danish testis cancer cohort treated with radiotherapy.
Material and methods
Patients
Clinical retrospective material of consecutive patients with malignant testicular tumours treated at Aarhus County Hospital between September 1964 and April 1973. A total of 94 patients, 55 with non-seminoma and 39 with seminoma living in Aarhus County were included. The selection criteria for this study were: (1) Alive 5 years after diagnosis; (2) No chemotherapy, or previous radiotherapy; (3) No previous malignancy. The median follow-up time was 25 years (range 6.6–32.9 years). The median age when radiotherapy was initiated was 34 years (range 18.8–64.2 years). The median age for patients with seminomas was 37 years (range 25.3–55.1 years), and with non-seminomas 31 years (range 18.8–64.2 years).
Data
The data was obtained from the case records where patients were followed one or twice a year for at least 5 years. Details of radiotherapy were available from the individual treatment sheets. Discharge letters of every relevant admission on hospitals and letters from private neurologist were obtained. Copies of death certificates from patients who died before 1998 were available. Fifty-eight (62%) family physicians were contacted by telephone with specific questions about the patient health and working capacity.
Treatment
The treatment of testicular cancer was orchidectomy and subsequently irradiation given either therapeutic or prophylactic to the regional lymph nodes. The radiation therapy was given with 2 field technique and included the para-aortic lymph nodes bilaterally and inguinal and iliac lymph nodes unilaterally. The upper border of the treatment field was usually placed at the lower margin of the eleventh thoracic vertebra (). Of the 94 patients, 89 received cobalt therapy and five were irradiated with photons from a linear accelerator. The radiation therapy was given 5 or 6 days a week, from the front and back alternately. Only 13 patients were irradiated back and front each day. The prescribed radiation dose in the para-aortic field including the spinal cord varied from 27 Gy to 54.5 Gy in 9 to 28 fractions in 13 to 47 days. Dose per fraction varied from 1.5 to 4 Gy.
Biological dose
To calculate the absorbed total spinal cord dose in each patient, we calculated the Biologically Equivalent Dose given in 2 Gy per fraction using the linear-quadratic model. A value for α/β = 2 Gy was assumed to be reasonable for late reaction of the spinal cord, and the reference depth from skin to spinal cord = 3 cm.
Statistical methods
Statistical analysis was performed using the SPSS 11.0 for Windows program package. The survival function and probability calculation were made according to the Kaplan-Meier method.
Results
Seven patients were identified with late neurological complications after irradiation (). One patient developed neurological symptoms 9 months after treatment, but in the six other cases we found a latency period between 10 and 20 years from radiotherapy to the initial neurological symptoms began. Furthermore we observed a slow progression of symptoms over 30 years with five of the patients developing paralysis. The clinical picture in all seven patients was dominated by muscle atrophy, flaccid paresis in the lower limbs, missing patellar- and Achilles reflex with absence of sphincter disturbances and sensory symptoms. These characteristics are all defined in the type of neuropathy with isolated motor symptoms, mostly described as PLMNS. In the two most severe cases fasciculations in the femoral and crural muscles are described and three patients suffered from pain in the loin and lower limbs. Four of the patients underwent a cerebrospinal fluid examination, which in three cases showed unspecific protein increase and in one case normal values.
Table I. Patients identified with late neurological complication after irradiation
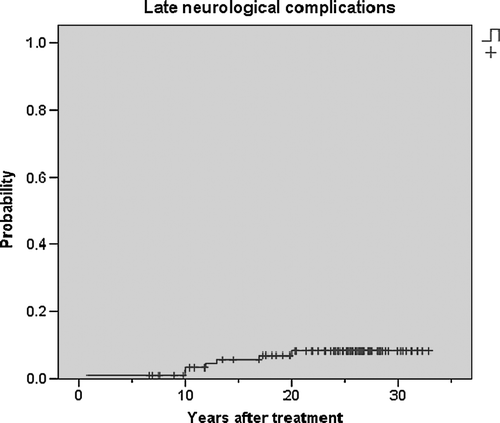
illustrates that the probability of developing neurological syndromes in this cohort increases up till 20 years after radiotherapy.
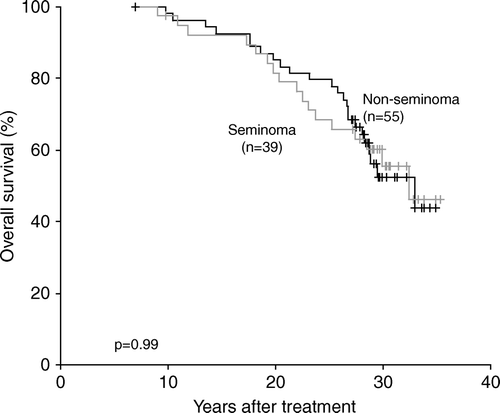
The overall survival for the patients divided by tumour type is shown in . As expected there is no difference in survival for seminoma versus non-seminoma 5 years after diagnosis. The relapse and death after testis cancer occur usually within the first years after diagnosis.
Dose-response
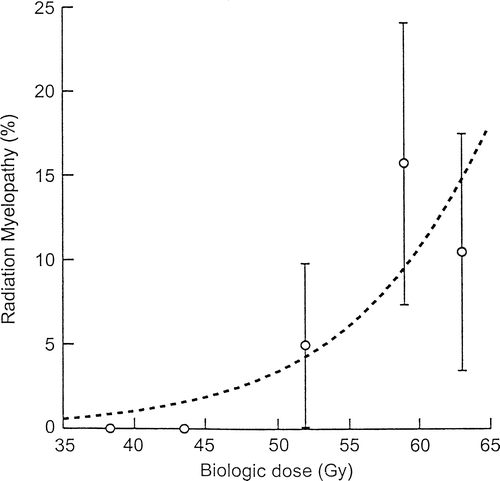
shows a significant correlation between the biologic spinal cord dose and the probability of developing neurological damage. The biologically equivalent dose, in 2-Gy fractions, range from 52 to 62 Gy to late reacting tissue, in the seven patients developing late spinal cord damage.
Secondary cancer
During follow-up also other serious problems were discovered. shows that 19 patients developed another primary cancer in the radiation field during the follow-up period. And 11 of these died due to the cancer. Five patients developed testis cancer on the contra lateral side in the follow-up period.
Table II. The number and type of secondary cancer in the radiation field
Other serious problems
Thirteen patients suffered from post irradiation intestinal damage. Four of them had peptic ulcer and at least two underwent surgeries, two suffered from malabsorbtion accompanied by grave loss of weight, four developed gangrene of the small intestine, three of them died. Three others had a gastrointestinal surgery due to ileus or stenosis. Nine patients were diagnosed with severe arteriosclerosis in the lower limbs. They were all diagnosed before they were 60 years and they all died early with a median age at 59 years (range 42–70 years). At least four of these underwent an arterioplastic surgery and one died of a mesenterial thrombosis.
Two patients underwent nephrectomy because of radiation nephritis, nine patients had medical treatment for hypertension, and one of them died of malignant hypertension.
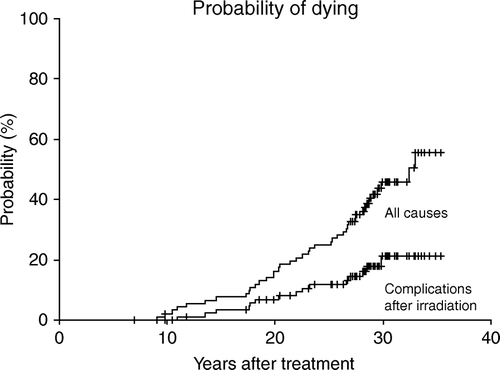
illustrates that the risk of dying of complications due to radiotherapy (n = 5) or the risk of dying of another primary cancer in the radiation field (n = 11) increases for three decades after treatment.
Discussion
Fifty percent of the patients in this cohort were still alive 30 years after treatment, and therefore they could express very slowly evolving injuries following radiotherapy.
Neurological syndromes
Seven patients were identified with late neurological syndromes after radiotherapy. A neurologist examined all these patients, other differential diagnoses were excluded, and the conclusions in the neurological letters were, that the neurological syndromes were due to earlier treatment with high doses of radiotherapy.
Nine other patients in this cohort developed neurological symptoms. Three of them had well-defined neurological diagnoses, but in the six other cases the exact diagnoses are lacking. Two patients were wheel chair users, but results from neurological examination were not available. One of them had emphysema, severe arteriosclerosis with clauditatio intermittence and he was an alcohol addict. He had disturbed gait, but no one had described the neurological status. He died 57 years old due to the alcohol abuse. The other patient, who was wheel chair user, suffered from severe adipositas, diabetes mellitus and nephropathy. The neurological symptoms were concluded to be due to the diabetes. He died 56 years old due to the chronic diseases. Three patients suffered from back pain and minor neurological symptoms in the lower limbs, but in all three cases the general practitioner has explained the symptoms as “chronic back problems”. One patient had four surgeries because of a discus prolapse or fibrosis, but continued to have back pain and decreased muscle power in the lower limbs. It is very likely, that some of these neurological syndromes to some extent were due to earlier radiotherapy, but in a retrospective material it is impossible to get closer to the truth. But we can conclude that at least seven patients suffer from neurological complications after radiotherapy.
All seven events had isolated motor dysfunction in the lower limb, two patients unilaterally and five bilaterally. These symptoms are known from earlier literature. Tallaksen et al. Citation[2] described a case report, and in a brief review they mention 30 other cases from eight different authors Citation[4], Citation[7–13]. The syndromes have many different names, but the frequently used name seems to be PLMNS. The clinical picture, though, are very similar with muscle atrophy in the lower limbs, decreased muscle power, paresis/paralysis of anterior muscles on crus, missing Achilles- and patellar reflex, drop foot often accompanied by pain and fasciculation's. Furthermore there is normal sensibility.
The localization of damage is discussed by several authors and they all seem to support localization to the anterior horn cells or the proximal segment of the nerve. Only two of the patients in this material underwent electrophysiological examination, and only a short conclusion in the neurological case record was available. None of the patients who died had an autopsy.
The cerebrospinal fluid findings (increased protein), also reported by most authors are unspecific and probably indicative of damage to the blood brain barrier.
Latency period
The latency period (from radiotherapy to the neurological symptoms initiated) in this material was median 12 years (range 9 months to 20 years) and judging from the literature this is uncommon. In 1969 Maier et al. Citation[3] described a latency period of 13 years in a single patient developing PLMNS, otherwise radiation myelopathy are reported usually to start within two years after treatment, progress over a couple of years and then reach a plateau. But in most of the studies describing PLMNS the follow-up period is less than 10 years, and that is an obvious explanation why the findings in this report are sole. Johansson Citation[6] described plexus brachialis injury in breast cancer patients after radiotherapy and found onset of neuropathy up till 17 years after completion of the irradiation with progression of the severity of the symptoms beyond 30 years after. These data demonstrate that follow-up more than 5 years is essential to evaluate late side effects.
The late damage in the spinal cord is not fully understood. But there is now increasing evidence indicating that the response of the CNS after radiotherapy is a continuous and interacting process Citation[5].
Secondary cancer
Nineteen patients developed secondary solid tumours in the treatment field. Travis et al. Citation[14] made a follow-up of long-term survivors after testicular cancer and reported that excess of leukaemia and cancers of stomach, bladder and possibly, pancreas were associated with radiotherapy. Furthermore they concluded that men with testicular cancer continued to have a significantly elevated risk of second malignant neoplasm for more than two decades following initial diagnosis. Møller et al. Citation[15] investigated 6 187 Danish men with testicular cancer in the period 1943–1987 and found an increased risk for leukaemia, gastric cancer, pancreatic cancer, bladder cancer, non-melanoma skin cancer and kidney cancer. Especially leukaemia was reported to appear in the first 10 years after testicular cancer diagnosis, whereas the excess incidence of the solid tumours was strongest 10–19 years after. In our material all patients dying within 5 years after diagnosis were excluded, and this could explain that we do not see any patients with leukaemia. No observed-to-expected calculation was made on this small material, and it is impossible to state that all cancers in the treatment field are due to earlier irradiation, but it is very likely that the majority of these cancers are. In a review Bokemeyer Citation[16] concludes that a three-to sevenfold increased risk seems to exist for the development of solid tumours arising in the previous radiation ports in patients treated for testicular cancer.
Five patients developed a new primary testicular cancer on the contra lateral side. It is well known, that patients with earlier history of testicular cancer have an increased risk of carcinoma in situ on the contra lateral side, followed by an increased, not treatment-related, risk of developing cancer Citation[17].
Other severe complications
Radiotherapy can induce and aggravate arteriosclerosis of the large arteries, with lesions sharply defined within the radiation field Citation[18]. Irradiation influences the permeability and cellular processes of the vessel wall which leads to development of the atheromatous plaque, which are not different from plaques in patients who never received radiotherapy. Gastrointestinal damage is a well-known complication after infradiaphragmatic radiotherapy. Dyspepsia and diarrhoea is common, but also more severe adverse effects such as peptic ulcer, malabsorbtion syndrome, ileus and stenoses are described Citation[19]. In the present cohort it is difficult to distinguish between arteriosclerosis and gastrointestinal damage and some of the complications mentioned above are probably a combination. In animal models histopathologic effects of chronic radiation damage of the rectum are changes in the thickness of the submucosal layer and degeneration of the vessel wall Citation[20]. Radiation nephritis is well known from the literature as both volume and dose related, and often accompanied by hypertension Citation[21]. It is impossible, though, to conclude that the hypertension is due to earlier radiotherapy.
Among 94 long-term survivors we identified 35 patients who suffered from different severe complications within the radiation field. Most often medical records contain only information about major treatment-related side effects and underreport minor somatic as well as sexual and psychosocial complications. Although important Citation[22], they can not be discussed in this retrospective study.
Why did it go so wrong?
In the 60th and 70th the treatments of choice for testicular cancer were orchidectomy and radiotherapy. Especially non-seminomas were relatively radioresitant and had to have high doses of radiotherapy to achieve tumour control. Furthermore the field size were large, and the patients young with a long survival time. The patients were treated according to the best knowledge available at that time.
The radiotherapy was given from the front and back alternately, leading to a poor dose distribution through the patients with high total dose and dose per fraction.
The possibility exists, that overlap of irradiated fields resulting in hot spot areas, may have occurred in an occasional case during this period, although there is no direct evidence of this from the data available.
Today the treatment technique has changed dramatically after introduction of cisplatin-based chemotherapy to primarily non-seminomas, and better radiation technique with eliminating the risk of hot spot areas and awareness of the risk with hypofractionation. The long-term overall survival for testis cancer patients today is 95%. However, there are still reported post-treatment long-term sequelae which emphasize the importance of long-term surveillance Citation[23].
Seven patients were identified with late neurological complications all described as a post-irradiation lower motor neuron syndrome (PLMNS), and a clear dose-incidence relationship was shown. The latency period, from irradiation to the initial neurological symptoms began, ranged from 9 months to 20 years with progression of symptoms beyond 25 years.
Furthermore many patients in the cohort suffered from solid tumours in the radiation field, severe arteriosclerosis and long-term gastrointestinal morbidity. The number and severity of treatment-related complications increased through three decades after radiotherapy, suggesting continued surveillance of survivors is important to evaluate late side effects.
Acknowledgements
This work was supported by the Danish Cancer Society.
References
- Grau C. Damage to the spinal medulla caused by radiation. Ugeskr Laeger 1993; 155: 208–11
- Tallaksen CM, Jetne V, Fossa S. Postradiation lower motor neuron syndrome–a case report and brief literature review. Acta Oncol 1997; 36: 345–7
- Maier JG, Perry RH, Saylor W, Sulak MH. Radiation myelitis of the dorsolumbar spinal cord. Radiology 1969; 93: 153–60
- Schiodt AV, Kristensen O. Neurologic complications after irradiation of malignant tumors of the testis. Acta Radiol Oncol Radiat Phys Biol 1978; 17: 369–78
- Wong CS, van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: Implications for neuroprotection. Mol Interv 2004; 4: 273–84
- Johansson S, Svensson H, Larsson LG, Denekamp J. Brachial plexopathy after postoperative radiotherapy of breast cancer patients–a long-term follow-up. Acta Oncol 2000; 39: 373–82
- Greenfield MM., Stark FM. Post-irradiation neuropathy. AJR Am Roentgenol 1948; 60: 617–622
- Gallego J, Delgado G, Tunon T, Villanueva JA. Delayed postirradiation lower motor neuron syndrome. Ann Neurol 1986; 19: 308–9
- Sadowsky CH, Sachs E, Jr, Ochoa J. Postradiation motor neuron syndrome. Arch Neurol 1976; 33: 786–7
- Lagueny A, Aupy M, Aupy P, Ferrer X, Henry P, Julien J. Post-radiotherapy anterior horn cell syndrome. Rev Neurol (Paris) 1985; 141: 222–7
- De Carolis P, Montagna P, Cipulli M, Baldrati A, D'Alessandro R, Sacquegna T. Isolated lower motoneuron involvement following radiotherapy. J Neurol Neurosurg Psychiatry 1986; 49: 718–9
- Horowitz SL, Stewart JD. Lower motor neuron syndrome following radiotherapy. Can J Neurol Sci 1983; 10: 56–8
- Berlit P, Schwechheimer K. Neuropathological findings in radiation myelopathy of the lumbosacral cord. Eur Neurol 1987; 27: 29–34
- Travis LB, Curtis RE, Storm H, Hall P, Holowaty E, Van Leeuwen FE, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst 1997; 89: 1429–39
- Moller H, Mellemgaard A, Jacobsen GK, Pedersen D, Storm HH. Incidence of second primary cancer following testicular cancer. Eur J Cancer 1993; 29A: 672–6
- Bokemeyer C, Schmoll HJ. Treatment of testicular cancer and the development of secondary malignancies. J Clin Oncol 1995; 13: 283–92
- von der Maase H, Rorth M, Walbom-Jorgensen S, Sorensen BL, Christophersen IS, Hald T, et al. Carcinoma in situ of contralateral testis in patients with testicular germ cell cancer: Study of 27 cases in 500 patients. Br Med J (Clin Res Ed) 1986; 293: 1398–401
- Lawson JA. Surgical treatment of radiation induced atherosclerotic disease of the iliac and femoral arteries. J Cardiovasc Surg (Torino) 1985; 26: 151–6
- Fossa SD, Aass N, Kaalhus O. Long-term morbidity after infradiaphragmatic radiotherapy in young men with testicular cancer. Cancer 1989; 64: 404–8
- Lundby L, Overgaard J, Laurberg S. Histopathological and morphometric analyses of late rectal injury after irradiation. APMIS 2000; 108: 216–22
- Krochak RJ, Baker DG. Radiation nephritis. Clinical manifestations and pathophysiologic mechanisms. Urology 1986; 27: 389–93
- Douchez J, Droz JP, Desclaux B, Allain Y, Fargeot P, Caty A, Charrot P. Quality of life in long-term survivors of nonseminomatous germ cell testicular tumors. J Urol 1993; 149: 498–501
- Fossa SD. Long-term sequelae after cancer therapy–survivorship after treatment for testicular cancer. Acta Oncol 2004; 43: 134–41