Abstract
The DBC2 (Deleted in breast cancer, RhoBTB2) has been identified as a tumor suppressor gene that has growth inhibitory function. To investigate whether genetic alterations of the DBC2 gene are involved in the development of gastric cancer, we analyzed mutations and allelic loss in the DBC2 gene in 95 primary gastric cancers by PCR-SSCP, sequencing and LOH analysis. In the mutational analysis, we found one missense somatic mutation (CGG→TGG, R275W) in the BTB/POZ domain of the gene in a patient with advanced gastric cancer and lymph node metastasis. In addition, we found one known polymorphism and three novel polymorphisms in the coding region of DBC2, which showed an amino acid change, and was detected in both the cancer cells and corresponding normal cells. On LOH analysis, 62 cases were heterozygous for at least one marker and 18 cases (29.0%) showed allelic loss at these markers. In conclusion, the mutations and allelic loss in the DBC2 gene are uncommon in gastric cancers in Korean patients. Further studies to identify the target gene at 8q21 responsible for the development of gastric cancer should be explored.
Introduction
Gastric cancer remains a leading cause of cancer death worldwide and continues to be responsible for the majority of cancer deaths in Asian countries Citation[1]. In Korea, it accounts for an estimated 20.9% of all malignancies, with 24.4% in the male population and 16.3% in the female population Citation[2]. Thus, gastric cancer remains a significant contributor to the world's health burden. However, little is known about the molecular genetic events during it's the development and progression of gastric cancer.
The frequent chromosomal losses in carcinomas that develop from certain epithelial cells have implicated putative tumor suppressor gene(s) located on the affected chromosome in carcinogenesis. Several cytogenetic studies have identified chromosome 8p21 as a common deletion region that contains one or more of the genes associated with the development or progression of gastric cancer Citation[3–6], lung cancer Citation[7] and head and neck cancer Citation[8]. In our previous report, we analyzed the genetic alteration and functional inactivation of DR4 and DR5, which are located at 8p21, and found a low frequency of mutations in the DR5 gene and no mutations in the DR4 gene Citation[9]. Therefore, another tumor suppressor gene(s) may be involved in gastric cancer, at the 8p21 location. Recently, Hamaguchi et al. identified a new tumor suppressor gene, the DBC2 (deleted in breast cancer, RhoBTB2) gene, which belongs to the Rho GTPase family and is located at 8p21 Citation[10]. The DBC2 gene demonstrated growth inhibitory activity in a breast cancer cell line and genetic alterations of the DBC2 gene were detected in nearly 10% of breast cancer samples. In addition, only one somatic mutation of the DBC2 gene was detected. Furthermore, 42% of tumors showed allelic loss in bladder cancer, suggesting an uncommon somatic mutation of the DBC2 gene in bladder cancer Citation[6], Citation[10].
The Rho family of small GTPase constitutes a subgroup of GTP-binding proteins of the Ras superfamily ubiquitously present in eukaryotic cells Citation[11]. Interestingly, Rho GTPase has been shown to be involved in the regulation of a broad range of cellular process like endocytosis, vesicle trafficking, morphogenesis, cytokinesis, transcriptional activation, cell cycle progression, and apoptosis Citation[12], Citation[13]. In addition, there is increasing evidence that Rho-regulated signal transduction pathways are associated with tumorigenesis and metastasis Citation[14]. Taken together, these facts led us to focus on the genetic alteration of the DBC2 gene in sporadic gastric cancer.
Here, to determine whether genetic alteration of the DBC2 gene on chromosome 8p21 could be involved in the development of gastric cancer. We evaluated gastric cancer samples for mutations and allelic losses in the DBC2 gene in Korean patients by SSCP-sequencing and loss of heterozygosity (LOH) analysis.
Material and methods
Tissue samples and microdissection
Ninety-five methacarn fixed paraffin-embedded gastric carcinoma specimens were obtained from the College of Medicine, The Catholic University of Korea. Hematoxylin & Eosin (H&E)-stained histological sections were reviewed in each case. The tumors were classified according to Lauren's criteria Citation[15]. Fifty-two carcinomas were of the intestinal-type and 43 tumors of the diffuse-type. Informed consent was provided according to the Declaration of Helsinki. Approval was obtained from the institutional review board of The Catholic University of Korea, College of Medicine. There was no evidence of familial cancer in any of the patients.
DNA extraction
The tumor cells were selectively procured from Hematoxylin & Eosin stained slides using a laser microdissection device (ION LMD, JungWoo International Co, Seoul, Korea). The surrounding normal gastric mucosal cells were also obtained to study the corresponding normal DNA from the same slides in all cases. We also extracted DNAs from metastasis negative surrounding lymph nodes in cases with sequence variations in both the cancer cells and the normal gastric mucosal cells, to rule out the possibility that the sequence variations were germline mutations. After microdissection, DNA extraction was performed by a modified single step DNA extraction method Citation[16].
Single strand conformation polymorphism (SSCP) and DNA Sequencing
Genomic DNAs from tumor cells and corresponding normal cells were amplified with 18 primer pairs covering the entire coding region of the DBC2 gene (). Oligonucleotide primers were designed with the program Oligo (National Biosciences, Plymouth, MN, USA) using sequences obtained from Genbank (accession No. NT_023666). The numbering of the cDNA from DBC2 was from the ATG start codon of the gene (NM_015178). Each polymerase chain reaction (PCR) was performed under standard conditions in a 10 µl reaction mixture containing 1 µl of template DNA, 0.5 µM of each primer, 0.2 µM deoxynucleotide triphosphate, 1.5 mM MgCl2, 0.4 unit of Ampli Taq gold polymerase (Perkin-Elmer, Foster City, CA, USA), 0.5 µCi of 32P-dCTP (Amersham, Buckinghamshire, UK), and 1 µl of 10X buffer. The reaction mixture was denatured for 12 min at 95°C, annealing for 30 s at 56–67°C, and extension for 30 s at 72°C. After amplification, PCR products were denatured for 5 min at 95°C at 1:1 dilution of sample buffer containing 95% formamide/20 mM EDTA/0.05% bromophenol blue/0.05% xylene cyanol and were loaded onto a SSCP gel (FMC Mutation Detection Enhancement system, Intermountain Scientific, Kayswille, UT, USA) with 10% glycerol. Samples were electrophoresed at 8 W at room temperature overnight Citation[17]. After electrophoresis, the gels were transferred to 3 MM Whatman paper and dried. Autoradiography was performed with Kodak X-OMAT film (Eastman Kodak, Rochester, NY, USA). For the detection of mutations, sequencing of the PCR products was carried out using the cyclic sequencing kit (Perkin-Elmer, Foster City, CA, USA) according to the manufacture's recommendations. Sequence variations were verified through triplicate experiments including PCR, SSCP and sequencing analysis.
Table I. Primer sequences for amplifying the coding region of the DBC2 gene.
LOH Analysis
The tumor and corresponding normal DNAs from each slide were amplified using a thermal cycler (MJ Research, Inc., Watertown, MA, USA) with the microsatellite markers D8S258 and NEFL. The PCR conditions for the LOH study were the same as the conditions described above. The reaction products (20 µl) were then denatured and electrophoresed in 6% polyacrylamide gels containing 7M urea. After electrophoresis, the gels were transferred to 3MM Whatman paper, dried and subject to autoradiography using Kodak X-OMAT film (Eastman Kodak, Rochester, NY, USA). Complete or near complete absence of 1 allele in the tumor DNA of informative cases, as defined by direct visualization, was considered as LOH.
Results
Mutational analysis
We analyzed mutations in the entire coding region of the DBC2 gene in 95 gastric cancers by PCR-SSCP and sequencing analysis. We found one missense mutation (case 66), a C to T transition that resulted in an arginine to tryptophan alteration at codon 275 in the BTB/POZ domain. The mutation was present in tumor tissue, but absent in the corresponding normal tissue, suggesting of somatic mutation (A). The case was a clinically advanced gastric cancer with lymph node metastasis (). In addition, three novel sequence variations were also detected, two at exon 5 and one at exon 6, in both corresponding normal and tumor DNA. All of them showed an amino acid change at codon 244 (Val→Leu, GTG→TTG), codon 263 (Pro→Leu, CCG→CTG) and codon 501 (Ala→Thr, GCT→ACT) (). To rule out the possibility of a somatic mutation in surrounding gastric mucosa and cancer cells, we extracted genomic DNAs from the surrounding lymph nodes and found identical sequence variations in the lymph node DNAs, indicating a single nucleotide polymorphism. One known polymorphism (S288S) was also identified Citation[6]. We repeated the experiments three times including microdissection and PCR, SSCP and sequencing to ensure the reliability of the results and the data were consistent.
Figure 1. Representative results showing SSCP, Sequencing and LOH analyses of the DBC2 gene in gastric cancer. A. SSCP analyses of case No. 66 showed aberrant band (arrow) as compared to SSCP from corresponding normal tissue. The mutations were missense mutation; a C to T at codon R275W in BTB/POZ domain of the gene. B. Representative results of loss of heterozygosity at microsatellite markers, D8S258 and NEFL (N, normal; T, tumor). Arrows indicate allele exhibiting LOH.
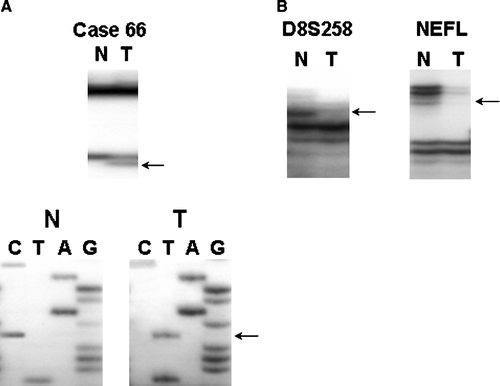
Table II. Clinicopathologic data of the gastric cancers with sequence variations of the DBC2 gene.
LOH Analysis
Ninety-five gastric cancer cases were analyzed for allelic loss of the DBC2 with highly informative microsatellite markers D8S258 and NEFL. Microsatillite instability was found in 17 and 11 cases at D8S258 and NEFL, respectively. For D8S258, 43 (55.1%) of 78 cases were informative with 10 (23.3%) of them showing an allelic loss. Thirty-nine cases were informative at NEFL and eight cases (20.5%) demonstrated an allelic loss. Sixty-two (78.6%) cases of 80 gastric cancers without a MSI were heterozygous at the D8S258 and/or NEFL markers and 18 (29.0%) cases showed a LOH at these markers (B). The case with the DBC2 somatic mutation also showed LOH at the NEFL marker. However, wild- and mutant-type alleles were seen on the SSCP gel of this case, indicating a somatic mutation in one allele and the presence of the remaining allele.
Discussion
Human carcinogenesis is generally regarded as a multistep process involving both activation of oncogenes and inactivation of tumor suppressor genes. Tumor suppressor genes are thought to participate in tumor development and progression when inactivated, commonly by gene mutation or allelic deletion. When a particular type of cancer exhibits frequent LOH, at a specific chromosomal region, we assume that a tumor suppressor gene important in the carcinogenesis of that tumor is present in that region. For example, LOH analysis in gastric cancers led to the identification of important loci critical to its development Citation[18], Citation[19]. Allelic losses of the chromosome region 8p21 have been reported as a frequent event in several cancers, including gastric cancer Citation[5], Citation[9]. These results strongly suggest that one or more tumor suppressor genes specific to several types of human cancers, especially to gastric cancer, may exist on 8p21. Thus, we focused our attention on another tumor suppressor gene at this chromosome region 8p21 to evaluate its role in gastric cancers.
Recently, mutation of the DBC2 (deleted in breast cancer, RhoBTB2) gene, which belongs to Rho GTPase family and is located on chromosome 8p21, has been identified as a candidate tumor suppressor gene in breast and bladder cancer Citation[10]. Additionally, the DBC2 gene, 0.1 Mb proximal to the DR5, is the nearest gene to this specific region of allelic loss. Therefore, it is reasonable to consider that inactivation of the DBC2 gene through primary structural changes, such as mutations and allelic loss, could be involved in the development or progression of various human cancers, including gastric cancer. In the present study, we found one missense mutation of the DBC2 gene, R275W, located in the BTB/POZ domain and an allelic loss in 18 (29.0%) of 62 informative cases with the D8S258 and/or NEFL markers. The case with the mutation was an advanced intestinal-type of gastric cancer with lymph node metastasis. Interestingly, the mutation site was a highly conserved region in rat, mouse and sheep homologues (data now shown). In mammals, the RhoBTB subfamily of Rho GTPases consists of three members (RhoBTB1, 2, 3) and is expressed in a wide range of tissues including brain, stomach, skeletal muscle, and placenta, in the human and mouse Citation[20]. The BTB domain (Broad-Complex, Tramtrack, and Bric á brac), also known as the POZ domain (poxvirus and zinc finger), is an evolutionarily conserved domain involved in protein-protein interaction and participates in homomeric and heterodimeric associations with other BTB domains Citation[21]. The function of the BTB/POZ domain is involved in transcription repression, cytoskeleton regulation, ion channel assembly and protein ubiquitination Citation[22–24]. In particular, induction of the wild-type DBC2 stopped cell proliferation, whereas induced expression of the mutant DBC2 in the BTB/POZ domain did not suppress cell growth Citation[10]. A recent report demonstrated that the down-regulation of cyclin D1 was an essential step for DBC2 growth suppressor function in tumor cells Citation[25]. Although we did not perform a functional analysis on this mutant, it is likely that the genetic alteration of the BTB domain in the DBC2 gene inhibits DBC2 activity as a growth inhibitor. However, our results suggest that the genetic alteration of the DBC2 gene might play a minor role in the development or progression of gastric cancer in Korean patients. Further studies on the DBC2 gene in a larger population are needed to broaden our understanding not only of the pathophysiological implication of Rho GTPase but also of the pathogenesis of the development of gastric cancer.
Acknowledgements
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R13-2002-005-02001- 0). The authors have no conflicts of interest to declare.
References
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer Statistics, 2005. CA Cancer J Clin 2005; 55: 10–30
- Shin HR, Jung KM, Won YJ, Park JG. 139 KCCR-affiliated Hospitals. 2002 Annual report of the Korea Central Cancer Registry: Based on registered data from 139 hospitals. Cancer Res Treat 2004;36:103–14.
- Baffa R, Santoro R, Bullrich F, Mandes B, Ishii H, Croce CM. Definition and refinement of chromosome 8p regions of loss of heterozygosity in gastric cancer. Clin Cancer Res 2000; 6: 1372–7
- Stamouli MI, Ferti AD, Panani AD, Raftakis J, Consoli C, Raptis SA, et al. Application of multiplex fluorescence in situ hybridization in the cytogenetic analysis of primary gastric carcinoma. Cancer Genet Cytogenet 2002; 135: 23–7
- Yustein AS, Harper JC, Petroni GR, Cummings OW, Moskaluk CA, Powell SM. Allelotype of gastric adenocarcinoma. Cancer Res 1999; 59: 1437–41
- Knowles MA, Aveyard JS, Taylor CF, Harnden P, Bass S. Mutation analysis of the 8p candidate tumour suppressor genes DBC2 (RHOBTB2)and LZTS1 in bladder cancer. Cancer Lett 2005; 225: 121–30
- Wistuba ll, Behrens C, Virmani AK, Milchgrub S, Syed S, Lam S, et al Allelic losses of a chromosome 8p21-23 are early and frequent events in the pathogenesis of lung cancer. Cancer Res 1999;59:1973–9.
- El-nagger AK, Coombes MM, Batsakis JG, Hong WK, Goepfert H, Kagan J. Localization of oral and laryngeal squamous cell carcinoma. Oncogene 1998; 16: 2983–7
- Park WS, Lee JH, Sin MS, Park JY, Kim HS, Kim YS, et al. In activation mutations of KILLER/DR5 gene in gastric cancers. Gastroenterology 2001; 21: 1219–25
- Hamaguchi M, Meth JL, von Klitzing C, Wei W, Esposito D, Rodgers L, et al. DBC2, a candidate for a tumor suppressor gene involved in breast cancer. Proc Natl Acad Sci USA 2002; 99: 13647–52
- Ridley AJ. Rho GTPases and cell migration. J Cell Sci 2001; 114: 2713–22
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev 1997; 11: 2295–322
- Siripurapu V, Meth J, Kobayashi N, Hamaguchi M. DBC2 significantly influences cell-cycle, apoptosis, cytoskeleton andmembrane-trafficking pathways. J Mol Biol 2005; 346: 83–9
- Aznar S, Local JC. Rho signals to cell growth and apoptosis. Cancer Lett 2001; 1665: 1–10
- Lauren P. The two histological main types of gastric carcinoma. Diffuse and so-called intestinal type carcinoma: An attempt at histoclinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49
- Lee JY, Dong SM, Kim SY, Yoo NJ, Lee SH, Park WS. A simple, precise and economical microdissection technique for analysis of genomic DNA from archival tissue sections. Virchows Arch 1998; 433: 305–9
- Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphism. PNAS 1989; 86: 2766–70
- Tokumaru Y, Nomoto S, Jeronimo C, Henrique R, Harden S, Trink B, et al. Biallelic inactivation of the RIZ1 in human gastric cancer. Oncogene 2003; 22: 6954–8
- Park WS, Oh RR, Park JY, Yoo NJ, Lee SH, Shin MS, et al. Mapping of a new target region of allelic loss at 21q2 in primary gastric cancers. Cancer Letter 2000; 159: 15–21
- Ramos S, Khademi F, Somesh BP, Rivero F. Genomic organization and expression profile of the small GTPases of the RhoBTB family in human and mouse. Gene 2002; 298: 147–57
- Ahmad KF, Engel CK, Prive GG. Crystal structure of the BTB domain from PLZF. Proc Natl Acad Sci USA 1998; 95: 12123–8
- Ahmad KF, Melnick A, Lax S, Bouchard D, Liu J, Kiang CL, et al. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol Cell 2003; 12: 1551–64
- Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci USA 2004; 101: 2046–51
- Wilkins A, Ping Q, Carpenter CL. RhoBTB2 is a substrate of the mammalian Cul3 ubiquitin ligase complex. Genes Dev 2004; 18: 856–61
- Yoshihara T, Collado D, Hamaguchi M. Cyclin D1 down-regulation is essential for DBC2's tumor suppressor function. Biochm Biophy Res Comm 2007; 358: 1076–9