To the Editor,
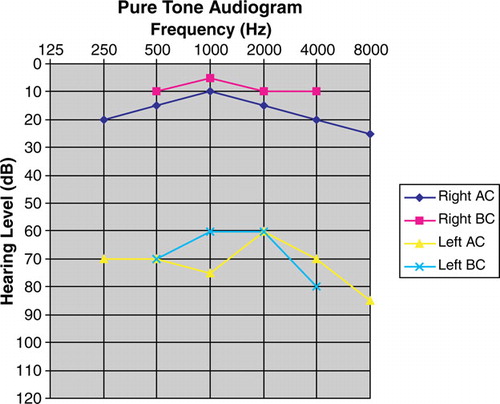
A 42 year old lady was diagnosed to have stage IIIA multiple myeloma of IgA subtype. She declined the offer of autologous bone marrow transplantation (ABMT) despite knowledge of the incurable nature of myeloma by conventional chemotherapy and the definitive survival benefit of ABMT. Intermittent melphalan and prednisolone (MP) was commenced, which rendered a partial response after six cycles. She remained asymptomatic but the disease progressed 6 months afterwards with rising serum IgA level to 56.4 g/L (normal: 0.68–3.78 g/L). Thalidomide was started at 200 mg/day. Despite the absence of neurological complication, thalidomide was stopped after 2 months because of pruritic skin rash. MP was resumed until static disease with serum IgA levels between 50–60 g/L, which began to rise after a period of 24 months. In view of a resurge of serum IgA to 76.4 g/L and dropping haemoglobin to 7 g/dL, high-does dexamethasone was started at 40 mg/day (on days 1–4, 9–12, 17–20) but was stopped after one month because of herpes zoster affecting the left third sacral (S3) dermatome. During the next 2 months when the patient was off therapy, disease progressed with further rise of serum IgA to 104 g/L. Intravenous bortezomib was commenced (at the dose of 1.3 mg/m2 on days 1, 4, 8 and 11 every 3 weekly), which resulted in a drastic response. After day 8 of the second cycle of bortezomib, she achieved complete remission with normalization of serum IgA level, as well as IgG and IgM levels. No serum M-protein was detectable by immunofixation. However, she complained of impaired hearing on left ear after completion of the second cycle of bortezomib, which was thus stopped. Pure tone audiogram confirmed left sensorineural deafness (). Two months after cessation of bortezomib, the serum IgA level resurged with re-appearance of M-protein. She received salvage therapy with VAD (vincristine, adriamycin and dexamethasone) but without any response. She died of progressive myeloma 8 months after cessation of bortezomib. Her left ear deafness persisted during the subsequent course of disease.
Figure 1. Pure tone audiogram showed sensorineural deafness of the left ear. Abbreviation: AC: air conduction; BC: bone conduction
Recent clinical trials confirmed the efficacy of bortezomib in MM Citation[1], Citation[2]. Subsequent to a phase II clinical trial (Summit) which showed a high efficacy of bortezomib in patients with refractory and relapsed MM Citation[1], a recent phase III trial (Apex) further attested the efficicacy of bortezomib in patients with relapsed/refractory MM, and showed a significantly higher response rate, longer time to progression and superior survivals in patients randomized to receive bortezomib compared with those receiving dexamethasone Citation[2]. More recently, Mateos et al. reported a very high CR (including near CR) rate of 43% in elderly patients using bortezomib in combination with MP as first line treatment Citation[3], thereby attesting the superior efficacy of bortezomib in less heavily treated or untreated patients. Therefore, bortezomib is not only effective in patients with relapsed/refractory MM; it is more effective in previously untreated patients. Major side-effects include sensory neuropathy, gastrointestinal upset and thrombocytopenia Citation[1], Citation[2]. Auditory side-effects were rarely described.
Our patient attained complete remission after one and a half cycle of bortezomib, consistent with the rapid response demonstrated in refractory myeloma patients receiving bortezomib as salvage therapy Citation[1]. While sensory neuropathy is a common complication of bortezomib, auditory complications were rarely reported. For instance, in the 192 MM patients receiving bortezomib as salvage therapy, peripheral sensory neuropathy was reported in 35% of patients, necessitating dose reduction in 12%, and discontinuation in 4–5% of patients Citation[1], Citation[2]. However, there was no report of auditory side-effects.
Drug-related ototoxicity usually results from cochlear neuroepithelial damage, and may occur in a dose-dependent or idiosyncratic manner Citation[4]. One patient has been reported to develop mild bilateral hearing loss after the third cycle of bortezomib, which increased in severity and necessitated cessation of bortezomib after the fourth cycle Citation[5]. Here we describe another patient with unilateral deafness associated with the use of bortezomib. Bortezomib was stopped immediately after the onset of unilateral deafness. Therefore, it is uncertain whether bilateral deafness might occur had bortezomib been continued.
In myeloma patients who developed peripheral neuropathy associated with bortezomib, cessation of bortezomib led to either resolution to baseline or improvement in 71% patients with severe peripheral neuropathy Citation[6]. As our patient died 8 months after cessation of bortezomib, it is uncertain whether the sensorineural might have improved had the patient survived longer.
In conclusion, despite being a rare complication, deafness may occur during the use of bortezomib in addition to sensory neuropathy.
References
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003; 348: 2609–17
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005; 352: 2487–98
- Mateos MV, Hernandez JM, Hernandez MT, Gutierrez NC, Palomera L, Fuertes M, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: Results of a multicenter phase 1/2 study. Blood 2006; 108: 2165–72
- Harpur ES. The pharmacology of ototoxic drugs. Br J Audiol 1982; 16: 81–93
- Engelhardt M, Muller AM, Maier W, Wasch R. Severe irreversible bilateral hearing loss after bortezomib (VELCADE) therapy in a multiple myeloma (MM) patient. Leukemia 2005; 19: 869–70
- Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 2006; 24: 3113–20