Abstract
Purpose. To determine the overall survival (OS) of breast cancer patients treated by Whole Brain Radiation Therapy (WBRT) and possible important prognostic factors for OS. Material and methods. The study population comprised 99 patients with brain metastases (BM) treated with WBRT in the period 1988 to 2004 at St. Olavs University Hospital, Trondheim, Norway. Prognostic factors as age, performance status, axillary lymph node involvement and extent of BM were evaluated. Results. Median survival (range) of the total population from start of irradiation was 5.3 (0.3–157) months. For patients >60 years, 40–60 years and <40 years median survival (range) were 4.5 (0.3–92), 6.8 (0.3–157) and 8.5 (0.8–11) months, respectively (NS, p=0.5), and for Karnofsky performance status (KPS) < or >70, were 3.7 (0.3–92) and 6.8 (1.0–157) months, respectively (NS, p=0.17). One,three, 12 and 24 month survival rate were 90, 64, 29 and 11%, respectively. Grouping patients according to Recursive Partitioning Analyses (RPA) classes, the median survival (range) were 8.0 (1.0–157), 6.5 (1.3–92) and 3.5 (0.3–92) months for RPA class 1, 2 and 3, respectively (NS, p=0.6). Conclusion. KPS and in particular the extent of BM were the most important prognostic factors. Grouping patients into RPA classes may be important when deciding whether breast cancer patients should be aggressively treated for their BM.
Brain metastases (BM) is not an uncommon location in breast cancer patients and some times it can be the first and only site of metastases Citation[1]. The incidence of BM has been estimated to 10–20% Citation[2], Citation[3], but could be found in 30% of breast cancer patients at autopsy Citation[2]. BM are most frequently diagnosed when patients present some type of cerebral symptoms as headache (24–48%) Citation[2–5], changes in cognitive function (24–34%), seizures (10%) Citation[3–6], and ataxia, loss of motor function, nausea and vomiting Citation[2], Citation[6]. Verification of the diagnosis is done by computed tomography (CT), magnetic resonance imaging (MRI) or by biopsy. MRI has proven to be superior to CT in detecting small BM, as well as leptomeningeal metastases Citation[2], Citation[7], Citation[8]. With an uncertain diagnosis a brain biopsy may be needed, although not always feasible.
The treatment strategy of patients with BM depends largely on location and number of BM, status of extra-cranial disease, performance status and age of the patients Citation[2], Citation[5], Citation[9]. In patients with relatively good performance status, well-controlled extra-cranial disease and few BM, a relatively aggressive local treatment with surgery or gamma knife radiosurgery can be performed Citation[10–12], generally considered only in patients with ≤4 BM. For the majority of patients, however, less aggressive methods are used as Whole Brain Radiation Therapy (WBRT) Citation[5], or even in some cases symptomatic treatment with corticosteroids only Citation[13]. Using WBRT, different total dose and fraction have been used. Multi-centre trials conducted by the Radiation Oncology Group (RTOG) using different fraction regimes (from 20 Gy over one week to 50 Gy over 4–5 weeks) have shown no significant differences in outcome Citation[14].
The aim of this retrospective study was to analyse breast cancer patients treated with WBRT for BM in a single institution to possible identify prognostic factors for determining short term survivors and to determine the possible correlation between prognostic factors and survival. Predicting short- (i.e. <3 months) and long-term survivors is a particularly compelling, yet challenging, goal of the clinician, and a central tenet of the personalized medicine paradigm for oncology.
Material and methods
Patients
The study population comprised breast cancer patients treated with WBRT at the Department of Oncology, St. Olavs University Hospital, Trondheim between January 1988 and September 2004. A total of 114 patients were identified in the departments database (Verification and Information System In Radiation therapy; VISIR), having registered whole brain as the treatment region and breast cancer as primary diagnose. BM was verified by CT or MRI. Patients with primary breast cancer without BM, such as cranial metastases (n = 10), meningeoma (n = 1), and glioblastoma (n = 1) were excluded. Primary diagnoses was verified, and patient with BM and primary lung (n = 1) and cervical cancer (n = 2) were also excluded, giving 99 evaluable patients. From the patients file the following data were extracted: date of birth and diagnosis of primary breast cancer, primary tumour size, grade and hormone receptor status, axillary nodal status, localisation and date of first distant metastases and date and method of BM diagnosis, as well as number of BM. Short-times survivors were specially paid attention to, and defined as patients who passed away, one or three months after initiation of WBRT.
Histopathology
The primary tissue samples were re-examined for tumour type, grade and ER status by the pathologist (AMB) according to the national guidelines based on the Bloom and Richardson grading system Citation[15]. Patients with positive ER and\or PgR status, defined by immunhistochemistry, were defined as patients with hormone receptor (HR) positive tumours.
Treatment
From VISIR the date at initiation of radiation therapy and amount of dose given was obtained. All patients received WBRT using 6-MV photons from a linear accelerator, where 30 Gy in 10 fractions over 2 weeks (n = 86) or 20 Gy in 5 fractions over one week (n = 13) were given. In 21 and two patients surgery and gamma knife radio surgery have been performed, respectively. Treatment outcome as overall survival (OS) was measured from initiation of WBRT to last clinical follow-up examination or death.
Performance status and Recursive Partitioning Analysis (RPA) prognostic classes
Patients’ performance status at starting WBRT was estimated based on information in patient files, using Karnofsky (KPS) index. Patients were grouped as proposed by Gaspar et al. Citation[14] into the RTOG RPA prognostic classes as follows; Class 1: patients <65 years and with KPS ≥70, without extracranial metastases and with controlled primary tumour, Class 3: patients with KPS <70, Class 2: all others.
Statistical analyses
Statistical analyses were performed using SPSS 13.0 (SPSS Inc. Chicago, IL).
Descriptive statistics are presented as median (range). The survival after starting WBRT was calculated according to the Kaplan-Meier methods. Uni- and multi-variate survival analyses were performed on the following factors using log-rank and Cox proportional regression test: hormone receptor status of the primary tumour, axillary lymph node status, extracranial metastases, KPS, RPA prognostic classes and number of BM., brain surgery, time from primary diagnosis to 1. relaps (DFI) and to detection of BM. Cox test was only done on factors having p < 0.2 on the log rank test.
P-values are two-sided and considered significant when p < 0.05.
Results
The characteristics of the 99 evaluable patients are given in . Median primary tumour size (range) was 3.0 (0–10) cm, tumour size was missing for four patients. No axillary lymph nodes were affected in 29 patients, for the positive axille the amount of nodes were 1–3 in 34 and >3 in 18 patients, and for 18 patients not known. The hormone receptor status was known for all but six patients. Due to degradation, only 41 samples could be reanalysed concerning hormone receptor status by the pathologist. The first hormone receptor evaluation was used in the case of the other 55 samples.
Table I. Characteristics of patients.
Median (range) time from primary diagnoses to first relapse and detection of BM were 22 (0–320) and 40.3 (0–380) months, respectively. The first relapse were located to brain (n = 31), bone (n = 30) or lung (n = 26). Twelve patients had multiple metastases including mediastinum, skin, pleura and liver, making it unable to point out the first relapse site. Between first relapse and BM median (range) time was 10 (0–111) months.
For primary tumour size, grade or hormonal receptor status, and the axillary pN status, no significant differences of median OS were observed (). The median OS of hormone receptor positive and negative patients were 5.2 (0.3–92) and 4.8 (0.3–157), respectively (NS, p = 0.5). Median age (range) at the start of WBRT was 57 (30–80) years.
Figure 1. Kaplan-Meier analyses of overall survival in relation with multiple and solitary BM. Median OS for multiple and solitary BM were 4.5 (normal) and 13.3 (bold) months, respectively.
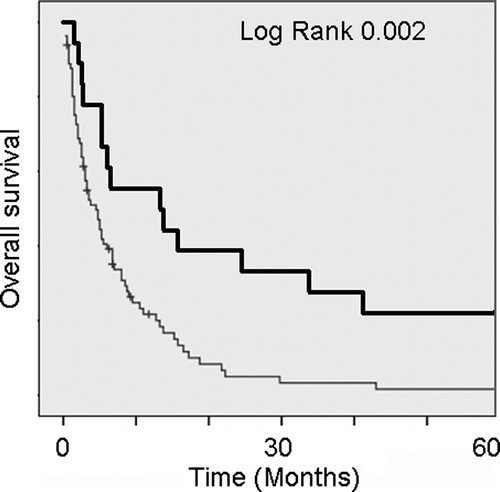
Table II. Univariate analyses of factors influencing survival in 99 breast cancer patients with brain metastases (BM).
The most usual symptoms at presentation before WBRT were urgent CNS symptoms, such as headache, seizures, loss of motor function and impaired mental status. Some patients, however, had less specific symptoms such as dizziness and impaired vision. Regarding symptoms after treatment, little information was given in the patient files.
Concerning BM, 18 patients had one BM, 81 patients had multiple BM, some uncountable number. After resection (n = 21) or gamma radiation knife (n = 2), everyone achieved WBRT.
The median time (range) from start of WBRT to last follow up or death was 5.3 (0.3–157) months. One- and two-years of survival rate were 29% and 11%, respectively, one and 3 months survival rate (short-time survivors) were 90% and 64%, respectively.
Absence of extracranial metastases, solitare BM and age below 40 are all associated with longer median time of survival but only significant for BM. Median (range) of OS of patients with KPS <70 and KPS ≥70 were 3.7 (0.3–92) and 6.8 (1.0–157) months, respectively (NS, p = 0.17), as shown in . Forty-two patients had KPS 60 with 5.2 (0.3–92) months median OS, and three patients had KPS ≤20 with a median OS of 3.0 (0.3–12) months. Median survival (range) for those with one and those with multiple BM were 13.3 (1.5–157) and 4.5 (0.3–84) months (p = 0.01), respectively. Median OS (range) of patients classified as RPA class 3 (n = 63), 2 (n = 19) and 1 (n = 17) 3.5 (0.3–92), 6.5 (1.3–92) and 8.0 (1.0–157) months (NS, p = 0.6), respectively (). Patients treated with WBRT before and after 1997 has a median OS (range) of 3.5 (0.3–157) months and 6.8 (0.3–92) months (NS, p = 0.2). Regarding response of chemotherapy at metastatic breast cancer, 11 patients with response had 7 (1.8–30), ten patients with stable disease had 10.5 (1.3–60) and 65 patient with non-response had 4.5 (0.3–60) months of OS, respectively (NS, p = 0.46). Fourteen patients did not receive any treatment before BM. Type of systemic treatment varied between Nolvadex (n = 51), Adriamycin (n = 14), FuMi regime (n = 4), CMF (n = 3), FEC (n = 9) and Taxol (n = 4) or none (n = 14), and by Kaplan-Meier method not significantly differentiating at OS (NS, p = 0.116). Finally, patients with viceral (n = 38) versus bone (n = 61) metastases, no significant difference in OS was found.
Patient with short DFI and time from primary tumor to BM had significant shorter OS using log rank. After Cox analysis only the factor BM was significant in relation to OS.
Discussion
Despite many therapy options developed throughout the years for systemic metastases, BM in breast cancer patients still remains without any effective systemic treatment. BM from breast cancer has today poor prognoses, and is often a terminal complication, as seen in our study as well, with a median OS of only 5.3 months. However, some patients are long-term survivors and seem to respond better to the treatment. One year after WBRT, 23 patients were still alive and five patients had more than 5 years survival. Four of these patients had undergone resection of BM and had only one BM, as well as long DFI. The main characterization was the picture of a limited metastatic situation in the brain, although many other factors may have contributed to good outcome, such as control of systematic disease situation, KPS and age at WBRT. When comparing the data of median OS related to different suggested prognostic factors: age, KPS and the extent of BM are the most reliable indicators. Patients ≥60 years, several BM and KPS ≤40 seem to have the worst prognosis, as shown in other studies Citation[16], Citation[17] only patients having single BM had significant longer OS both with log rank and Cox analysis in our study. Having extracranial metastases seem to influence the severeness of the disease and sub sequentially reduces OS, not shown in our study. This could lead to a debate as to whether these patients should undergo an intensive radiotherapy treatment, when it is likely that they will die within few weeks after treatment start, as discussed by others Citation[18]. The usual WBRT dose was 30 Gy although in patients in poor state 20 Gy was chosen, due to short expected survival. These patients were pre-selected due to their poor health state and expected short survival, and because of this pre-selection, the schedule of 20 Gy is not a comparable value of better or worse treatment.
The RPA prognostic classes derived from the RTOG prospective trials on BM were identified as a tool for comparison of treatment results and stratification of patients for clinical studies. This classification is based on the presence of three prognostic factors; performance status, presence of extracranial disease and age. Regarding the RPA prognostic classes in our study, no significant difference with respect to median survival times were seen, possible due to low number of patients in the three groups. However, the majority of the patients (64%) were classified as class 3 indicating the low performance status in general in patients with BM.
Patients having multiple BM (82%) had significant shorter median survival (p < 0.01, as shown in Fig 1), and was the only prognostic factor significant in our study.
This is not surprising, since only those patients having 3–4 or less BM are suitable for resection.
BM was often the first and only manifestation of relapse, although bone or lung metastases were often observed as the first site as well, as shown by others Citation[14]. High histological grade has been associated with short OS Citation[14], Citation[17], not found in our study. It is not unlikely that the incidence of BM will increases as systemic treatment for both metastatic breast cancer and in the adjuvant setting improves and thereby prolonging OS. BM causes decreased survival and may change dramatically the quality of life of the patients Citation[1]. Early detection or possible prevention of BM by identifying risk factors for BM relapses might provide the opportunity to select patients who probably benefit of prophylactic treatment at an early stage of the disease. Better understanding of the biology of BM is needed.
Despite great changes of therapeutic regimes during 1988 until 2004, no significant difference of patients treated before or after 1997 appeared (p = 0.2). The low volume in the patient cohort could be the reason of this, since a prolonged OS is expected due to better systemic disease control. In this study however, neither type of chemotherapy nor metastatic pattern of the disease had any significantly influence of OS.
The results presented in this study, showing that patients having the worst prognoses are those with KPS < 70 and multiple BM, only significant different for BM, not for the different RPA classes possible due to the low number of patients in RPA class 1 and 2. Small patient cohort like this is a great challenge in a single regional institution study and influences the results of significance. However, we still believe that regional and single institutional studies are important and reflects the national patient cohort situation.
The overall conclusion is that previous experience and findings of prognostic factors will be important in addition to knowledge of the tumours molecular characteristics in future patients. All this information will hopefully contribute to establish an individual therapy of these patients, in order to improve OS.
Acknowledgements
The study was supported by grants from the Central Norway Region Health Authority and The Norwegian Women's Public Health Association, Trondheim. We thank engineer Torbjørn Tveit at the Department of Oncology, for providing the files from VISIR. The skilled assistance of Dr. Ivar Thor Jonsson, former at the Department of Oncology, Trondheim University Hospital, Trondheim is appreciated.
References
- Higashi H, Fukutomi T, Watanabe T, Adachi I, Narabayashi M, Shibui S, et al. Seven cases of breast cancer recurrence limited to the central nervous system without other visceral metastases. Breast Cancer 2000; 7: 153–6
- Patanaphan V, Salazar OM, Risco R. Breast cancer. Metastatic patterns and their prognosis. South Med J 1988; 81: 1109–12
- Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer 1991; 49: 650–5
- Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 1996; 78: 1781–8
- Forsyth PA, Posner JB. Headaches in patients with brain tumors: A study of 111 patients. Neurology 1993; 43: 1678–83
- Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin 2003; 21: 1–23
- Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol 2004; 22: 3608–17
- Davis PC, Hudgins PA, Peterman SB, Hoffman JC, Jr. Diagnosis of cerebral metastases: Double-dose delayed CT vs contrast-enhanced MR imaging. AJNR Am J Neuroradiol 1991; 12: 293–300
- Akeson P, Larsson EM, Kristoffersen DT, Jonsson E, Holtas S. Brain metastases: Comparison of gadodiamide injection-enhanced MR imaging at standard and high dose, contrast-enhanced CT and non-contrast-enhanced MR imaging. Acta Radiol 1995; 36: 300–6
- DiStefano A, Yong YY, Hortobagyi GN, Blumenschein GR. The natural history of breast cancer patients with brain metastases. Cancer 1979; 44: 1913–8
- Hendrickson FR, Lee MS, Larson M, Gelber RD. The influence of surgery and radiation therapy on patients with brain metastases. Int J Radiat Oncol Biol Phys 1983; 9: 623–7
- Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990; 322: 494–500
- Gerosa M, Nicolato A, Foroni R, Zanotti B, Tomazzoli L, Miscusi M, et al. Gamma knife radiosurgery for brain metastases: A primary therapeutic option. J Neurosurg 2002;97(5 Suppl):515–24.
- Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997; 37: 745–51
- Boogerd W, Vos VW, Hart AA, Baris G. Brain metastases in breast cancer; natural history, prognostic factors and outcome. J Neurooncol 1993; 15: 165–74
- Cho SY, Choi HY. Causes of death and metastatic patterns in patients with mammary cancer. Ten-year autopsy study. Am J Clin Pathol 1980; 73: 232–4
- Priestman TJ, Dunn J, Brada M, Rampling R, Baker PG. Final results of the Royal College of Radiologists' trial comparing two different radiotherapy schedules in the treatment of cerebral metastases. Clin Oncol (R Coll Radiol) 1996; 8: 308–15
- Evans AJ, James JJ, Cornford EJ, Chan SY, Burrell HC, Pinder SE, et al. Brain metastases from breast cancer: Identification of a high-risk group. Clin Oncol (R Coll Radiol) 2004; 16: 345–9