Abstract
Background. Local progression of advanced gastric cancer often manifests as bleeding, dysphagia/obstruction, or pain. We evaluated the magnitude and durability of palliation with radiotherapy (RT). Material and methods. From 1996 to 2004, 37 gastric cancer patients were treated with palliative RT (median dose 35Gy in 14 fractions). Nearly two-thirds of all patients received concurrent chemoradiation therapy (CRT). Index pre-treatment symptoms were gastric bleeding, dysphagia/obstruction, and pain in 54%, 43%, and 19% of patients, respectively. Results. The rates of control for bleeding, dysphagia/obstruction, and pain were 70% (14/20), 81% (13/16), and 86% (6/7), respectively. These symptoms were controlled without additional interventions for a median of 70%, 81%, and 49% of the patient's remaining life, respectively. Patients receiving CRT had a trend towards better median overall survival than those receiving RT alone (6.7 vs. 2.4 months, p=0.08). Lower (<41 Gy) biologically effective dose (BED, assuming an alpha/beta ratio of 10 for early responding tissues) predicted for poorer local control (6-month local control 70% vs. 100%, p=0.05) while T4 tumors had a trend towards inferior local control (6-month LC 56% vs. 100%, p=0.06). Discussion. Palliative RT controls symptoms for most of the remaining life in the majority of gastric cancer patients. The role of a higher dose of RT (BED ≥41 Gy), especially in patients with T4 tumors, remains to be established. In order to accurately define the role for radiotherapy in palliation of these symptoms, prospective randomized studies need to be conducted.
Although the incidence of gastric cancer has declined in developed countries in the past few decades, it is still the fourth most common cancer worldwide (with an estimated 934 000 new cases annually in 2002), and the second most common cause of cancer deaths (700 000 deaths annually) Citation[1]. In the USA, an estimated 22 280 new cases and 11 430 deaths Citation[2] occurred due to gastric cancer in 2006. Even after decades of research the prognosis remains poor, with 5-year survival rates of approximately 20% Citation[3], Citation[4]. Surgical resection is the only curative treatment. The combination of chemotherapy and radiation therapy improves median survival in resected gastric cancer patients Citation[5]. Randomized trials comparing chemotherapy versus best supportive care Citation[6–8] have established the role of chemotherapy in the treatment of patients with metastatic gastric cancer. For patients with symptomatic unresectable disease, in the presence or absence of metastatic disease, palliative measures help reduce the morbidity associated with local progression of the tumor.
Radiation treatment alleviates symptoms such as dysphagia, bleeding, and pain and is recognized as a viable non-invasive palliative therapeutic option. In contrast to other palliative options such as surgical bypass, photodynamic therapy, and endoscopic stenting for gastric outlet obstruction, radiation therapy has the added advantage of reducing tumor burden while relieving symptoms. Although palliative irradiation has been widely employed in the treatment of inoperable gastric cancer around the world for decades, there are not many reports dealing with this subject. Further, the magnitude and the durability of palliation remain inadequately characterized in the literature. This study was undertaken to retrospectively evaluate the clinical symptoms, disease control, and overall survival among patients with advanced gastric cancer being treated palliatively with radiation therapy, with or without chemotherapy.
Material and methods
Patients
We retrospectively reviewed the charts of consecutive unselected patients with biopsy-proven gastric cancer treated with palliative radiation therapy to the stomach at our institution between 1996 and 2004. The institutional tumor registry and the departmental radiation therapy database were reviewed. The institutional review board approved this analysis. We excluded patients treated with curative-intent chemoradiation therapy, most of who were treated on a series of successive pre-operative chemoradiation therapy protocols. Three patients with previous partial gastrectomy for peptic ulcer disease and eight patients with coexisting or prior unrelated malignancy were included. Patient and tumor characteristics of the 37 patients included in this study are outlined in . No patient had received prior abdominal radiation therapy.
Table I. Patient, tumor, and treatment characteristics stratified by treatment group.
Pretreatment evaluation
Pretreatment evaluation included recording of medical history, physical examination, and routine laboratory studies. Baseline staging investigations included abdominal and pelvic computed tomography (CT) scan and esophagogastroduodenoscopy (EGD) in all patients, and endoscopic ultrasonography (EUS) in 14 patients. Assessment for peritoneal involvement included laparoscopy in 15 patients and laparotomy in four patients. Cytology studies were done in ten patients. Percutaneous jejunostomies or gastrostomies were placed in 18 patients at laparoscopy. Complete staging could not be completed in 14% of patients prior to initiating radiation therapy. Baseline regional nodal status was designated as either “node-positive” or “node-negative” based on abdominal CT and EUS when available.
Symptom and toxicity assessment
The most frequent indications for palliative radiation therapy were bleeding, dysphagia/obstruction, and epigastric/abdominal pain. If the patient no longer complained of these symptoms during follow-up and did not require an intervention such as stenting, neurolysis, transfusion, or coagulation after treatment to the primary tumor, radiation therapy was assumed to have relieved the symptoms. Otherwise, the symptoms were considered to be uncontrolled by radiation therapy. Duration of symptom control was coded as the time until the patient was free of initial symptom(s) or the earliest time that the patient needed a post-radiation intervention to re-address the symptom(s). Acute and late toxicities were assessed according to the National Cancer Institute-Common Terminology Criteria for Adverse Events (RTOG/NCI-CTCAE) v3.0 and Radiation Therapy Oncology Group scoring systems.
Treatment
Treatment details are listed in . Radiation therapy was administered using megavoltage x-rays to a local field that encompassed the primary tumor only. The entire stomach was radiated only in patients with linitis plastica. Regional lymph nodes were radiated (in three patients) only if they were adjacent to the primary tumor and assumed to contribute to the index symptom. The choice of customized radiation therapy field arrangements was dictated by the location of the tumor within the stomach and its proximity to dose limiting structures including the spinal cord, liver, and kidneys. The most common field arrangement was an opposed antero-posterior field. The most common radiation fractionation regimen was 35 Gy in 14 fractions. To account for differences in fractionation regimen and to compare radiation regimens, the BED was calculated using the linear-quadratic formalism and an alpha/beta ratio of 10 for early responding tissue (tumor).
Table II. Prognostic variables for local control and overall survival.
Among the 24 patients who received concomitant chemotherapy with radiation therapy, most patients received single agent chemotherapy predominantly with fluoropyrimidines. Fifteen patients (11 chemoradiation patients and 4 radiation alone patients) received additional chemotherapy after completing chemoradiation therapy.
During treatment, patients were assessed weekly for symptoms and signs of acute toxicity. Patients were also followed by a dietary team for management of their nutritional intake (oral and via percutaneous jejunostomies/gastrostomies).
Follow-up
Patients were evaluated clinically approximately every 2 months. Local and regional response or progression of the primary tumor or regional nodes was documented using abdominal CT scans. Lesions that appeared suspicious on CT were determined to be malignant if subsequent radiological evidence of disease progression was demonstrated. Peritoneal failures were reported as distant failure. If no imaging follow-up was available post-treatment, the patient was censored on the last date of radiation treatment. Local response was also gauged by endoscopy. Follow-up information also was obtained from the M. D. Anderson Tumor Registry, which collects information on patients annually through letters, phone calls, and Bureau of Vital Statistics records.
Statistical analysis
Local control, regional control, and distant control were assessed using radiographic data collected after treatment. Times to these events, overall survival (OS), and symptom control, were calculated from the start of radiation therapy and summarized with Kaplan-Meier estimators. Patients who were alive at the time of our analysis were censored for survival. Comparisons between survival experiences were performed with a log-rank test. Proportions were compared by χ2 tests or Fisher exact tests and means were compared using t-tests. Demographic, patient-specific, tumor-specific, and treatment-specific variables were investigated for their prognostic significance by univariate analysis. Since the sample sizes are quite small, these results are exploratory in nature rather than confirmatory. All tests were two-sided and a p-value of 0.05 or less was considered to be statistically significant.
Results
Control of symptoms
With a median follow-up of 3.1 months, rates of symptom control were 70% (14/20) for bleeding, 81% (13/16) for dysphagia, and 86% (6/7) for pain. Actuarial control of bleeding and dysphagia/obstruction were sustained for a median duration of 11.4, and 6.2 months, respectively. The median duration of pain control had not been reached at last follow-up. In patients presenting with bleeding, dysphagia/obstruction, and pain as their index symptom, these symptoms were controlled without additional interventions for a median of 70%, 81%, and 49% of their remaining life, respectively.
Survival and disease progression
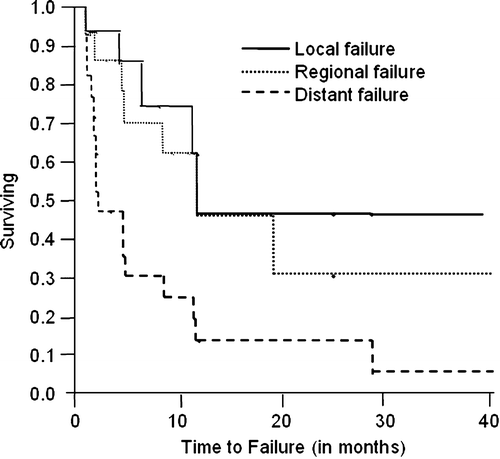
Five (26%) of 19 assessed patients progressed at the primary tumor, 17 (89%) progressed in regional lymph nodes, and 7 (32%) of 22 progressed distantly. Median actuarial time to progression was 11.9 months for local disease, 11.9 months for nodal disease, and 4.6 months for distant metastases (). Median actuarial OS was 5.2 months (95% CI: 2.7–6.8 months). Patients receiving radiation therapy with concurrent chemotherapy had a trend towards improvement in median OS over those who did not receive concurrent chemotherapy (6.7 vs. 2.4 months, p=0.08).
shows median local control and OS for selected groups of variables. On univariate analysis, there was a trend towards poorer OS among patients with signet ring cell features on pathology than those without these features (6-month OS 13% vs. 48%, p = 0.06). None of the other variables were significantly related to survival outcome. Local control was inferior in patients treated with a BED of less than 41 Gy (6-month local control 70% vs. 100%, p = 0.05) and there was a trend towards inferior local control among patients with T4 tumors (6-month LC 56% vs. 100%, p = 0.06). No other factors impacted local control rates.
Toxicity
There was no treatment-related mortality. Grade 3 nausea occurred in 2 (15%) of 13 patients who received radiation without concurrent chemotherapy. Five (21%) of 24 CRT patients experienced grade 3 toxicity (2 neutropenia, 2 nausea and 1 dehydration).
Discussion
This report documents the frequency and duration of symptomatic relief achieved by a palliative course of radiation therapy, with or without chemotherapy, in patients with symptomatic advanced gastric cancer. Palliative radiation therapy improves symptoms such as bleeding, dysphagia, and pain in the vast majority of patients with advanced disease not suitable for definitive treatment. The palliation offered is not only well tolerated, but also durable for many months, and for the majority of the patient's remaining life. These results compare favorably to other palliative treatment options available to patients with symptomatic unresectable gastric cancers. The addition of chemotherapy to radiation therapy does not increase toxicity significantly and merits consideration even in the palliative setting.
Little data exists in the literature regarding the effects of palliative radiation therapy on patients with symptomatic advanced gastric cancer. In one of the earliest studies, Mantell et al. noted an improvement in dysphagia among 13 of 17 patients with inoperable gastric carcinoma treated with a course of palliative radiotherapy Citation[9]. More recently, a study by Tey et al. reported on the efficacy and tolerability of radiation therapy in the local palliation of gastric cancer Citation[10]. Among 33 patients with locally advanced or recurrent gastric cancer, a palliative benefit was noted in 54.3% of patients (13/24) with bleeding, 25% of patients (2/8) with obstruction, and 25% of patients with pain (2/8). This palliation was durable and lasted for a median duration of 140 days, 102 days, and 105 days for dysphagia, bleeding, and pain, respectively. However, the radiation regimens ranged from 8 Gy in 1 fraction to 40 Gy in 16 fractions. Further, a correlation between radiation dose and local control was not discernable. Nevertheless, the finding that palliation lasted for the majority of the patient's remaining life in the current study is consistent with that in the study by Tey et al. Two studies have highlighted the benefits of palliative chemoradiation on malignant dysphagia in patients with advanced esophageal cancer. Harvey et al. prospectively evaluated a cohort of 106 patients Citation[11] offered palliative chemoradiation due to locally advanced or metastatic esophageal cancer, advanced age, or co-morbidity. Patients were scored based on their degree of dysphagia before and monthly after treatment, and evaluated for toxicity during and after therapy. Following treatment, 49% of patients no longer had dysphagia, and 51% maintained improved swallowing until the time of last follow-up or death. Only 5% of patients failed to complete therapy and treatment-related mortality was 6%. In 79 patients treated with palliative chemoradiation for incurable esophageal cancer, Burmeister et al. reported 3-year survival rates of 8.5% with durable palliation of dysphagia Citation[12]. Radiation dose was 30–35 Gy in 3 weeks and concurrent chemotherapy included a single dose of cisplatin (80 mg/m2) followed by a continuous infusion of 5-FU at 800 mg/m2/day for 4 days. Moderate grade 3 (28% hematologic and 9% gastrointestinal) and grade 4 (28% hematologic) toxicities were noted. The increased rate of toxicity may have been due to the use of larger radiation fields that included all macroscopically visible disease with 5 cm margins and draining lymph nodes. In the current study, we defined palliation as the lack of need for additional surgical and/or other interventional procedures. Some patients may have derived additional benefits from subsequent chemotherapy following palliative radiation therapy, however. The finding that advanced T stage correlated with poorer local control is intuitive and argues for the consideration of higher doses of palliative radiation therapy in these patients. This is especially true in light of the dose-response relationship noted for local control in this series. Doses above the BED value of 41 Gy are readily achievable with a fractionation schedule of 35 Gy in 14 fractions while the commonly used fractionation schedule of 30 Gy in 10 fractions has a BED value just below this value. It is worth noting that the higher BED did not improve OS, suggesting that the local control benefit noted with higher BED was less likely to be due to patient selection (i.e. higher doses or more intensive radiation fractionation schedules delivered to patients with better performance status).
As with most retrospective analyses, there are multiple caveats to the interpretation of these findings. First, the results of our study may not be generalizable to all patients with symptomatic unresectable gastric cancer since some of these patients may have came to our institution seeking aggressive treatment. Second, as opposed to definitive treatment comparisons from prospective trials, retrospective analyses of differences in outcomes between chemoradiation therapy and radiation therapy are subject to selection bias. It is clearly possible that patients with better performance status and less co-morbidities were offered chemotherapy with their radiation therapy. Besides these selection biases, there are uncertainties with evaluation of symptom relief from review of charts that might lead to overestimation of symptom control. Therefore, these analyses are exploratory in nature, and serve to generate testable hypotheses, not prove hypotheses. Nevertheless, especially since prospective randomized trials are uncommon in this setting, retrospective studies may be one of the few available ways to document the magnitude and durability of palliative benefit from radiotherapy. In order to accurately define the role for radiotherapy in palliation of these symptoms, prospective randomized studies need to be executed, e.g. comparing stent placement alone versus stent placement and radiation therapy when obstruction is the primary symptom or argon plasma coagulation alone versus argon plasma coagulation and radiation therapy when bleeding is the primary symptom.
In conclusion, our series demonstrates durable control of the common symptoms of dysphagia/ obstruction and gastric bleeding for most of the remaining life in the majority of patients treated with palliative radiation therapy. In order to accurately define the role for radiotherapy in palliation of symptoms, prospective randomized studies need to be executed, e.g. comparing stent placement alone versus stent placement and RT when obstruction is the primary symptom or argon plasma coagulation alone versus argon plasma coagulation and RT when bleeding is the primary symptom. Radiation therapy with a BED ≥41 Gy may provide better local control and this may be most pertinent in T4 tumors that have a lower rate of local control. However, the role of dose escalation for improved tumor control remains to be established.
This paper was presented in part at the Annual Meeting of the American Radium Society, 2006. There is no conflict of interest and financial funding.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin 2006; 56: 106–30
- Wanebo HJ, Kennedy BJ, Chmiel J, Steele G, Jr, Winchester D, Osteen R. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 1993; 218: 583–92
- Allum WH, Powell DJ, McConkey CC, Fielding JW. Gastric cancer: A 25-year review. Br J Surg 1989; 76: 535–40
- Macdonald JS. Role of post-operative chemoradiation in resected gastric cancer. J Surg Oncol 2005; 90: 166–70
- Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993; 72: 37–41
- Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995; 71: 587–91
- Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997; 8: 163–8
- Mantell BS. Radiotherapy for dysphagia due to gastric carcinoma. Br J Surg 1982; 69: 69–70
- Tey J, Back MF, Shakespeare TP, Mukherjee RK, Lu JJ, Lee KM, et al. The role of palliative radiation therapy in symptomatic locally advanced gastric cancer. Int J Radiat Oncol Biol Phys 2007; 67: 385–8
- Harvey JA, Bessell JR, Beller E, Thomas J, Gotley DC, Burmeister BH, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004; 17: 260–5
- Burmeister BH, Denham JW, O'Brien M, Jamieson GG, Gill PG, Devitt P, et al. Combined modality therapy for esophageal carcinoma: Preliminary results from a large Australasian multicenter study. Int J Radiat Oncol Biol Phys 1995; 32: 997–1006