Abstract
Osteonecrosis of the jaw (ONJ) has recently been reported as a potentially serious complication of prolonged treatment with intravenous bisphosphonates. We studied its frequency in prostate cancer patients receiving intravenous zoledronate. The medical and dental records of 52 consecutive patients with prostate cancer and bone metastases treated at our institute between January 2002 and October 2005 were reviewed. All patients received intravenous zoledronate 4 mg every 3 or 4 weeks and concomitant conventional prostate cancer treatments. We analysed the association of ONJ with the number of administrations of zoledronate and exposure to chemotherapy. At a median follow-up of 7 months (range 1–41) after the initiation of zoledronate, ONJ occurred in six patients (12%, 95% C.I. 5.4–23.0%). All six ONJ cases occurred after the 9th administration of zoledronate. The median number of zoledronate administrations was 17 (range 9–24) and 8 (range 1–32) for patient developing and not developing ONJ, respectively (p =0.02). Chemotherapy with docetaxel was also associated with a strong, but not statistically significant, trend towards increased risk of ONJ (OR 3.8, 95% C.I. 0.4–35.6, p =0.24). The length of exposure to zoledronate was associated with an increased frequency of ONJ in prostate cancer patients. A possible role of chemotherapy with docetaxel as a cofactor for ONJ merits further evaluation.
The skeleton is the most frequently involved site of metastatic progression in patients with prostate cancer. Bone metastases, together with treatment-induced bone loss, threaten prostate cancer patients with complications that result in significant morbidity Citation[1]. Zoledronate, one of the most biologically active bisphosphonates, is effective in reducing skeletal-related events (SREs) and increases bone mineral density associated with androgen-deprivation therapy Citation[2].
Recently, several reports have described a serious complication of treatment with intravenous bisphosphonates, particularly with zoledronate Citation[3–7]: avascular osteonecrosis of the jaw (ONJ) has been reported in 0.15 to 10% of patients exposed to both zoledronate and pamidronate in different settings, including breast cancer, multiple myeloma and prostate cancer Citation[6], Citation[8].
ONJ results in bone destruction and causes severe morbidity and impairment of quality of life because of its chronic course and lack of effective treatments. Signs and symptoms that may occur before the appearance of clinical evident ONJ include changes in the health of periodontal tissues, non-healing mucosal ulcers, loose teeth and unexplained soft-tissue infection Citation[9].
The real frequency of bisphosphonates-related ONJ is probably unknown at present for several reasons. The most important is probably that almost all reports are based on retrospective analyses triggered by the occurrence of unexpected serious dental complications. As a consequence, a wide range of entities encompassing osteonecrosis, osteomyelitis, exposed bone, bone necrosis, sequestrum, impaired healing post-dental procedure, has been considered ONJ.
Considering all the sites of osteonecrosis, cancer patients have been found to have a three-fold increase in the risk of developing these complications compared with a cancer-free population Citation[10]. Among the potential causes, including cancer-specific factors and comorbidities, chemotherapy may act as a cofactor Citation[4], Citation[11], Citation[12].
In a previous report we described the frequency of ONJ in breast and prostate cancer patients treated with zoledronic acid at our institution Citation[13]. This report focuses on prostate cancer patients. With additional follow-up and further events occurring in this series of patients, we sought to redefine the frequency of ONJ and to study possible associations with local risk factors, the number of administrations of zoledronate and with the type and duration of chemotherapy.
Material and methods
The medical records of 52 consecutive prostate cancer patients with bone metastases receiving zoledronate between January 2002 and October 2005 were reviewed. The median age was 68 years (range 49–84). Zoledronate was administered at doses of 4 mg every 3 or 4 weeks following a policy of initiation at the first evidence of bone metastases and continuation until patient performance allowed it Citation[14]. All patients received complete androgen blockade with bicalutamide and luteinizing hormone-releasing hormone analogs (LHRH-A), and 41 (78.8%) of them, who became hormonorefractory, maintained LHRH-A and were treated with chemotherapy (median time on chemotherapy 6 months, range 1–26 months).
A total of 19 (36%) patients received one line, 18 (35%) received two lines, and four received three lines of chemotherapy. Thirty-one patients received at least one line of chemotherapy containing docetaxel, which was administered weekly in 16 patients (36 mg/m2/wk times 6, followed by a 2-week rest) and once every 3 weeks (75 mg/m2) in the remaining 15 patients. The use of corticosteroids was common both in patients treated with endocrine therapy and in patients receiving chemotherapy. No patient received radiation therapy involving the head and neck.
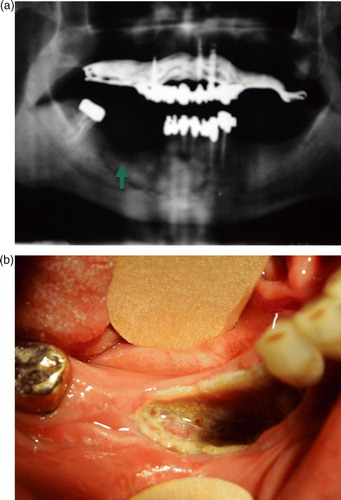
We considered ONJ as bone necrosis in non-metastatic site, documented by X-ray (a) or CT-scan together with an exposed bone in the oral cavity (b), with or without associated infection. Biopsy of the lesions was not routinely performed because of difficulties in bone repair after a traumatic procedure in patients with ONJ, as suggested by the initial reports describing this entity Citation[15]. All patients discontinued zoledronate therapy after presentation of symptoms and received conservative treatment and multiple courses of antibiotics.
Figure 1. A case of ONJ in a patient treated with zoledronate (24 administrations) and docetaxel (75 mg/m2 q3wks for a total of 5 months of treatment). Panel a) panoramic radiograph of the mandible showing classic lytic bone radiolucency in the site of a prior tooth extraction (green arrow). b) Bone necrosis exposure in the mandibula at the corresponding X-ray site.
The observed frequency of ONJ is reported as a percentage, together with its 95% confidence interval (C.I.). The difference in the number of zoledronate administrations between patients developing or not developing ONJ was evaluated by the Mann Whitney U test. Logistic regression analysis was also used to explore the potential association of ONJ with the number of zoledronate administrations (as a continuous variable) and with the use of chemotherapy, in particular docetaxel. The results are reported in terms of odds ratio (OR) together with 95% C.I.
Significance, assessed by the Wald statistics, was set at p < 0.05.
Due to the exploratory purpose and the retrospective design, we did not perform multivariate analyses.
Results
Overall, the median number of administrations of zoledronate was 8.5 (range: 1–32). At a median follow-up of 7 months (range 1–41) after the initiation of zoledronate, ONJ occurred in six patients (12%, 95% C.I. 5.4%–23%).
Five of these patients had ONJ of the mandible and one of the maxilla. For all six patients we identified periodontal injuries preceding the onset of osteonecrosis: three had undergone tooth extraction and in the other three ONJ arose in edentulous areas subject to chronic mechanical stimulus caused by dental prosthesis.
All patients presented with an area of painful exposed bone and in five cases ONJ was associated with chronic infection. In all six cases, ONJ was diagnosed after the 9th cycle of zoledronate (). The median number of zoledronate administrations was 17 (range 9–24) and 8 (range 1–32) for patients developing and not developing ONJ, respectively (Mann Whitney U test, p = 0.02). All cases of ONJ except one occurred after (4 cases), or during chemotherapy with docetaxel (1 case). Only one of these patients received docetaxel on a weekly schedule.
Table I. Characteristics of patients with ONJ.
Logistic regression analysis showed that the number of zoledronate administrations was significantly associated with the risk of ONJ (OR 1.1, 95% C.I. 1.0–1.3, p = 0.04). This would suggest that each additional administration of zoledronate is associated with a roughly 10% increase in the risk of ONJ. Having received chemotherapy with docetaxel was also associated with an increased risk of ONJ, but it did not achieve statistical significance (OR 3.8, 95% C.I. 0.4–35.6, p = 0.24). Four of the five patients developing ONJ had been treated with docetaxel for over 3 months. Conversely, other chemotherapies as well as the overall time on chemotherapy, obtained by summing-up the duration in months of all the chemotherapy lines received by patients, did not affect the risk of ONJ (data not shown).
After antibiotic treatment, only one patient experienced a major improvement of ONJ-related symptoms, together with a partial healing of the bone lesion. The remaining five patients showed minor improvements and persistence of clinical symptoms until death from progressive prostate cancer.
Discussion
In our retrospective analysis we found a 12% frequency of ONJ in prostate cancer patients treated with zoledronate from the onset of symptomatic skeletal metastases. We found a significant association of ONJ with the number of zoledronate administrations, confirming that the length of exposure to this drug may be the major risk factor for ONJ in prostate cancer patients. Additionally, for all the six patients developing ONJ we identified well known precipitating factors (tooth extractions and chronic mechanical stimulus by tooth prosthesis). Unfortunately, because of the retrospective nature of this analysis, we were not able to adequately collect information about local risk factors in patients who did not develop ONJ. Despite this limitation, however, we speculate that although prevalence of local risk factors may be high in patients not developing ONJ, it is certainly lower than the 100% we found in patients who subsequently developed ONJ.
All ONJ cases occurred after the 9th administration of zoledronate. Interestingly, for patients receiving ≥ 9 administrations, we could not find a significant trend towards an increased risk of ONJ with subsequent administrations of zoledronate. Whether this finding underlies a threshold effect of zoledronate on ONJ, rather than a cumulative effect of repeated administrations, is not ascertainable because of the low number of events.
We found a strong, albeit not statistically significant, association of ONJ with docetaxel chemotherapy, with an almost four-fold increase in the risk for treated patients. No association was found with other types of chemotherapy. This finding might be related to the antiangiogenetic properties of both zoledronate Citation[16], Citation[17] and docetaxel Citation[18], which may result in an additive or even synergistic effect on jaw vascularisation and determine a predisposition to bone necrosis after traumatic injuries Citation[5], Citation[11], Citation[19–21].
Although at present any comparison between different published reports is inappropriate, the frequency on ONJ in our report seems to be higher than that recently observed by Bamias et al. Citation[8], who described ONJ in three of 46 patients (6.5%) with prostate cancer receiving bisphosphonates. In the same paper, the authors also reported a marginally significant association of ONJ with the type of bisphosphonate that was used, suggesting that zoledronate was most frequently associated with this complication. The use of zoledronate as the only bisphosphonate in our series might therefore justify the higher incidence of ONJ, compared with other reports of patients receiving different bisphosphonates. Obviously, this finding deserves further investigation.
Patients with prostate cancer usually have additional potential risk factors for ONJ that may be intrinsic to the disease, or related to co-morbidities (anaemia, platelets disorders and haemostasis), or related to the frequent use of corticosteroids. The association of these factors with ONJ needs to be further elucidated.
Both zoledronate and docetaxel are important components in the management of patients with hormonorefractory prostate cancer with bone metastases, with confirmed advantages on survival and quality of life Citation[22], Citation[23]. Similarly to that suggested by guidelines for multiple myeloma and breast cancer, it is reasonable to treat patients with zoledronate for as long as it is tolerated or until the patient experiences a substantial decline in performance status Citation[24]. Additionally, zoledronate has been recently recommended to prevent treatment-induced bone loss in non-metastatic prostate cancer patients Citation[2].
On the other hand, bisphosphonate-related ONJ is a clinical entity causing severe morbidity and an impairment of quality of life, and its true pathogenesis and incidence will remain unknown until results of prospective studies, with adequate follow-up, become available.
For these reasons, physicians need to implement measures of oral hygiene and prevention in their cancer patients with bone metastases, regardless of the primary site of disease, in order to fully exploit the therapeutic potential of bisphosphonates and to minimize the risk of ONJ.
At our institution, zoledronate candidates undergo a basic routine dental examination, panoramic radiograph and tooth extractions (if necessary) and initiation of zoledronate is postponed until tissue healing. During treatment, patients undergo regular dental consultations, the frequency of which varies according to the presence of local risk factors for ONJ. As high concentrations of bisphosphonates are maintained in the bone for long periods of time after zoledronate discontinuation, with a consequent long-term inhibition of bone remodelling Citation[25], it is reasonable to continue regular dental consultations after discontinuation of zoledronate.
In conclusion, ONJ occurred in 12% of prostate cancer patients treated with zoledronate at our institution, with all the cases occurring after at least nine administrations of this bisphosphonate.
In our opinion, given the relevant benefits of zoledronate in this setting of patients, efforts should be focused on both preventing the development of ONJ as well as on clarifying its pathogenesis. In addition, with regard to this last issue, the possible additive antiangiogenic effects of zoledronate and docetaxel deserve further evaluation.
References
- Green JR. Skeletal complications of prostate cancer: Pathophysiology and therapeutic potential of bisphosphonates. Acta Oncol 2005; 44: 282–92
- Saad F, McKiernan J, Eastham J. Rationale for zoledronic acid therapy in men with hormone-sensitive prostate cancer with or without bone metastasis. Urol Oncol 2006; 24: 4–12
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J Oral Maxillofac Surg 2004; 62: 527–34
- Migliorati CA, Schubert MM, Peterson DE, Seneda LM. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone. Cancer 2005; 104: 83–93
- Purcell PM, Boyd IW. Bisphosphonates and osteonecrosis of the jaw. Med J Aust 2005; 182: 417–8
- Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 2005; 353: 99–102
- Sanna G, Zampino MG, Pelosi G, Nole F, Goldhirsch A. Jaw avascular bone necrosis associated with long-term use of biphosphonates. Ann Oncol 2005; 16: 1207–8
- Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J Clin Oncol 2005; 23: 8580–7
- Ficarra G, Beninati F, Rubino I, Vannucchi A, Longo G, Tonelli P, et al. Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. J Clin Periodontol 2005; 32: 1123–8
- Balcewicz-Sablinska MK, Gonzáles-Pérez A, Garcia Rodríguez LA. Risk of osteonecrosis in cancer patients. Ann Epidemiol 2004; 14: 593 (P006)
- Wang J, Goodger NM, Pogrel MA. Osteonecrosis of the jaws associated with cancer chemotherapy. J Oral Maxillofac Surg 2003; 61: 1104–7
- Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005; 63: 1567–75
- Ortega C, Faggiuolo R, Vormola R, Montemurro F, Nanni D, Goia F, et al. Jaw complications in breast and prostate cancer patients treated with zoledronic acid. Acta Oncol 2006; 45: 216–7
- Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003; 21: 4042–57
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J Oral Maxillofac Surg 2003; 61: 1115–7
- Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, et al. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 2002; 62: 6538–44
- Wood J, Bonjean K, Ruetz S, Bellahcene A, Devy L, Foidart JM, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther 2002; 302: 1055–61
- Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res 2001; 61: 3369–72
- Clezardin P, Ebetino FH, Fournier PG. Bisphosphonates and cancer-induced bone disease: Beyond their antiresorptive activity. Cancer Res 2005; 65: 4971–4
- Brubaker KD, Brown LG, Vessella RL, Corey E. Administration of zoledronic acid enhances the effects of docetaxel on growth of prostate cancer in the bone environment. BMC Cancer 2006; 6: 15
- Tarassoff P, Csermak K. Avascular necrosis of the jaws: Risk factors in metastatic cancer patients. J Oral Maxillofac Surg 2003; 61: 1238–9
- Saad F, Lipton A. Zoledronic acid is effective in preventing and delaying skeletal events in patients with bone metastases secondary to genitourinary cancers. BJU Int 2005; 96: 964–9
- Tannock IF, de Wit R., Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004; 96: 879–82
- Coleman RE. Bisphosphonates in breast cancer. Ann Oncol 2005; 16: 687–95