Abstract
Purpose. Intravenous (IV) contrast at the time of CT-Simulation facilitates radiotherapy contouring, but may introduce a discrepancy between planned and delivered dose due to density variation in blood vessels. Here, the effect of physiologic and non-physiologic extremes of IV contrast densities on intensity modulated radiotherapy (IMRT) plans for patients with head and neck cancer was investigated. Methods and materials. This planning study was conducted using IV contrast CT scans of ten patients with squamous cell cancer of the head and neck treated with IMRT. The target volumes and normal tissues, including the blood vessels of the head and neck, were contoured and IMRT plans were created according to RTOG Protocol 0022. The density within the blood vessels was then virtually altered to mimic non-contrast and extreme (bone and air) densities. The dose was then recalculated using the same IMRT plan. Plans obtained with and without density overrides were then compared. Results. The change in planning target volume (PTV) coverage for plans with and without IV contrast was minimal. The volume of the PTVs covered by the 93% and 100% isodoses changed on average by 0.57%. The minimum dose to PTVs varied by a maximum of 0.17 Gy. The maximum point dose to critical organs changed by a maximum of 0.12 Gy (brainstem). Non-physiologic extremes of density within blood vessels also resulted in minimal changes in tumor or normal tissue dosimetry. Conclusion. The use of IV contrast at time of CT-simulation does not significantly affect dose calculation in head and neck IMRT plans.
Although advances in radiotherapy such as hyperfractionated strategies Citation[1] and combined radiation and chemotherapy Citation[2] have led to improved outcomes in head and neck cancer, toxicity following radiotherapy remains substantial. Intensity modulated radiotherapy (IMRT), with steep dose gradients tightly conforming to target volumes, allows for greater sparing of normal tissues, leading to reduced toxicity Citation[3], Citation[4]. Preservation of saliva flow is possible with IMRT for patients who require bilateral neck radiation therapy, and who would have a high probability of permanent xerostomia following conventional radiation therapy Citation[5]. Appropriate contouring of target volumes including the primary tumor, gross lymph nodes, microscopic nodal volumes at risk, and normal organs, is essential for a safe and effective IMRT.
In 2003, an Intergroup consensus document was developed to provide guidelines on the definition of microscopic nodal areas at risk in the node negative neck. Many of the boundaries of nodal volumes at risk are based on the position of blood vessels Citation[6]. Intravenous (IV) contrast can facilitate nodal volume contouring and the delineation of gross nodal and primary tumor volumes that often enhance with IV contrast, while reducing observer contouring variability Citation[7].
IV contrast has the potential disadvantage that it could introduce dose artifacts in an IMRT plan due to its higher density than blood. At an ASTRO IMRT practicum in February 2007, attendees expressed this putative concern as a reason to avoid IV contrast or to modify the density of IV contrast at the time of CT simulation for head and neck cancer IMRT planning (verbal communication Quynh-Thu Le, Program Co-chair, March 2007). We have also observed this to be a recurring theme at other similar meetings.
The purpose of this study is to model the effects of both physiologic and non-physiologic extremes of IV contrast densities in IMRT plans from patients with head and neck cancer. We hypothesized that the use of IV contrast would not substantially alter dose distributions for IMRT plans for head and neck cancer patients.
Material and methods
The study was approved by the Princess Margaret Hospital/University Health Network institutional Research Ethics Board. The patient population investigated for this planning study comprised ten patients with squamous cell cancer of the head and neck, nine with oropharyngeal cancer (six tonsil, two base of tongue, one soft palate), and one with retromolar trigone cancer, all suitable for parotid sparing IMRT delivered in 30 fractions, as described in RTOG 0022 Citation[8].
To investigate the impact of IV contrast on IMRT plans, planning was conducted on IV contrast-enhanced CT scans, acquired with patients immobilized in an S-frame thermoplastic mask, in their treatment position (slice thickness of 1.5 mm). One patient underwent planning CT scan in the Radiation Therapy Department (GE, PET-CT Discovery) and nine patients had diagnostic CT scans in the Diagnostic Imaging Department on a Siemens Biograph Duo PET-CT hybrid Scanner. All patients received 60 cm3 of IV contrast (Visipaque) according to standard institutional protocol (2 cm3/s with a 25 s scan start delay). The diagnostic CT images were imported into the treatment planning system (Pinnacle3, version 7.6, Philips, Madison, WI) for this study. The Hounsfield units of both planning and diagnostic CT scans were converted to relative electron density using the corresponding conversion table acquired with a CT calibration phantom (RMI, Gammex, Middleton, WI) on each CT scanner.
For all patient scans, the regions of interest were contoured by a single observer (a senior radiation oncology resident, MF) and then verified by a radiation oncologist specializing in the treatment of head and neck cancer (LAD). The primary tumor and grossly involved lymph nodes formed the gross tumor volume (GTV). The clinical target volume (CTV) around the primary and nodal GTVs consisted of a minimum of a 1.0 cm expansion in areas at risk for microscopic disease (CTV66). A second CTV (CTV54) was contoured for the nodal areas at risk of harboring subclinical cancer. CTV54 most often included the lymph node levels II-IV, including level II nodes at the base of skull and retropharyngeal nodes for the side of the neck containing gross nodes. For the side of the neck not containing gross nodal disease, the caudal extent of the CTV54 included the jugulodigastric level II nodes (determined by the axial CT slice in which the posterior belly of the digastric muscle crossed the jugular vein), while the nodes cranial to this were not targeted.
A 0.5 cm margin was added to each CTV to obtain planning target volumes (PTVs). The planning target volume encompassing the primary tumor and any lymph node metastases was planned to receive 66 Gy in 30 fractions (PTV66). The PTV surrounding the microscopic nodal areas at risk were planned to receive 54 Gy in 30 fractions (PTV54). For optimization, PTVs were modified back from the build up regions near skin (3 mm from skin surface).
Organs at risk (OARs) contoured included the spinal cord, brainstem, mandible and parotid glands. Expansions of 0.3 cm around the brainstem and 0.5 cm around the spinal cord were added to obtain planning organ at risk volumes (PRVs). The external and internal carotid arteries and jugular veins and other major blood vessels, excluding the vertebral artery, were contoured.
Goals of IMRT planning for this study were consistent with the RTOG Protocol 0022 Citation[8], designed for early stage oropharyngeal cancer treated with IMRT delivered in 30 fractions. The prescription isodoses (66 or 54 Gy) had to encompass at least 95% of the corresponding PTV. No more than 1% of either PTV54 or PTV66 could receive <93% of the prescribed dose (50.2 and 61.4 Gy respectively). No more than 20% of PTV66 could receive 72.6 Gy (>110% of the prescribed dose).
IMRT plans were optimized with the heterogeneity correction enabled (0.4×0.4 cm dose grid). The densities within the blood vessels were then altered in the planning system. To model the clinically relevant “Without Contrast” scenario where IV contrast is given at the time of simulation but not treatment, the density within the blood vessels was changed to a water equivalent 1.00 g/cm3 (Hounsfield unit (HU) of 0). To model two non-physiologic extremes, the density within the blood vessels was altered to 1.682 g/cm3 and 0.001 g/cm3 for cortical bone (+1000 HU) and air (−1000 HU) respectively.
Using the same beam arrangements and multi-leaf collimator (MLC) segments obtained in the IV contrast optimization, the dose was recalculated for non-contrast and non-physiological extremes. Comparisons between these plans and the IV contrast plan were then made, specifically evaluating the differences in the volume of the PTV66 covered by the 93%, 100% and >110% isodose lines, the volume of the PTV54 covered by the 93% and 100% isodose lines, the maximum and minimum dose (0.1 cm3) for the PTV66 and PTV54, the maximum dose (0.1 cm3) to the brainstem, spinal cord, mandible and corresponding PRVs as well as the mean dose to the parotid glands.
Results
For all IV contrast IMRT plans, target coverage and normal tissue avoidance criteria as specified in RTOG 0022 were met. For example, the volume of PTV66 covered by 93% of prescription dose averaged 99.94±0.06%, with 96.15±1.23% receiving 66 Gy. The corresponding values for PTV54 were 99.57±0.20%, and 96.47±1.08% respectively. The doses to the organs at risk are summarized in . In terms of parotid sparing, the ipsilateral (node positive side of the neck) parotid mean dose ranged from 29.1 to 58.9 Gy, while the contralateral (node negative side of the neck) parotid mean doses were less than or equal to 26 Gy, in eight of ten of the patients. The mean parotid doses of the other two patients (node negative side) were 29.5 Gy and 30.8 Gy.
Table I. Compilation of original planned dose (with IV contrast) and dose variation for different contrast scenarios for PTVs and OARs. The ±values represent 1 standard deviation. The perturbation of the minimum dose (0.1 cm3) to both PTVs was not assessed due to the presence of blood vessel in these regions of interest, which brought the minimum dose to zero.
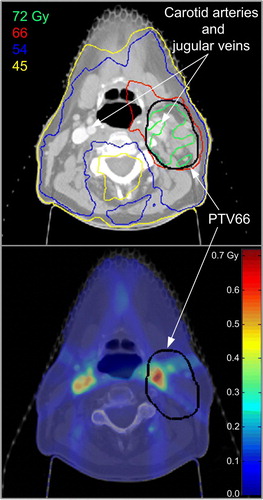
Dose variations for both PTVs and organs at risk (OARs) for the different contrast scenarios compared to IV contrast IMRT plans are summarized in . When we modeled the blood vessels without IV contrast (density of 1.00 g/cm3), the parameters for assessing plan quality changed minimally. The volume of PTV66 covered by the 93%, 100% and >110% isodose lines increased by an average of 0.01%, 0.11% and 0.57% respectively with a standard deviation ≤0.58%. The greatest change in the mean dose covering PTV66 corresponded to an increase of 0.11 Gy for the scenario without IV contrast. The greatest change in the maximum point dose to PTV66 was 0.38 Gy or 0.58% of the prescribed dose (66 Gy). The volume of PTV54 covered by the 93% and 100% isodose lines varied on average by 0.01% and 0.08% respectively with a standard deviation ≤0.05%. The maximum variation in terms of minimum dose to the PTVs (0.1 cm3) for a given patient was −0.17 Gy and 0.17 Gy for PTV54 and PTV66, respectively. illustrates the original dose distribution (with IV contrast) and the dose difference (dose without IV contrast minus dose with IV contrast) for the patient with the largest dose variation in PTV66. The presence of the IV contrast in the carotid arteries and jugular veins appears to have had a shielding effect on the IV contrast dose distribution as demonstrated by the increased dose most obvious around the vessels. The dose recalculated without IV contrast was higher than the original dose distribution with IV contrast by 0 to 0.71 Gy. For the plans without IV contrast, mimicking physiologic density of vessels (density of 1.00 g/cm3), there was little change in dose to OARs. The largest variation of maximum point doses to an OAR was observed for the brainstem of patient #9 with an increase of 0.12 Gy over the course of 6 weeks of radiation treatment. The mean dose to either parotid gland changed by less than 0.05 Gy.
Figure 1. a) Original dose distribution (with IV contrast) for the patient with the largest dose variation in terms of maximum point dose (0.1 cm3) to PTV66. The dose difference for the plan without IV contrast minus the plan with IV contrast is overlaid on the CT data, showing the largest differences in dose are near the blood vessels, less than 0.7 Gy over 30 fractions (b).
Taking the vessels to non-physiologic extremes of density (similar to cortical bone and air) perturbed the dose distribution only slightly more than with the IV contrast scenario. In the non-physiologic extremes of densities, the PTV66 volumes receiving 93% and 100% of the prescription changed by ≤±1.10%. The PTV66 volume that received >110% of the prescription dose was more sensitive to the change in blood vessel densities with an average change of ±1.55% with bone-equivalent or air-equivalent densities. The largest variation of maximum point dose (0.1 cm3) and mean dose to PTV66 observed for all ten patients was −0.39 and −0.60 Gy, respectively. The minimum point (0.1 cm3) dose to PTV66 was largely insensitive to non-physiologic contrast perturbations, with the greatest change being −1.07 Gy for the bone-equivalent density.
Although for PTV54, the bone-equivalent density had a greater effect than the air-equivalent density on the volume receiving full dose, the degree of change was still minimal (−0.64±0.46%). The largest variation observed in minimum point dose (0.1 cm3) for PTV54 in the bone-equivalent scenario was a reduction of 0.14 Gy. For the air-equivalent density, the minimum point dose to both PTVs was zero for many patients, which reflected that an “air” filled blood vessel was present within the target volume. The variation of minimum point dose to both PTVs for the “air” scenario was artificially large due to the “air” filled blood vessels and was not reported in .
The greatest absolute change in maximum point dose to OARs occurred with the air-equivalent vessels, when the dose to cord increased by 0.73 Gy. The mean doses to both the contralateral and ipsilateral parotid glands changed by an average of ≤±0.06 Gy (SD = 0.05 Gy) for both non-physiologic scenarios.
Discussion and conclusion
Although IV contrast facilitates head and neck cancer target delineation for conformal radiotherapy and IMRT Citation[7], it is not used routinely for IMRT head and neck cancer radiation planning. This may be in part due to the potential for IV contrast reactions, and contrast-induced nephropathy Citation[9], but also due to concern that IV contrast may lead to dosimetric alterations in head and neck cancer IMRT planning. We hypothesized that the dosimetric changes due to IV contrast for IMRT treatment planning would not be clinically significant, and thus should not be a reason to avoid IV contrast for CT planning if otherwise desirable.
Limited published information exists on the effects of contrast agents on external beam treatment planning in general and specifically for IMRT. Phantom and Monte Carlo simulation studies have been performed to assess the impact of CT contrast agents on dose distribution calculation Citation[10], Citation[11]. Weber et al. studied the effects of bladder opacification on dose calculations of prostate cancer patients treated with a 6-field conformal plan and found a slight reduction of dose to the prostate and the rectum (0.03–0.14% and 1.13%, respectively) Citation[12]. Wertz et al. demonstrated that intravenous contrast at the time of CT-simulation for 25 patients with skull base tumors resulted in a mean change in tumor tissue homogeneity of 19 HU (±7 HU) with a maximum change of 36 HU Citation[13]. These values are similar to those found elsewhere Citation[14].
More recently, the insensitivity of IMRT dose distribution to IV contrast perturbation was reported in two studies for head and neck patients Citation[15], Citation[16]. Our results investigating the impact of IV contrast on head and neck IMRT planning are consistent with both studies. As hypothesized, there were no clinically significant changes in dose to either targets or normal tissues when IV contrast was used for IMRT planning of head and neck cancers. In the present study, the use of CT density override, instead of acquiring two CT datasets with and without IV contrast for each patient, eliminated confounding factors such as patient deformation and source-skin distance variation, observed elsewhere Citation[16]. For example, the variation in PTV maximum and mean dose observed by Choi et al. Citation[16] for plans with and without contrast (0.21 to 0.59 Gy) is larger than results reported here (≤0.6±0.11 Gy) and could be due in part to source-to-skin distance differences (−6 to 16 mm) observed between the two CT scans acquired with and without IV contrast. In our present study, the comparison of PTV coverage in terms of volume of PTV covered, in addition to point dose comparisons, confirms the insensitivity of the head and neck IMRT plans to blood vessel density inhomogeneities. Furthermore, we have included additional testing of non-physiologic extreme density scenarios as a novel approach in the assessment of IV contrast effect on dose calculation. Even with air or bone-equivalent densities, heterogeneity in vessels produced little dosimetric perturbation due to the limited cross-section of the blood vessels. Our findings reinforce the conclusion that the dosimetric impact of IV contrast on HN IMRT plans is negligible.
Conflict of Interest
No conflict of interest for this work.
Disclaimer
ASTRO grants the authors permission to reference the ASTRO IMRT Symposium as specified in the language provided to them and to publish this article with that reference to the ASTRO IMRT Symposium. ASTRO makes no representation or warranties and disclaims all warranties, express or implied, related to the material, including but not limited to warranties of non-infringement, merchantability or fitness for a particular purpose.
Acknowledgements
The authors would like to thank Stephen Breen and Gavin Disney for their support and helpful discussion.
References
- Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006; 368: 843–54
- Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: An individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 2006; 64: 47–56
- Eisbruch A, Dawson LA, Kim HM, Bradford CR, Terrell JE, Chepeha DB, et al. Conformal and intensity modulated irradiation of head and neck cancer: The potential for improved target irradiation, salivary gland function, and quality of life. Acta Otorhinolaryngol Belg 1999; 53: 271–5
- Pow EH, Kwong DL, McMillan AS, Wong MC, Sham JS, Leung LH, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 2006; 66: 981–91
- Eisbruch A, Ship JA, Dawson LA, Kim HM, Bradford CR, Terrell JE, et al. Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World J Surg 2003; 27: 832–7
- Gregoire V, Daisne JF, Geets X, Levendag P. Selection and delineation of target volumes in head and neck tumors: Beyond ICRU definition. Rays 2003; 28: 217–24
- Breen S Publicover J De Silva S Pond G Brock K O'Sullivan B et al. Intra- and inter-observer variability in GTV delineation on FDG-PET-CT images of head and neck cancers. Int J Radiat Oncol Biol Phys ( in press).
- RTOG. Phase I/II study of conformal and intensity modulated irradiation for oropharyngeal cancer. Protocol No. 0022. Philadelphia: Radiation Therapy Oncology Group; 2001.
- Goldenberg I, Matetzky S. Nephropathy induced by contrast media: Pathogenesis, risk factors and preventive strategies. Cmaj 2005; 172: 1461–71
- Ramm U, Damrau M, Mose S, Manegold KH, Rahl CG, Bottcher HD. Influence of CT contrast agents on dose calculations in a 3D treatment planning system. Phys Med Biol 2001; 46: 2631–5
- Kassas B, Mourtada F, Horton JL, Lane RG. Contrast effects on dosimetry of a partial breast irradiation system. Med Phys 2004; 31: 1976–9
- Weber DC, Rouzaud M, Miralbell R. Bladder opacification does not significantly influence dose distribution in conformal radiotherapy of prostate cancer. Radiother Oncol 2001; 59: 95–7
- Wertz H, Jakel O. Influence of iodine contrast agent on the range of ion beams for radiotherapy. Med Phys 2004; 31: 767–73
- Clement O, Robert P, Cuenod CA, Siauve N, Sobotka A, Kahn E, et al. Functional imaging of tumors using CT and iodinated contrast media of different molecular weights. Acad Radiol 2002; 9(Suppl 1)S212–S214
- Liauw SL, Amdur RJ, Mendenhall WM, Palta J, Kim S. The effect of intravenous contrast on intensity-modulated radiation therapy dose calculations for head and neck cancer. Am J Clin Oncol 2005; 28: 456–9
- Choi Y, Kim JK, Lee HS, Hur WJ, Hong YS, Park S, et al. Influence of intravenous contrast agent on dose calculations of intensity modulated radiation therapy plans for head and neck cancer. Radiother Oncol 2006; 81: 158–62