Abstract
We aimed to investigate the mechanisms of cisplatin resistance using an in vitro cancer model. A derivative breast cancer cell line (MCF-7CR) was established which demonstrated significant resistance to cisplatin at clinically relevant low concentrations compared to the MCF-7 parental cell line. Expression microarray analysis was used to identify targets from a 3k cancer-related oligonucleotide platform which were differentially expressed between the derivative and parental cell lines. Real-time quantitative PCR was used to confirm the difference in expression of a subset of genes which demonstrated significant up- or down-regulation. Using expression microarray analysis a total of 28 genes were identified to be differentially expressed (by at least 2-fold) between the MCF-7 and MCF-7CR cells. Real-time quantitative PCR expression analysis confirmed the differential expression of a selection of these genes (ACTG2, ARHD, CTSL, GSTM3, GSTM4 and EHF) between the two cell lines. An in vitro model of cisplatin resistance has been established and expression microarray analysis revealed 28 genes which may be associated with cisplatin resistance.
One of the most problematic areas in the management of breast cancer is the treatment of patients with poor prognosis metastatic breast cancer. These patients have circulating tumour cells in peripheral blood and liver or bone metastases. Approximately 17–65% of patients with advanced metastatic breast cancer will initially respond to chemotherapy treatment Citation[1], Citation[2] but many will subsequently relapse Citation[3]. Cisplatin has been used as a first-line single agent therapy in metastatic breast cancer patients and achieves response rates of approximately 50% Citation[4] but the cisplatin/docetaxel combination is emerging as a promising therapy for first-line treatment of metastatic breast cancer patients, achieving response rates of 64% in one study Citation[5]. The potential of cisplatin/docetaxel therapy for first-line treatment of metastatic breast cancer patients was realised after its initial success as a salvage regimen for metastatic breast cancers which had proven unresponsive to anthracycline-based therapy Citation[6–8].
The ability to identify metastatic breast cancer patients who would respond to a particular treatment regime would be advantageous. This would avoid the needless treatment of patients who would not benefit from the treatment, sparing them the associated side effects. The identification of novel genes associated with resistance to chemotherapy in poor prognosis metastatic breast cancer may also lead to the development of new treatment agents. Cisplatin is also widely used in other tumour types. We aimed to establish an in vitro model and utilise microarray (MA) analysis to identify changes in gene expression associated with the development of cisplatin resistance.
Materials and methods
Establishment of cisplatin-resistant cell line
Cell lines were maintained in RPMI 1640 media (Invitrogen, Paisley, UK) supplemented with 10% foetal calf serum (Invitrogen), 2 mM glutamine (Invitrogen), 100 U/ml penicillin (Invitrogen) and 100 µg/ml streptomycin (Invitrogen). A humidified atmosphere of 37°C and 5% CO2 was provided for cell growth. MCF-7CR was established from the surviving population of parent MCF-7 breast cancer cells (initially derived from a metastatic infiltrating ductal carcinoma) after treatment of the parent population with seven cyclic 24 h treatments with a final concentration of 50 µM cisplatin (#P4394, Sigma-Aldrich, Dorset, UK) in the media, followed by approximately 30 days of drug-free culture. The concentration of cisplatin used to establish the resistant cell line was based upon reported clinically achievable plasma concentrations Citation[9], Citation[10] as well as our own dose response observations in order to apply a degree of clinical relevance to this in vitro study.
Assessment of in vitro cisplatin resistance
Cells were plated in triplicate into 96-well tissue culture plates at a concentration of 10 000 cells/well in 200 µl media. Cisplatin was added at a final concentration in the range of 3.3–117 µM 24 h post-plating and the cells were incubated for 24 h in 5% CO2 at 37°C. Cell survival was measured using the MTT assay as previously described Citation[11]. Absorbance was read at an excitation wavelength of 570 nm on a Multiscan MS plate reader (Thermo Electron Corporation, Waltham, MA, USA). Cell survival was measured as a percentage of the survival of the untreated control cells. For both cell lines the concentration of cisplatin which resulted in 50% growth inhibition (IC50) was determined. The ANOVA statistical test was used to determine significant resistance of MCF-7CR over the dose range of 0–117 µM.
RNA extraction & quantitation
Total RNA was extracted using the Qiagen RNeasy® Mini Animal Cell RNA Extraction Kit (#74104 Qiagen Ltd, Crawley, UK) according to the manufacturer's protocol. RNA was quantified and assessed using the RNA 6000 Nano Kit and Bioanalyser (Agilent Technologies UK Ltd, West Lothian, UK) according to manufacturer's protocols and an RNA integrity number of ≥ 8.5 was used to select those samples of sufficient quality for MA analysis.
Reverse transcription PCR (RT-PCR)
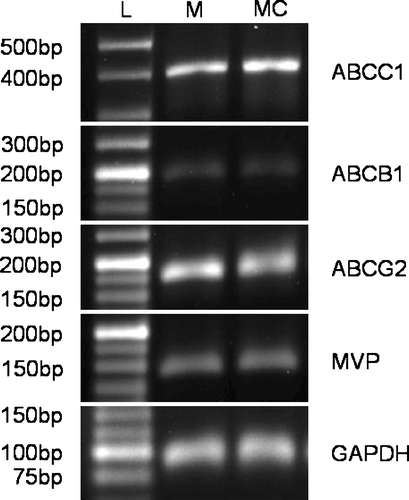
RT-PCR was carried out using the using the Power SYBR® Green Supermix (#4367659, Applied Biosystems, Warrington, UK) following the manufacturer's protocols and using the reagents therein. Reactions were carried out using the equivalent of 50 ng template RNA in 25 µl reactions using primers for the ABCB1, ABCC1, ABCG2 and MVP encoding drug efflux pumps (). RT-PCR was carried out using a GeneAmp PCR System 2400 PCR machine (Applied Biosystems) using the following program: 1 cycle of 95°C 10 min; 40 cycles of 95°C 15 s, 60°C 1 min; final hold at 4°C. Samples were electrophoresed in 3% agarose gels at 80 V for 60–80 min. Products were visualised by scanning with a Pharos FX™ Molecular Imager (Bio-Rad Laboratories, Hemel Hempstead, UK), at a resolution of 50 µm using a wavelength suitable for detection of ethidium bromide.
Table I. Primers used successfully for RT-qPCR and RT-PCR. Sequences were selected using a design service (Sigma-Aldrich), RTPrimerDB (http://medgen.ugent.be/rtprimerdb/) or PrimerBank (http://pga.mgh.harvard.edu/primerbank/).
Microarray slides
Expression MA slides were constructed using the Array-Ready™ Human Cancer Subset v3.0 (#806103 Operon, Cologne, Germany). The subset consisted of 2 876 oligonucleotides (70 mer) with 12 control housekeeping genes designed from the UniGene Database Version Hs 147 and the Human Reference Sequence (RefSeq) Database (www.ncbi.nlm.nih.gov). A QArrayMini microarray printer equipped with 16 split tungsten MA pins of 100 µm diameter (Genetix, New Milton, UK) was used to print oligonucleotides in a triplicate random pattern onto epoxy-coated glass microscope slides (Nexterion slide E #1066643, Schott AG, Mainz, Germany). Slides were printed at 8°C in 50% humidity and quality checked before use. The full list of genes on the MA can be viewed at: http://omad.operon.com/download/index.php
Expression microarray analysis
Indirect cDNA labelling was performed using the FairPlay II® Microarray Labelling Kit (#252006, Stratagene, La Jolla, CA, USA) according to the manufacturer's protocol using Cy3 and Cy5 monofunctional reactive dyes (#PA23001, #PA25001, GE Healthcare, Buckinghamshire, UK). Post-labelling, the samples were combined and concentrated to 40 µl using a Microcon® Centrifugal Filter Device (Millipore, Watford, UK) according to the manufacturer's protocol. Slides were washed in 50 ml centrifuge tubes on an end-over-end rotary mixer (12 rpm) at room temperature. Slides were washed once in Triton X-100 (0.1% v/v) for 5 min, twice in two separate washes of 1 mM HCl for 2 min, once in 100 mM KCl solution for 10 min and finally in distilled water for 1 min. Slides were blocked by incubating in 1× Nexterion® Blocking Solution (#1066071, Schott) for 15 min at 50°C, followed by rinsing in distilled water for 1 min at room temperature, again utilising 50 ml centrifuge tubes and a rotary mixer (12 rpm). Slides were dried by centrifugation at 200 g for 5 min. Once dried, a Hybriwell™ adhesive coverslip (#HBW2240, Grace Bio-Labs, Oregon, USA) was applied to the slide. The labelled, pooled cDNA (40 µl) was mixed with 5 µl of 7.5% w/v (0.2% final concentration) BSA (#15260037 Invitrogen, Paisley, UK), 2 µl of 1 µg/ul human Cot-1 DNA (#15279011, Invitrogen), 2 µl of 1 µg/ul Oligo d(A) (#POLYA.GF, Invitrogen) and 151 µl Nexterion® Hybridisation Buffer E (#1066075, Schott), to make a total hybridisation volume of 200 µl. The hybridisation mix was denatured at 95°C for 3 min in a thermal cycler before application to the MA slide. Slides were sealed into humidified hybridisation chambers (MWG-Biotech AG, Ebersberg, Germany) and incubated at 65°C for 16–18 h in a dark hybridisation oven. Post-hybridisation, the coverslip was removed and slides were rinsed in filtered 4× SSC using a 50 ml syringe. Slides were washed in 50 ml centrifuge tubes for 5 min in 2× SSC/0.1% w/v SDS (pre-warmed to 42°C), 5 min in 2× SSC/0.1% w/v SDS, 1 min in 0.2× SSC and 1 min in 0.1× SSC. Slides were dried by centrifugation at 200 g for 5 min. Images were acquired using the GenePix™ 4100A Personal Microarray Scanner in combination with Genepix™ Pro® v4.1 image collection software (Molecular Devices Corporation, Sunnyvale, CA, USA). The MA slides were scanned at a resolution of 5 µm/pixel with the photomultiplier tube gains manually set for each dye to ensure a normal ratio across the whole array of 1 (±0.1). After application of the .gal file the results files were imported into Acuity™ v4.0 (Molecular Devices Corporation) and normalised using the non-linear Lowess normalisation method. Once the data for each slide had been imported into Acuity™, a dataset was created by filtering each array to include only those features with the parameter “Flags ≥ 0”. This ensured that only those features which were “found”, and not “bad” or “not found”, were included in the analyses. Once filtered, the data were analysed according to the following parameters: Low inter-array variance (p ≤ 0.05) and absolute log (635/532) value ≥ 1.0 in three of four independent experiments. A log ratio value of ≥ 1 was chosen for the cut-off as this represented an actual fold change in expression of 2-fold or greater, which is a standard for MA analysis Citation[12].
Real-time quantitative PCR (RT-qPCR)
Two micrograms of total RNA were treated with 2 U of amplification grade DNase 1 (#AMP-D1, Sigma-Aldrich) at room temperature for 15 min to remove any DNA contamination and then reverse transcribed into cDNA using the iScript™ cDNA Synthesis Kit (#170-8890 Bio-Rad Laboratories) following the manufacturer's protocol. RT-qPCR was carried out with 50 ng equivalent RNA template using the iQ™ SYBR® Green Supermix (#170-8880, Bio-Rad Laboratories) following the manufacturer's protocols in a 20 µl reaction volume on a MyiQ™ Single-Colour Real-Time PCR Detection System (Bio-Rad Laboratories). Primer details are shown in and were synthesised by Applied Biosystems. Data was collected using the MyiQ™ Optical System Software (Bio-Rad Laboratories) and data analysis was carried out according to the method of Pfaffl (2001) Citation[13]. In order to assess the relative changes in gene expression between samples, the amplification efficiency of each gene-specific primer set was determined for all of the target genes and the GAPDH reference gene. The final efficiency value was obtained by averaging three independent replicate standard curves (range 50 ng–5×10−03 ng equivalent RNA template) for each primer set and changes in expression of each gene were then calculated using the equation:where, Etarget and Eref are the relative real-time PCR efficiencies of the target and reference (GAPDH) primer sets respectively and ΔCTtarget and ΔCTref are the difference between the control and sample threshold cycle values for the target and reference (GAPDH) genes.
Results
Establishment of MCF-7CR cells
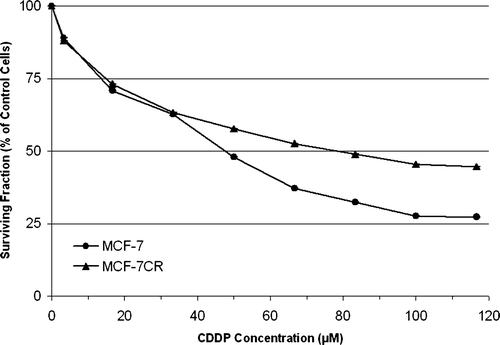
The novel cisplatin -resistant MCF-7CR cell line was established after seven sequential treatments with 50 µM (final concentration) cisplatin, each for 24 h. demonstrates the relative resistance of MCF-7CR, when compared with parental MCF-7 cells, to cisplatin determined using the MTT assay. The IC50 values of MCF-7 and MCF-7CR were 48 µM and 79 µM respectively, making MCF-7CR approximately 1.6-fold more resistant to cisplatin than the MCF-7 parent line. Phenotypic characterisation of MCF-7 and MCF-7CR cells using western blotting and immunocytochemistry revealed similar positive expression for ER and PgR and no change in the wild type p53 status (data not shown). Other phenotypic changes observed in MCF-7CR included a decrease in cell area from a mean of approximately 942 µm2 to approximately 552 µm2. Importantly, RT-PCR indicated no difference in expression of the major drug pumps ABCB1 (MDR1, P-gp), ABCC1 (MRP1), ABCG2 (BCRP1), or MVP (LRP) between MCF-7 and MCF-7CR ().
Figure 1. Relative resistance of MCF-7CR and MCF-7 cells to cisplatin (CDDP) over the concentration range 0–117 µM (final concentration in media). Each point represents the means of at least 3 individual experiments and error bars* represent the standard error of the mean for each point. The IC50 values of MCF-7 and MCF-7CR were 48 µM and 79 µM respectively, making MCF-7CR approximately 1.6-fold more resistant to cisplatin than the MCF-7 parent line. The MCF-7CR cells were significantly more resistant to cisplatin than MCF-7 cells over the dose range 50–117 µM (p < 0.05 by ANOVA). *Error bars are less than the size of the data point symbols.
Expression microarray analysis
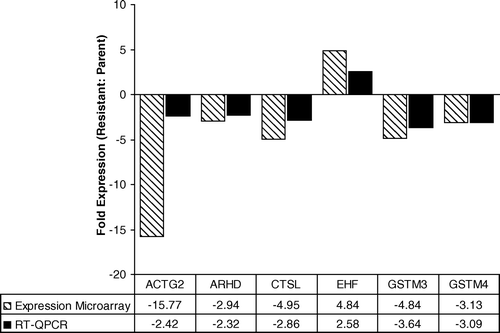
Using the standard 2-fold change in expression as our threshold, expression microarray analysis revealed significant changes in expression of 28 genes (). Eight genes demonstrated an increase in expression (ranging from 2.77 to 5.27 fold change) in MCF-7CR relative to MCF-7. A total of 20 genes demonstrated a reduced level of expression (ranging from 2.34 to 15.77 fold change) in MCF-7CR relative to MCF-7.
Table II. Expression values for each of the genes identified as significantly associated with the observed in vitro resistance of MCF-7CR to cisplatin using expression MA analysis. Gene ontology information was derived from the GOA database (http://www.ebi.ac.uk/GOA/). *Target selected for RT-qPCR confirmation.
RT-qPCR
To validate the MA data, seven genes were selected from the list of 28 for confirmation using RT-qPCR. Analysis of EHF, ACTG2, CTSL, GSTM3, GSTM4, and ARHD revealed a high degree of concordance in the direction of change (increase or decrease) in expression (). The change in expression was at least 2-fold when quantitated using RT-qPCR for each of these genes, although a higher level of expression change was suggested by the MA analysis in each case (). After numerous attempts using several primer designs we were unable to generate any data for PRL using RT-qPCR.
Discussion
Establishment of MCF-7CR cells
The aim of this study was to establish an in vitro model of cisplatin resistance and to identify changes in gene expression associated with the selection of a cisplatin -resistant cell population. We utilised a cyclical treatment protocol and worked with a low dose range to simulate the clinical scenario as closely as possible. A major challenge for this study was the correlation between drug concentrations which can be used in vitro and the relative drug concentrations which are administered to a breast cancer patient. As such, the concentration of cisplatin used to establish the derivative cell line was selected based on data for peak plasma concentrations of cisplatin in cancer patients of 6.3–12.6 µM Citation[14] in combination with information from our initial dose response curve. Cells were monitored after each cycle as we were interested only in producing a low-level of resistance, which may be more clinically relevant than thousand-fold changes. Therefore the selection of MCF-7CR cells was halted after the seventh cycle of treatment as the cells were demonstrating significant resistance. Analysis of the expression of the major drug transporters associated with multidrug resistance (ABCB1, ABCC1, ABCG2, MVP) revealed no differential expression, indicating that the observed difference in sensitivity to cisplatin is independent of the expression of these genes.
Expression MA analysis
Using MA analysis with a 3k cancer-related gene expression platform, a total of 28 genes were identified with significant difference in expression level (at least 2-fold) between MCF-7 cells and the MCF-7CR subpopulation. Of these genes, eight demonstrated an increase in expression and 20 demonstrated a decrease in expression in the cisplatin -resistant cell line. Six genes were tested using RT-qPCR to successfully validate our MA data. Cisplatin is a DNA cross-linking agent which elicits cell death by inducing the formation of inter- and intra-strand crosslinks and bulky adducts within the DNA molecule, resulting in gross distortion of the DNA molecule and inhibition of repair and replication. Gene ontology analysis revealed that most of the 28 genes identified in the MA analysis belonged to the broad functional categories of cell cycle regulation/cell proliferation, signal transduction and metabolism.
Cell cycle regulation/cell proliferation
Of the genes with a known role in cell cycle regulation/cell proliferation, PRL and EDN1 demonstrated increased expression whilst CCNB1, MDK, MYBL2, MAD2L1) demonstrated decreased expression in MCF-7CR cells. Most of these genes have roles in promoting cell proliferation or progression through the cell cycle. Both PRL (prolactin) and EDN1 (endothelin 1) encode secreted proteins known to promote cell proliferation Citation[15], Citation[16]. Endogenously expressed prolactin has been demonstrated to protect established breast cancer cell lines from ceramide-dependent apoptosis Citation[17], a pathway invoked by many chemotherapy drugs Citation[18]. The reduction in expression of cell proliferation-associated genes suggests an inhibition of cell cycle progression and in MCF-7CR, possibly increasing the time for DNA repair. The reduction in expression of CCNB1 (cyclin B1) suggests inhibition of the G2/M phase transition of the cell cycle and resistance to apoptosis Citation[19].
Signal transduction
In total, three genes (DUSP4, HRMT1L2 and ARHD) associated with signal transduction demonstrated differential expression between MCF-7 and MCF-7CR. DUSP4 (MKP2) is a dual specificity protein kinase phosphatase which is associated with overexpression in breast cancers Citation[20], and known to be a regulator of the MAP kinase signalling pathway Citation[21]. ARHD was isolated from a human placental cDNA library and, demonstrating significant homology to the Rho genes, was initially designated Rho-related protein HP1 (RhoHP1) Citation[22]. Rho proteins are essential for FAS-induced apoptosis through modification of the actin cytoskeleton Citation[23].
Metabolism
In total, nine genes which demonstrated a change in expression between MCF-7 and MCF-7CR appear to have functional roles in metabolism. Of these nine genes, only two (CHST1 and SLC7A1) demonstrated increased expression in MCF-7CR cells. Two genes which demonstrated a reduction in expression in MCF-7CR belong to the same class of glutathione-S-transferase (GST) enzymes. GSTM3 and GSTM4 belong to the “mu-class” of GST enzymes, of which there are five in total. Generally, the GST enzymes function by conjugating glutathione to electrophilic xenobiotics, making them more water soluble and therefore easier to excrete. In an early yeast cell culture model, overexpression of members of the GST family induced resistance to the chemotherapy drugs chlorambucil and doxorubicin Citation[24]. Since this initial study, several researchers have investigated the role of overexpression of specific GST class enzymes in the resistance of human cancer cell lines to cytotoxic drugs. A model involving MCF-7 breast cancer cell lines stably transfected to overexpress GST mu class cDNAs established no role for GST mu enzymes in conferring resistance to a wide range of drugs including cisplatin Citation[25]. We have identified downregulation of both GSTM3 and GSTM4 in association with cisplatin resistance in both MA and confirmatory RTqPCR analyses. Although the functional significance of this finding is not clear as yet, our proteomics study of the same cell lines has independently identified the significant downregulation of the GSTM3 protein in MCF-7CR with a complete loss of expression demonstrated by western blotting (unpublished data). The importance of these findings will require further investigations.
Conclusions
This study has established a model of low level cisplatin resistance based upon clinical parameters. The observed resistance was independent of the expression of major drug pumps. Using a cancer specific expression MA platform we identified the differential expression of 28 genes which may be involved in the development of cisplatin resistance in breast cancer. Functional studies using gene silencing methods and/ or pharmacological inhibitors will determine whether such proteins are the cause or result of resistance to this agent. In addition, these targets must be validated on clinical samples. This is because the microenvironment influences the behaviour of tumour cells and cultured cells do not have this influence. Thus, although in-vitro cell culture models represent a good starting point they are not truly indicative of the in-vivo situation. The identification of novel biomarkers that are associated with cisplatin resistance may allow treatment to be tailored on an individual patient basis and provide novel targets for future therapeutic intervention. Ultimately, the ability to overcome resistance to cisplatin may represent a major advance in the effective management of cancer today.
Acknowledgements
We gratefully acknowledge Mrs Barbara Smith and Dr Naveed Aziz at the University of York Technology Facility, York, UK, for their assistance in the manufacture of the expression MA slides described in this study.
References
- Robain M, Pierga JY, Jouve M, Asselain B, Dieras V, Beuzeboc P, et al. Predictive factors of response to first-line chemotherapy in 1426 women with metastatic breast cancer. Eur J Cancer 2000; 36: 2301–12
- Dose Schwarz J, Bader M, Jenicke L, Hemminger G, Jänicke F, Avril N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PET. J Nucl Med 2005; 46: 1144–50
- Pusztai L, Asmar L, Smith TL, Hortobagyi GN. Relapse after complete response to anthracycline-based combination chemotherapy in metastatic breast cancer. Breast Cancer Res Treat 1999; 55: 1–8
- Martin, M. Platinum compounds in the treatment of advanced breast cancer. Clin Breast Cancer 2001;2:190–208; Discussion 209.
- Vassilomanolakis M, Koumakis G, Barbounis V, Demiri M, Panopoulos C, Chrissohoou M, et al. First-line chemotherapy with docetaxel and cisplatin in metastatic breast cancer. Breast 2005; 14: 136–41
- Vassilomanolakis M, Koumakis G, Barbounis V, Demiri M, Pateras H, Efremidis AP. Vinorelbine and cisplatin in metastatic breast cancer patients previously treated with anthracyclines. Ann Oncol 2000; 11: 1155–60
- Rosati G, Riccardi F, Tucci A, De Rosa P, Pacilio G. A phase II study of paclitaxel/cisplatin combination in patients with metastatic breast cancer refractory to anthracycline-based chemotherapy. Tumori 2000; 86: 207–10
- Vassilomanolakis M, Koumakis G, Demiri M, Missitzis J, Barbounis V, Efremidis AP. Vinorelbine and cisplatin for metastatic breast cancer: A salvage regimen in patients progressing after docetaxel and anthracycline treatment. Cancer Invest 2003; 21: 497–504
- Moriuchi S, Shimizu K, Miyao Y, Yumada M, Ohkawa M, Hayakawa T. In vitro assessment for neurotoxicity of antitumor agents before local administration into central nervous system. Anticancer Res 1996; 16: 135–40
- Schondorf T, Kurbacher CM, Gohring UJ, Benz C, Becker M, Sartorius J, et al. Induction of MDR1-gene expression by antineoplastic agents in ovarian cancer cell lines. Anticancer Res 2002; 22: 2199–203
- Keepers YP, Pizao PE, Peters GJ, van Ark-Otte J, Winograd B, Pinedo HM. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur J Cancer 1991; 27: 897–900
- Schena M, Shalon D, Heller R, Chai A, Brown PO, Davis RW. Parallel human genome analysis: Microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci USA 1996; 93: 10614–9
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45
- Iwasaki I, Sugiyama H, Kanazawa S, Hemmi H. Establishment of cisplatin-resistant variants of human neuroblastoma cell lines, TGW and GOTO, and their drug cross-resistance profiles. Cancer Chemother Pharmacol 2002; 49: 438–44
- Liby K, Neltner B, Mohamet L, Menchen L, Ben-Jonathan N. Prolactin overexpression by MDA-MB-435 human breast cancer cells accelerates tumor growth. Breast Cancer Res Treat 2003; 79: 241–52
- Alberts GF, Peifley KA, Johns A, Kleha JF, Winkles JA. Constitutive endothelin-1 overexpression promotes smooth muscle cell proliferation via an external autocrine loop. J Biol Chem 1994; 269: 10112–8
- Perks CM, Keith AJ, Goodhew KL, Savage PB, Winters ZE, Holly JM. Prolactin acts as a potent survival factor for human breast cancer cell lines. Br J Cancer 2004; 91: 305–11
- Ogretmen B, Hannun YA. Updates on functions of ceramide in chemotherapy-induced cell death and in multidrug resistance. Drug Resist Updat 2001; 4: 368–77
- Porter LA, Cukier IH, Lee JM. Nuclear localization of cyclin B1 regulates DNA damage-induced apoptosis. Blood 2003; 101: 1928–33
- Wang HY, Cheng Z, Malbon CC. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett 2003; 191: 229–37
- Guan KL, Butch E. Isolation and characterization of a novel dual specific phosphatase, HVH2, which selectively dephosphorylates the mitogen-activated protein kinase. J Biol Chem 1995; 270: 7197–203
- Shimizu F, Watanabe TK, Okuno S, Omori Y, Fujiwara T, Takahashi E, et al. Isolation of a novel human cDNA (rhoHP1) homologous to rho genes. Biochim Biophys Acta 1997; 1351: 13–6
- Subauste MC, Von Herrath M, Benard V, Chamberlain CE, Chuang TH, Chu K, et al. Rho family proteins modulate rapid apoptosis induced by cytotoxic T lymphocytes and Fas. J Biol Chem 2000; 275: 9725–33
- Black SM, Beggs JD, Hayes JD, Bartoszek A, Muramatsu M, Sakai M, et al. Expression of human glutathione S-transferases in Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. Biochem J 1990; 268: 309–15
- Townsend AJ, Tu CP, Cowan KH. Expression of human mu or alpha class glutathione S-transferases in stably transfected human MCF-7 breast cancer cells: Effect on cellular sensitivity to cytotoxic agents. Mol Pharmacol 1992; 41: 230–6