Abstract
To investigate whether adjuvant treatment with CMF or tamoxifen predisposes to an unfavorable cosmetic outcome or increased breast morbidity after radiotherapy in breast conservation. Data from 266 patients who entered a randomized breast conservation trial (DBCG-82TM protocol) was analyzed. The patients were treated with lumpectomy and axillary dissection followed by external beam radiotherapy to the residual breast. High-risk patients (n = 94), as well as 31 low-risk patients, received additional radiation to the regional lymph nodes. Adjuvant systemic treatment was given to all high-risk patients: premenopausal patients (n = 67) received eight cycles of CMF intravenously (600/40/600 mg per m2) every fourth week; postmenopausal patients (n = 27) received 30 mg of tamoxifen daily for one year. Clinical assessments included cosmetic outcome, breast fibrosis, skin telangiectasia, and dyspigmentation which were scored on a 4-point categorical scale after median 6.6 years. The observations were analyzed in multivariate logistic regression analysis which included potential risk factors on outcome related to systemic treatment, surgery, radiation technique, tumor, and patient characteristics. In premenopausal patients, systemic treatment with CMF independently predicted a fair/poor cosmetic outcome, RR = 2.2 (95% CI 1.2–4.2), as well as increased skin telangiectasia, RR = 3.3 (1.4–8.2). There was no impact of tamoxifen treatment on cosmetic outcome in postmenopausal patients (p = 0.32). However, univariate analysis showed that tamoxifen was significantly associated with breast fibrosis (p <0.004), as was radiation to the regional lymph nodes (p <0.0001). A strong interaction between axillary irradiation and tamoxifen treatment occurred since 26 of 27 high-risk postmenopausal patients had received both tamoxifen and axillary irradiation. In multivariate regression analysis, axillary irradiation independently predicted moderate/severe breast fibrosis with a relative risk of 5.0 (2.0–12.5) and 9.6 (3.3–27.7) in premenopausal and postmenopausal patients, respectively. To circumvent the strong interaction between tamoxifen treatment and axillary irradiation, a subsequent analysis omitting axillary treatment from the multivariate regression showed a significant effect of both tamoxifen and CMF on the occurrence of breast fibrosis with relative risks of 5.3 (CI 1.8–15.8) and 4.4 (1.8–10.3), respectively. Adjuvant systemic treatment with CMF given sequentially to radiotherapy independently predicted an adverse cosmetic outcome as well as increased skin telangiectasia after breast conserving treatment. Due to a strong interaction between tamoxifen administration and radiation to the regional lymph nodes, the effect of tamoxifen on the development of fibrosis could not be fully discerned in this study. Axillary irradiation increased the incidence of moderate to severe breast fibrosis in both premenopausal and postmenopausal patients.
Large randomized clinical trials have established radiotherapy in conjunction with adjuvant systemic treatment as standard treatment in breast cancer after both mastectomy and lumpectomy Citation[1–6]. While the effect on overall survival has been recognized, there has been less focus on late toxicity after combined treatment Citation[7].
Reports on late toxicity after combined treatment in breast cancer have generally been of retrospective nature, and often, only univariate analysis has been applied which has not taking into account the confounding interaction between systemic treatment and other factors which potentially are associated with poor cosmesis in high-risk patients such as surgical factors, individual patient factors, and loco-regional radiation treatment.
The adverse effects of combining loco-regional radiotherapy with either CMF or tamoxifen after mastectomy have previously been reported by Bentzen et al. Citation[8], Citation[9] who demonstrated an enhanced risk of normal-tissue reactions from either systemic modality in combination with post-mastectomy radiation. In this context, tamoxifen has been implicated as a TGFβ-inducer and thus a significant factor in the pathogenesis of radiation-induced fibrosis Citation[10], Citation[11].
Combined systemic treatment and radiation is increasingly employed in various cancer diseases to improve local control and survival rates. However, such improvements might be at the expense of an increase in complication rates. We previously reported clinical results regarding cosmetic outcome and normal-tissue reactions in breast conservation in relation to different radiation techniques Citation[12]. To extend our knowledge on the causes of adverse reactions after breast-conserving treatment, we analyzed the follow-up data from a national prospective breast conservation trial (DBCG-82TM protocol) with respect to combined radiotherapy and adjuvant systemic treatment.
Patients and methods
The study population included patients from a randomized national trial comparing breast conservation therapy with mastectomy (DBCG-82TM protocol) as reported previously see Citation[12–14]. It was decided to perform a prospective assessment of cosmetic outcome and late effects in patients belonging to the breast conservation arm of the trial.
From the original group of 450 patients undergoing BCT, 343 were alive and recurrence-free at the time of the investigation, and 266 (78%) accepted to participate in the follow-up study. This was done as a single follow-up interview performed by the same oncologists who visited nine centers throughout Denmark. The interview consisted of a study-specific structured questionnaire and a physical examination. Self-assessments as well objective observations on cosmetic outcome were obtained. Specific skin and breast changes including dyspigmentation, skin telangiectasia, and breast fibrosis were scored as described below.
The clinical examination was ‘blinded’ in the sense that no information was available for the observer at the time of the interview regarding individual radiation treatment techniques, extent of surgery, or whether adjuvant treatment had been given. Individual data on systemic treatment, radiation treatment, as well as patient-related characteristics related to tumor size or axillary lymph node status were obtained from the DBCG secretariat.
The interview and the examination were performed after a median follow-up time of 6.6 years (range 3.5–10.5 years). A discussion regarding the expression of normal-tissue reactions in relation to the observation time is presented elsewhere Citation[12].
The patients were treated according to protocol guidelines. For a detailed description of the treatment protocol Citation[12–14]. Only major technicalities are presented here. Surgery consisted of a complete tumor resection ensuring tumor-free margins at gross examination of the specimen. Axillary dissection was mandatory and included level I and II through a separate transverse incision. However, if the tumor was located in the axillary tail, the protocol recommended a continuous extension of the breast incision to the axilla for an ‘en bloc’ dissection of the axillary contents and the tumor-bearing area.
Treatment allocation is shown in . All the patients had irradiation of the residual breast with a median target absorbed dose of 50 Gy given in 25 fractions, 5 fractions per week (90%), or 48 Gy in 22 fractions, 4 fractions per week (10%). The recommended radiation treatment technique was opposing tangent fields (6–10 MV) with a boost dose of minimum 10 Gy to the scar and the tumor bed, either with tangential photons or a single electron field. However, the protocol also allowed radiation treatment to be given through a single direct anterior electron field (6–20 MeV) to the whole breast defining the 85% isodose curve at the pleural surface.
Figure 1. Diagram of patient allocation according to risk status and menopausal status. Number of low-risk patients receiving nodal irradiation is indicated (axilla).
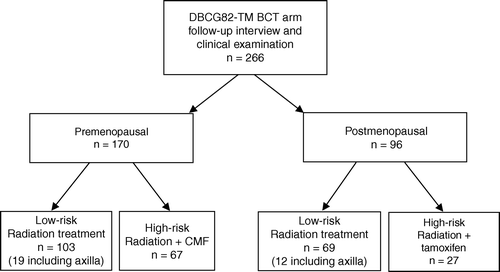
High-risk patients received an additional direct anterior photon field (6–16 MV) of 50 Gy/25 fractions to cover the regional lymph nodes in the axilla and periclavicular region while the tangents covered the internal mammary chain. It should be noted that if an ‘en bloc’ dissection to the axilla had been performed, additional axillary irradiation was recommended by the protocol guidelines irrespectively of the risk status of the patient. As a result, 18% (31/172) of low-risk patients received axillary radiation treatment.
High-risk patients
High-risk patients were defined as patients with a tumor diameter >5 cm, and/or invasion to the skin or pectoral facia, and/or involvement of axillary lymph nodes.
High-risk patients received adjuvant systemic treatment in conjunction with loco-regional radiotherapy. Premenopausal high risk patients received eight cycles of CMF which consisted of cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2, and 5-FU 600 mg/m2, all given intravenously as a bolus injection day 1 every fourth week. CMF was administered sequentially to radiotherapy, i.e., the first cycle was given one week prior to the initiation of radiotherapy while the second cycle was planned 1–2 weeks after completion of radiation treatment.
Postmenopausal high-risk patients received 30 mg of tamoxifen daily from the time of radiation treatment and continued for 12 months.
Clinical endpoints
The clinical endpoints were scored on 4-point ordinal scales. Cosmetic outcome was scored independently by the oncologist and the patient as excellent, good, fair, or poor by comparison to the un-treated breast. Breast size was categorized as A, B, C, or D.
The objective changes in the breast tissues and skin were scored outside the lumpectomy scar and radiation boost field–and away from any supraclavicular treatment fields–as follows: Breast fibrosis: 0. None 1. Barely palpable, but increased density 2. Definite increased density and firmness 3. Very marked density with retraction or fixation. Skin telangiectasia: 0. None 1. <1/cm2 2. 1–4/cm2 3. >4/cm2. Dyspigmentation: 0. None 1. Mild 2. Moderate 3. Severe.
Lung symptoms of any kind were also recorded. This was done to explore the prevalence of radiation pneumonitis, but no detailed radiological changes were obtained.
Statistics
Premenopausal and postmenopausal patients were analyzed separately to compare whether CMF or tamoxifen were associated with excess breast morbidity compared to patients who did not receive adjuvant systemic treatment. For this analysis, multivariate logistic regression was used. Skin or breast complications which in itself affected cosmetic outcome, such as dyspigmentation or skin telangiectasia, were not included as co-variates in the models concerning cosmesis.
First, univariate analyses were applied (χ2 analysis and Mann-Whitney rank sum test) to explore which patient-associated characteristics or treatment-related factors that most likely were associated with the study endpoints. This included adjuvant systemic treatment, radiation technique and dose, addition of boost, age, breast size, tumor size, and collagen vascular disease. Also, the independent effect of axillary irradiation was analyzed even though this treatment was almost exclusively confined to high-risk patients receiving systemic treatment as well. Potential explanatory factors were then incorporated in the multivariate linear regression models. The dependent factors were dichotomized (grade 0 and 1 versus grade 2 and 3).
The impact of the co-factors was described in terms of relative risk (RR) and 95% confidence intervals (CI) were estimated. A two-sided p-value of less than 0.05 was considered significant. P-values are two-tailed.
Results
Demographic data
The distribution of the 266 participating patients in the different treatment groups is shown in . The low-risk group of both premenopausal and postmenopausal patients had slightly longer observation times. Among premenopausal patients, there was a larger but non-significant proportion of the CMF group who received breast radiation with electrons (p = 0.13).
Table I. Distribution of potential confounders of the systemic effect on cosmesis and complications in 266 women undergoing BCT.
The prevalence of combined grade 2 and grade 3 adverse reactions is shown in as well as significance values from univariate analysis (Mann-Whitney U tests using the individual graded scores from 0 to 3). This showed that in premenopausal patients, systemic treatment was significantly associated with cosmetic outcome (p = 0.002), subcutaneous fibrosis (p < 0.001), skin telangiectasia (p = 0.001), and dyspigmentation (p = 0.001). The frequencies of excellent/good cosmetic scores in relation to systemic treatment are illustrated in .
Figure 2. Frequency of patients with excellent or good cosmetic outcome in relation to systemic treatment, A) in premenopausal patients, B) in postmenopausal patients.
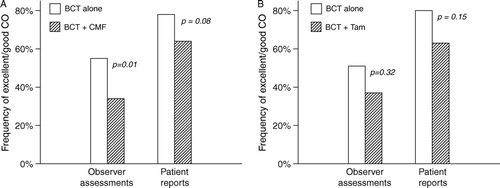
Table II. Prevalence of grade 2 or grade 3 reactions in patients receiving breast irradiation (RT) with or without adjuvant systemic treatment. p-values derived from Mann-Whitney U tests using all the graded responses (from 0 to 3).
On multivariate logistic regression analysis in premenopausal patients, CMF independently predicted a fair/poor cosmetic outcome (OR = 2.2; 95%CI 1.2–4.2) and increased skin telangiectasia (OR = 3.3; 1.4–8.2). The first finding was independent of whether objective or patient assessed cosmetic scores were used.
Cosmetic outcome was not related to endocrine treatment with tamoxifen in postmenopausal patients (p = 0.32). However, univariate analysis showed that tamoxifen was significantly associated with breast fibrosis (p = 0.004) (). This also applied to axillary irradiation which was equally associated to the occurrence of fibrosis (p < 0.0001). However, there was a strong interaction between axillary irradiation and tamoxifen treatment since 26 of 27 high-risk postmenopausal patients who received tamoxifen also received axillary irradiation.
In multivariate regression analysis, complementary axillary radiation was significantly related to increased breast fibrosis in both premenopausal and postmenopausal patients with a relative risk of 5.0 (2.0–12.5) and 9.6 (3.3–27.7), respectively. To circumvent the strong interaction between tamoxifen treatment and axillary irradiation, a subsequent analysis was performed omitting axillary treatment from the multivariate regression, but including the other above mentioned co-variates. This demonstrated a significant effect of both tamoxifen and CMF on the occurrence of breast fibrosis with relative risks of 5.3 (CI 1.8–15.8) and 4.4 (1.8–10.3), respectively.
Only 14 patients (5%) complained of lung symptoms. These were all of mild degree and not exclusively indicative of radiation pneumonitis. Due to the very low prevalence of clinical symptoms indicating pneumonitis, no further statistical analysis was considered regarding this endpoint.
Discussion
Normal-tissue reactions to radiotherapy are caused by a variety of treatment related factors as well as inherent patient factors Citation[15], Citation[16]. Due to the complexity of radiation-induced complications, normal-tissue reactions have been difficult to predict Citation[17–19] and the picture becomes even more complex when radiation treatment is combined with systemic treatment.
During the last decade, chemoradiation has become standard practice in the treatment of several solid malignancies due to improved survival rates. The side-effects have increased correspondingly, however, often only a few years of follow-up data has been available and the long-term effects are infrequently reported Citation[7].
In breast cancer, combined radiotherapy and chemotherapy have been employed for several decades and with improved overall survival rates Citation[3], Citation[4]. Due to a more favorable prognosis in the adjuvant setting of breast cancer compared to other diseases treated with chemoradiation, the prevalence is high and longer observation time is available in breast cancer. For this reason the present investigation has allowed us to throw light on the impact of either CMF chemotherapy or tamoxifen treatment on long-term morbidity after radiotherapy in breast conservation. We analyzed the effect of chemoradiation separately in premenopausal and postmenopausal patients due to the difference in age as well as different mechanisms of action of CMF and tamoxifen.
Induction of fibrosis
Several lines of evidence point to TGFβ as a key cytokine in the role of transformation of tissue fibrosis Citation[10], Citation[11], Citation[20–22]. Tamoxifen has been shown to cause induction of extracellular TGFβ in murine Citation[23] as well as in human in vivo studies as demonstrated by Butta et al. Citation[24] who found that three months of tamoxifen treatment in breast cancer patients caused a consistent induction of extracellular TGFβ deposits in breast specimens compared with matched pretreatment samples from the same individuals.
Some of the first clinical data to support the relationship between tamoxifen treatment and radiation-induced fibrosis comes from our own group Citation[9]. In an analysis of 196 breast cancer patients undergoing post-mastectomy radiotherapy, tamoxifen treatment was significantly related to marked lung fibrosis as measured by optical density changes on X-ray films of the apical lungs.
In the present study, breast fibrosis seemed to be significantly enhanced by both CMF and tamoxifen in univariate analysis, but this could not be confirmed on multivariate regression that included axillary radiation as a co-variate. Axillary irradiation surprisingly caused significantly more breast fibrosis in both premenopausal and postmenopausal patients, and this finding was observed despite that breast morbidity was assessed away from possible sites of overlapping treatment fields.
Other studies have shown that the use of a multiple-field radiation techniques including axillary irradiation is associated with a significantly worse cosmetic outcome and breast retraction compared with the use of tangential treatment portals alone, perhaps due to radiation-induced damage to regional lymphatics causing loco-regional edema Citation[25–28].
An additional multivariate analysis omitting axillary treatment, but including all other factors which possibly could affect breast morbidity as described above, showed that both CMF and tamoxifen were associated with the risk of developing moderate to severe breast fibrosis suggesting a relationship between systemic treatment and transformation of fibrosis. Axillary irradiation and tamoxifen treatment were both prescribed to high-risk patients, i.e., 26 of 27 high-risk postmenopausal patients who received tamoxifen also received axillary irradiation. Due to this strong interaction between axillary irradiation and tamoxifen treatment, one must be careful drawing firm conclusions about which factors that induce subcutaneous fibrosis since the effect of tamoxifen could not be distinguished from that of axillary radiation in this study.
We found a prevalence of pulmonary symptoms of 5% which covered symptoms of any cause for which reason we did not analyze the effect of systemic treatment on pneumonitis in this study. Despite a high incidence of reported pulmonary changes on chest x-ray films and CT-scans Citation[9], Citation[29], Citation[30], only a small percentage of patients seem to have symptomatic pneumonitis after radiotherapy for breast cancer, around 1% Citation[30], Citation[31]. Therefore, the likely effect of tamoxifen on radiation-induced pulmonary fibrosis seems to be of little clinical concern but more of biological relevance to help explaining mechanistic elements of the pathogenesis of lung fibrosis. In this respect there are discrepancies in the evidence of an association between tamoxifen treatment and the development of lung fibrosis Citation[9], Citation[29], Citation[30], Citation[32], Citation[33].
Tamoxifen, cosmetic outcome, and normal-tissue reactions
Tamoxifen treatment did not affect cosmetic outcome in conjunction with radiation in postmenopausal patients, who were evaluated 6.5 years after breast conservation. Published reports regarding cosmetic outcome and skin reactions after tamoxifen treatment and irradiation seem as conflicting as those describing tamoxifen-induction of fibrosis. The inconsistency of the data lies primarily in the retrospective nature of the studies but also the general use of univariate analysis which fails to isolate the independent effect of tamoxifen by not controlling for treatment-related factors or patient-specific characteristics.
Azria et al. Citation[34] analyzed the effect of tamoxifen on late effects during a prospective study dealing with predictive testing of lymphocyte radiosensitivity in breast cancer patients. Multivariate analysis showed that grade 2 or greater skin/subcutaneous fibrosis–but not telangiectasia–was significantly increased in patients treated with tamoxifen despite that tamoxifen-treated patients were characterized by older age, irradiation of significant larger breast volumes, larger disease stages, and higher node positivity. Two additional studies have also shown that patients who received tamoxifen were more often axillary node positive and consequently comprised a greater percentage of patients who received radiation treatment to both the breast and the axilla Citation[35], Citation[36].
Fowble et al. made a retrospective study of 154 tamoxifen-treated women undergoing breast conservation without additional chemotherapy. Skin complications were not evaluated; however, breast edema was increased after a median of 5.3 years observation time but only on univariate analysis. There was apparently no adverse effect of tamoxifen on cosmesis.
Wazer et al. also looked at cosmetic outcome in relation to tamoxifen treatment in breast conservation Citation[25], Citation[36]. In the first study, it was indicated by univariate analysis that tamoxifen treatment was related to radiation-induced fibrosis; however, in the following update the authors corrected for confounding factors by applying multivariate regression analysis and found no adverse effect of tamoxifen on cosmesis. This is in accordance with Harris et al. who retrospectively analyzed the difference between adjuvant tamoxifen given concurrently or sequentially to radiotherapy in breast conservation, with or without chemotherapy Citation[37]. After 5 years, cosmetic data was available for just over half of the patients and no effect of the tamoxifen sequence was found on complication rates or cosmesis. However, the incidence of complications was low which might reduce the ability to detect a difference in this study group.
Two similar investigations also studied tamoxifen in breast conservation, with or without additional chemotherapy, and hormonal therapy did not affect the cosmetic results in any of these investigations Citation[27], Citation[38].
Altogether, the literature as well as our own data show that tamoxifen treatment with radiotherapy does not seem to impact on the cosmetic outcome in breast conservation treatment.
CMF, cosmetic outcome, and normal-tissue reactions
In the present study, CMF given sequentially to breast irradiation independently had a negative impact on the overall cosmetic outcome scores after breast conservation in premenopausal patients. This observation was independent of whether the patient evaluations or observer assessments were used. The impact of CMF on cosmetic outcome was demonstrated after correcting for other confounding factors impacting on breast cosmesis associated with surgery, radiotherapy, as well as patient-related factors (listed in ).
Telangiectasia of the skin was also significantly increased in the present study after combined CMF and radiation. Whether the mechanism of increased telangiectasia is a pure late skin reaction of CMF in combination with radiotherapy or a consequential complication due to excess acute reactions (moist desquamation) is uncertain from the present analysis since no systematic recording of acute effects had been done.
As described above, breast fibrosis was only significantly more common after combined CMF and radiation when axillary irradiation as a co-factor was omitted from multivariate analysis. Again, CMF administration in high-risk patients was associated with axillary radiation treatment which in itself impacted on breast fibrosis, but the number of patients in the subgroups of the multivariate analyses allows one to make more firm conclusions regarding CMF and fibrosis as opposed to the present tamoxifen data.
Previous reports on cosmetic outcome and late normal-tissue reactions to combined systemic treatment and radiotherapy in breast conservation have been contradictory. Some have reported significant adverse effects on cosmetic outcome after combined treatment Citation[25], Citation[28], Citation[39–41] while other studies did not find any detrimental effect on cosmesis from adding chemotherapy to radiation Citation[38], Citation[42–44]. However, as described previously, only few studies have applied multivariate analysis to analyze the independent effect of chemotherapy on treatment complications after radiotherapy.
Great controversies also exist regarding the sequencing of systemic treatment and radiotherapy in relation to complication rates as well as to treatment efficacy Citation[45]. Abner et al. Citation[43] did a retrospective case-control analysis comparing the outcome of 340 patients after a median of 60 months of observation. At 3 years, fair/poor cosmetic outcome, breast edema, and retraction were more common in patients receiving any kind of chemotherapy while skin telangiectasia was only increased in patients having concurrent chemoradiation. The proportion of excellent results also decreased more in the concurrent group compared to both the sequentially treated patients and the control group (radiotherapy alone). This is in agreement with Moro et al. Citation[41] and Vass et al. Citation[28] who presented multivariate analyses on 164 patients and 142 patients, respectively, of whom 28% and 37% had received concomitant CMF as part of a breast-conserving schedule. Concomitant chemoradiotherapy negatively impacted on cosmesis after a median of 38 months and 3 years, respectively. In contrast, the study by Taylor et al. Citation[27] also suggested an effect of concurrent chemotherapy and radiation on the cosmetic results, however, when the analysis was completed with other factors in a multivariate analysis, adjuvant chemotherapy failed to demonstrate a significant effect on cosmesis.
Regarding late normal-tissue reactions, concurrent administration of chemotherapy and radiation was shown to cause significantly higher complication rates in a randomized trial Citation[46]. This compared concurrent and sequential mitoxantrone/5-FU/cyclophosphamide and significantly more subcutaneous fibrosis, skin telangiectasia, pigmentation, and breast atrophy was recorded in the concurrent arm. Also, Ray et al. Citation[39] found in a univariate analysis of 134 patients that adjuvant chemotherapy given either before, during, or after radiotherapy had a negative impact upon cosmesis as well as increased fibrosis and breast retraction.
Conclusion
The present study group belonged to a prospective study with a fairly long observation time to allow cosmetic outcome and late normal-tissue complications to be assessed properly. Our results add to the findings of an impaired cosmetic outcome and increased rate of clinically observed normal-tissue reactions in the irradiated breast after combined systemic treatment and local radiotherapy, and confirm the previous findings in breast cancer patients treated according to DBCG guidelines Citation[8].
Tamoxifen treatment does not seem to affect cosmetic outcome in breast conservation. Previous reports on fibrosis, cosmetic outcome, and pneumonitis following combined tamoxifen and radiation treatment are conflicting. There is some evidence of a combined effect on pulmonary fibrosis while the impact of tamoxifen on skin or breast fibrosis is less substantiated. In contrast, larger studies applying multivariate analyses have confirmed a significant effect of chemotherapy in conjunction with radiation treatment on both cosmetic outcome and breast complications, especially when the combination is given concomitantly Citation[47].
Acknowledgements
This work was supported by the Danish Cancer Society and the Danish Medical Research Council.
References
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 1997; 337: 949–55
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353: 1641–8
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- van der Hage JA, Putter H, Bonnema J, Bartelink H, Therasse P, van de Velde CJ. Impact of locoregional treatment on the early-stage breast cancer patients: A retrospective analysis. Eur J Cancer 2003; 39: 2192–9
- Huang EH, Tucker SL, Strom EA, McNeese MD, Kuerer HM, Buzdar AU, et al. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol 2004; 22: 4691–9
- Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst 2005; 97: 116–26
- Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol 2000; 18: 1220–9
- Bentzen SM, Overgaard M, Thames HD, Christensen JJ, Overgaard J. Early and late normal-tissue injury after postmastectomy radiotherapy alone or combined with chemotherapy. Int J Radiat Biol 1989; 56: 711–5
- Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst 1996; 88: 918–22
- Canney PA, Dean S. Transforming growth factor beta: A promotor of late connective tissue injury following radiotherapy?. Br J Radiol 1990; 63: 620–3
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994; 331: 1286–92
- Johansen J, Overgaard J, Rose C, Engelholm SA, Gadeberg CC, Kjær M, et al. Cosmetic outcome and breast morbidity in breast-conserving treatment. Results from the Danish DBCG-82TM national randomized trial in breast cancer. Acta Oncol 2002; 41: 369–80
- Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson CK, Andersen KW, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: Six years of life-table analysis. Monogr Natl Cancer Inst 1992; 11: 19–25
- Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials. J Clin Oncol 2001; 19: 1688–97
- Turesson I, Nyman J, Holmberg E, Odén A. Prognostic factors for acute and late skin reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys 1996; 36: 1065–75
- Safwat A, Bentzen SM, Turesson I, Hendry JH. Deterministic rather than stochastic factors explain most of the variation in the expression of skin telangiectasia after radiotherapy. Int J Radiat Oncol Biol Phys 2002; 52: 198–204
- Johansen J, Bentzen SM, Overgaard J, Overgaard M. Relationship between the in vitro radiosensitivity of skin fibroblasts and the expression of subcutaneous fibrosis, telangiectasia, and skin erythema after radiotherapy. Radiother Oncol 1996; 40: 101–9
- Russell NS, Begg AC. Editorial radiotherapy and oncology : Predictive assays for normal tissue damage. Radiother Oncol 2002; 64: 125–9
- Andreassen CN, Alsner J, Overgaard M, Overgaard J. Prediction of normal tissue radiosensitivity from polymorphisms in candidate genes. Radiother Oncol 2003; 69: 127–35
- Martin M, Lefaix JL, Pinton P, Crechet F, Daburon F. Temporal modulation of TGF-β1 and β-actin gene expression in pig skin and muscular fibrosis after ionizing radiation. Radiat Res 1993; 134: 63–70
- Illsley MC, Peacock JH, McAnulty RJ, Yarnold JR. Increased collagen production in fibroblasts cultured from irradiated skin and effect of TGF beta1-clinical study. Br J Cancer 2000; 83: 650–4
- Andreassen CN, Alsner J, Overgaard J, Herskind C, Haviland J, Owen R, et al. TGFB1 polymorphisms are associated with risk of late normal tissue complications in the breast after radiotherapy for early breast cancer. Radiother Oncol 2005; 75: 18–21
- Barcellos-Hoff MH. Radiation-induced transforming growth factor β and subsequent extracellular matrix reorganization in murine mammary gland. Cancer Res 1993; 53: 3880–6
- Butta A, MacLennan K, Flanders KC, Sacks NPM, Smith I, McKinna A, et al. Induction of transforming growth factor β1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res 1992; 52: 4261–4
- Wazer DE, DiPetrillo T, Schmidt-Ullrich R, Weld L, Smith TJ, Marchant DJ, et al. Factors influencing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol 1992; 10: 356–63
- Olivotto IA, Rose MA, Osteen RT, Love S, Cady B, Silver B, et al. Late cosmetic outcome after conservative surgery and radiotherapy: analysis of causes of cosmetic failure. Int J Radiat Oncol Biol Phys 1989; 17: 747–53
- Taylor ME, Perez CA, Halverson KJ, Kuske RR, Philpott GW, Garcia DM, et al. Factors influencing cosmetic results after conservation therapy for breast cancer. Int J Radiat Oncol Biol Phys 1995; 31: 753–64
- Vass S, Bairati I. A cosmetic evaluation of breast cancer treatment: A randomized study of radiotherapy boost technique. Int J Radiat Oncol Biol Phys 2005; 62: 1274–82
- Huang E-Y, Wang CJ, Chen HC, Sun LM, Fang FM, Yeh SA, et al. Multivariate analysis of pulmonary fibrosis after electron beam irradiation for postmastectomy chest wall and regional lymphatics: evidence for non-dosimetric factors. Radiother Oncol 2000; 57: 91–6
- Koc M, Polat P, Suma S. Effects of tamoxifen on pulmonary fibrosis after cobalt-60 radiotherapy in breast cancer patients. Radiother Oncol 2002; 64: 171–5
- Lingos TI, Recht A, Vicini F, Abner A, Silver B, Harris JR. Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 1991; 21: 355–60
- Fu XL, Huang H, Bentel G, Clough R, Jirtle RL, Kong FM, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys 2001; 50: 899–908
- Li C, Wilson PB, Levine E, Barber J, Stewart AL, Kumar S. TGF-beta1 levels in pre-treatment plasma identify breast cancer patients at risk of developing post-radiotherapy fibrosis. Int J Cancer 1999; 84: 155–9
- Azria D, Gourgou S, Sozzi WJ, Zouhair A, Mirimanoff RO, Kramar A, et al. Concomitant use of tamoxifen with radiotherapy enhances subcutaneous breast fibrosis in hypersensitive patients. Br J Cancer 2004; 91: 1251–60
- Fowble B, Fein DA, Hanlon AL, Eisenberg BL, Hoffman JP, Sigurdson ER, et al. The impact of tamoxifen on breast recurrence, cosmesis, complications, and survival in estrogen receptor-positive early-stage breast cancer. Int J Radiat Oncol Biol Phys 1996; 35: 669–77
- Wazer DE, Morr J, Erban JK, Schmid CH, Ruthazer R, Schmidt-Ullrich RK. The effects of postradiation treatment with tamoxifen on local control and cosmetic outcome in the conservatively treated breast. Cancer 1997; 80: 732–40
- Harris EE, Christensen VJ, Hwang WT, Fox K, Solin LJ. Impact of concurrent versus sequential tamoxifen with radiation therapy in early-stage breast cancer patients undergoing breast conservation treatment. J Clin Oncol 2005; 23: 11–6
- Markiewicz DA, Schultz DJ, Haas JA, Harris EE, Fox KR, Glick JH, et al. The effects of sequence and type of chemotherapy and radiation therapy on cosmesis and complications after breast conservation therapy. Int J Radiat Oncol Biol Phys 1996; 35: 661–8
- Ray GR, Fish VJ, Marmor JB, Rogoway W, Kushlan P, Arnold C, et al. Impact of adjuvant chemotherapy on cosmesis and complications in stages I and II carcinoma of the breast treated by biopsy and radiation therapy. Int J Radiat Oncol Biol Phys 1984; 10: 837–41
- de la Rochefordiere A, Abner AL, Silver B, Vicini F, Recht A, Harris JR. Are cosmetic results following conservative surgery and radiation therapy for early breast cancer dependent on technique?. Int J Radiat Oncol Biol Phys 1992; 23: 925–31
- Moro G, Stasi M, Borca VC. Does concomitant chemoradiotherapy influence cosmetic outcome in conservative treatment of breast cancer?. Tumori 1997; 83: 743–7
- Borger JH, Keijser AH. Conservative breast cancer treatment: Analysis of cosmetic results and the role of concomitant adjuvant chemotherapy. Int J Radiat Oncol Biol Phys 1987; 13: 1173–7
- Abner AL, Recht A, Vicini FA, Silver B, Hayes D, Come S, et al. Cosmetic results after surgery, chemotherapy, and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys 1991; 21: 331–8
- Fehlauer F, Tribius S, Holler U, Rades D, Kuhlmey A, Bajrovic A, et al. Long-term radiation sequelae after breast-conserving therapy in women with early-stage breast cancer: An observational study using the LENT-SOMA scoring system. Int J Radiat Oncol Biol Phys 2003; 55: 651–8
- Whelan T, Levine M. Radiation therapy and tamoxifen: Concurrent or sequential? That is the question. J Clin Oncol 2005; 23: 1–4
- Toledano A, Garaud P, Serin D, Fourquet A, Bosset JF, Breteau N, et al. Concurrent administration of adjuvant chemotherapy and radiotherapy after breast-conserving surgery enhances late toxicities: Long-term results of the ARCOSEIN multicenter randomized study. Int J Radiat Oncol Biol Phys 2006; 65: 324–32
- Recht A. Integration of systemic therapy and radiation therapy for patients with early-stage breast cancer treated with conservative surgery. Clin Breast Cancer 2003; 4: 104–13