Abstract
We compared the effect of a group-based 12-week supervised exercise programme, i.e. aerobic and resistance exercise, and group sports, with that of the same programme combined with cognitive-behavioural training on physical fitness and activity of cancer survivors. One hundred and forty seven cancer survivors (all cancer types, medical treatment ≥3 months ago) were randomly assigned to physical training (PT, n=71) or PT plus cognitive-behavioural training (PT+CBT, n=76). Maximal aerobic capacity, muscle strength and physical activity were assessed at baseline and post-intervention. Analyses using multilevel linear mixed-effects models showed that cancer survivors’ physical fitness increased significantly in PT and PT+CBT from baseline to post-intervention. Changes did not differ between PT and PT+CBT. Physical fitness of cancer survivors was improved following an intensive physical training programme. Adding a structured cognitive-behavioural intervention did not enhance the effect.
Many cancer survivors experience serious physical and psychological complaints caused by the disease and consequent treatment that may persist for many years Citation[1]. Due to these complaints, the activity level of cancer survivors often diminishes. Inactivity itself leads to a progressive decline in physiological functioning characterized by decreased physical capacity, reduced muscle strength and rapid fatigue during exertion Citation[2], Citation[3]. Several physical training programmes have been developed for cancer survivors aiming at breaking this vicious cycle. Reviews of the effectiveness of exercise interventions after cancer treatment demonstrate a beneficial effect on physical fitness Citation[4–6]. Two shortcomings of available studies have been summarized Citation[4]. Firstly, most research was performed in breast cancer survivors limiting generalization to other types of cancer. Secondly, the majority of exercise interventions focused on cardiovascular training. However, it is argued that muscle strength exercises should be included because such exercise may counteract cancer-related decreased muscle strength Citation[6]. The few studies that combined aerobic and resistance exercise reported positive effects on muscle strength in cancer survivors Citation[3], Citation[7–11] but only two Citation[7], Citation[11] were randomised controlled trails.
Positive effects on physical outcomes were not only reported following exercise interventions, but also following psychosocial interventions. It is reported that physical activity increased after cognitive-behavioural interventions based upon principles of self-management in various diseases Citation[12–14]. Moreover, Courneya and associates observed significant improvements in cardiovascular endurance in cancer survivors receiving cognitive-behavioural therapy Citation[15]. Several authors suggested that adding a behavioural intervention to a structured exercise programme may help facilitate exercise adoption Citation[13], Citation[16]. A cognitive-behavioral intervention might positively influence physical activity behaviour and exercise compliance by increasing self-efficacy and decreasing perceived barriers to exercise such as cancer-related distress and fatigue Citation[17]. These favourable findings imply that adding a cognitive-behavioural intervention to physical training might enhance the effect on physical outcomes obtained by exercise alone.
Therefore, in the present randomised controlled trial we combined an extensive, supervised exercise programme including aerobic training, resistance exercise and group sports with a cognitive-behavioural intervention, which was aimed at solving cancer-related problems that limit patients to be physically active in everyday life. Patients were randomly assigned to physical training (PT) or PT plus cognitive-behavioural training (PT + CBT). We hypothesized that PT + CBT and PT participants would experience significant improvements in physical fitness and activity from baseline to post-intervention. We also expected PT + CBT participants to benefit more than PT participants.
Participants and methods
A randomised controlled multicenter trial was conducted with four participating centres experienced in oncological rehabilitation: Erasmus University Medical Center, Rotterdam; University Medical Center Groningen, Groningen; Hilversum Hospital, Hilversum; and Rehabilitation Center De Hoogstraat, Utrecht, all in The Netherlands.
Inclusion criteria were: last cancer treatment completed at least 3 months before study entry; age ≥18 years; and estimated life expectancy at least one year. Moreover, subjects needed to be referred by a medical specialist or a general practitioner who judged whether rehabilitation was indicated. The latter meant a minimum of three positive findings on the following questions:
Physical complaints like aching muscles, problems with coordination, headache, nausea, heart palpitations, shortness of breath;
Reduced physical capacity compared with before the illness, e.g., less able to walk or cycle;
Psychological problems like increased anxiety level, depression, uncertainty, lack of energy or nervousness;
Increased levels of fatigue;
Sleep disturbances;
Problems in coping with reduced physical and psychosocial functioning due to cancer.
Patients were excluded if they had cognitive disturbances, serious psychopathology or emotional instability that might impede participation, or if they needed intensive medical treatment or rehabilitation. The medical ethics committee of the University Medical Centre Utrecht and the local research ethics committees approved the study that was performed according to the Helsinki Declaration of 1975, as revised in 1983.
Recruitment and allocation
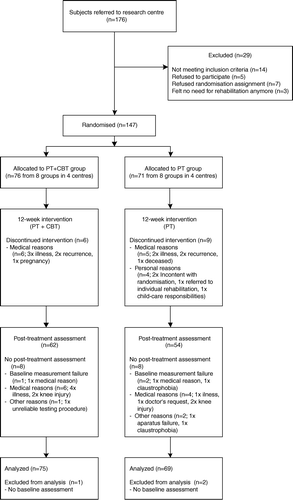
Cancer survivors were informed about the study by various methods, including leaflets handed out by oncologists and general practitioners, information in the local newspapers and through the website. Those expressing interest were sent an information letter, an informed consent form, an intake questionnaire and referral papers. After written consent, eligible subjects were scheduled for baseline measurements and randomised to either PT or PT + CBT. In each centre consecutive groups of eight to 12 eligible subjects were assigned to the randomly determined treatment to ascertain adequate numbers of participants in each group. An independent researcher randomly determined the sequence of interventions at each centre, using a randomisation list. The number of PT and PT + CBT groups were balanced in each centre. shows the flow of participants through the trial. Until the first session, participants were blinded to the intervention they were allocated to. A power analysis for a comparison between the randomized groups on the primary outcome maximal exercise capacity estimated a sample size of 64 participants in each group to detect a moderate effect-size (d = 0.50) with a power of 0.80 and a two-tailed alpha of 0.05. Accounting for an estimated dropout of 10% 71 participants in each group were needed.
Intervention
Both components, PT and the cognitive-behavioural intervention were based on the principles of self-management: i.e. goal selection, information collection, information processing and evaluation, decision making, action and self-reaction Citation[18]. Rehabilitation took place in groups of 8–12 cancer survivors. PT was supervised by two physical therapists and CBT by a psychologist and a social worker. All therapists were experienced professionals and in the field of cancer rehabilitation. The experience of PT therapists ranged from 2.5 to 6.3 years (median 5.1 years) and of CBT therapists from 2.4 to 11.3 years (median 4.4 years). All therapists received group training to apply the standardized protocols: PT therapists during one day, CBT therapists during two days.
Physical training (twice weekly, two hours per session)
In accordance with the principles of self-management, participants used heart rate monitors, the Borg Scale for dyspnea and fatigue and training logs to monitor and evaluate their performance, and received feedback, information and support from their therapists in regulating their performance.
Bicycle training (30 minutes)
Patient's main baseline physical problems were defined by assessing aerobic capacity, testing muscle strength, and medical history. Based on these, participants chose, in cooperation with the therapists, their individual goals during the first four weeks, to be trained from week five onwards: i.e. (a) improving exercise capacity, (b) improving muscle strength, (c) coping with fatigue or (d) handling physical role limitations. Training intensity was determined using the Karvonen formula Citation[19] that uses the maximum heart rate (HRmax) obtained from baseline graded exercise testing and the heart rate (HR) at rest (HRrest) to calculate the training HR (HRtr). During the first four weeks training was performed at a HRtr of (HRrest+40% to 50% of (HRmax−HRrest)). Training intensity from Week 5–12 depended on the individual training goal as mentioned above. The training programme of participants who chose improvement of exercise capacity gradually increased to a HRtr of (HRrest+80% of (HRmax−HRrest)) at Week 12, whereas the programmes of patients with other goals were aimed at a gradual increase to (HRrest+70% of (HRmax−HRrest)).
Muscle strength training (30 minutes)
Resistance exercise of lower and upper extremities was based on the baseline 1-Repetition Maximum (1-RM). Training intensity started at 30% of the 1-RM during the first week and was increased until 60% of 1-RM in week 12 for participants aiming at improving muscle strength and until 50% of 1-RM for patients with other goals.
Group sport (60 minutes)
Group sports, such as swimming, badminton and soccer, which were outlined in the training protocol, aimed at enjoying sports and overcoming any lack of confidence patients may have felt about exercising their body. Sports were performed while participants were still able to talk, which implicates a moderate intensity level Citation[20].
From week six on, the patients started a home-based walking programme as described in detail by Winningham Citation[21] to provide an additional training stimulus. Based on performance status and age, subjects started walking for 5 to 20 minutes once per week increasing the walking time by 30 seconds to two minutes per week. Walking speed was regulated by the subjects’ heart rate depending on their age, e.g. the target pulse of persons aged between 40 and 50 years was from 110–120 beats per minute. Therefore, participants wore heart rate recorders or counted their pulse rate during walking.
Cognitive-behavioural training (once a week, two hours per session)
Cognitive-behavioural training
This was aimed at training self-management skills to solve personal problems associated with physical and psychosocial consequences of cancer limiting patients to be physically active in daily living using a cognitive-behavioural problem-solving protocol for individual cancer patients Citation[22] and a group problem-solving protocol Citation[23]. The content of CBT is outlined in .
Table I. Content of the cognitive-behavioural training*.
Outcomes
Socio-demographic and medical data were collected using a self-report questionnaire. Medical data were confirmed by the referring physicians. Information about pre-cancer and pre-recruitment activity levels were assessed at intake.
Cardiopulmonary outcomes were change in peak oxygen consumption (VO2peak), peak power (Wpeak) and exercise duration evaluated by a symptom limited graded exercise test. Changes in muscle strength were determined in all centres except one. All assessments were conducted at baseline (T0) and immediately after the 12-week rehabilitation (T1). T0 and T1 tests were consistently performed by the same assessor who was not involved in the intervention. Participants were asked not to eat or drink (except water) during the two hours before exercise testing.
Exhaustive graded exercise test
Participants cycled at 60 rates per minute (rpm) with no workload for one minute to adapt to the cycle ergometer Citation[24]. The exercise test started with a workload of 20 Watt and the load was increased every minute by 10, 15 or 20 Watt (depending on the subject's fitness) until voluntary exhaustion. Increments were estimated using formulas provided by Wassermann et al. Citation[24]. Subjects were encouraged during the test. The test ended when the patient was restricted by clinical symptoms, when the cycling rate was lower than 60 rpm, or by the physician's intervention. HR was recorded during the whole test (Polar S610i, Polar Electro Inc., Helsinki, Finland). Expired gases were analysed using Oxycon Delta, Oxycon Champion (Jäger, Höchberg, Germany), Metamax MMX (Cortex Biophysics GmbH), or K4b2 (Cosmed, Rome, Italy) in the four study centres, respectively. Differences in measured oxygen uptake and carbon dioxide output between analysis systems in the different centres were small (−3.4% to 2.4% difference from overall mean at 150 W) and fell within the range of day-to-day variability Citation[25] (data not shown). VO2peak was calculated as the mean of VO2-values during the final 30 s of exercise. Wpeak was defined as workload at exhaustion.
Muscle strength measurement
Maximum voluntary isometric muscle strength of elbow flexor and extensor muscles and the knee extensor muscles was determined using a hand-held dynamometer (Strength Evaluating & Testing (microFET), Hoggan Health Industries, USA). The ‘break method’ was applied: the examiner gradually overcomes the strength produced by the patient until the extremity gives way Citation[26]. All measurements were performed three times, with recovery intervals of at least 10 s. Peak strength was recorded, and the mean values of three technically correct measurements were taken for analysis.
Physical activity
Physical activity was assessed through the 12-item Physical Activity Scale for the Elderly (PASE), a valid and reliable questionnaire Citation[27], Citation[28]. Questions deal with physical activities, such as leisure, sports, occupational, housework, and gardening. The questionnaire records the frequency of participation in these activities over the preceding 7 days. Scoring procedures were derived from motion sensor counts, physical activity diaries and a global activity self-assessment. The total PASE-score is computed by multiplying the amount of time spent in each activity by the item weights and summing over all activities. The PASE generates a single composite score of physical activity that ranges from 0–400.
Adherence to intervention
The exercise trainers and psychologists filled in a Case Record Form for each subject every session to monitor adherence to the intervention. Moreover, after each PT and CBT session, therapists filled in a general form to monitor whether the session was performed as described in the protocol.
Data analysis
Analyses (R software, version 2.3.1) were performed according to the intention-to-treat principle. Missing values of outcome variables were imputed by the mean of the predicted distribution given the hierarchical structure and specific characteristics of the person (age, gender, weight, and group allocation) using Bayesians statistics. Subjects with missing baseline values were not taken into account (n = 3; missing due to untreated hypertension, lymphedema in both legs, and claustrophobia caused by the mask covering nose and mouth). The reasons for these missing values were unrelated to non-compliance, withdrawal, or losses to follow-up and were not affected by the treatment these patients were assigned to. Therefore, post-randomisation exclusion was appropriate Citation[29].
The baseline status of the randomised participants was compared to that of those who discontinued intervention using independent Student's t-tests or Mann-Whitney tests for continuous data and χ2 tests for categorical data. Differences in socio-demographic and medical characteristics of PT + CBT and PT were tested with ANOVA and χ2 tests.
Changes in outcome variables between study groups were compared using linear mixed-effects models while taking the different levels (centre, group and individual) into account. The Akaike Information Criterion was used as a measure of how well our different models fit the data. A lower value on the Akaike Information Criterion indicated a better model fit. Additionally, an analysis was performed for complete cases. Furthermore, presence or absence of breast cancer and its interaction with treatment allocation was included as covariate to investigate whether the effects of rehabilitation were different between breast cancer patients and others.
Two-sided significance tests were used (α < 0.05).
Results
Participant recruitment took place between February 2004 and December 2005. Measurements started in March 2004 and the last follow-up measurements were performed in April 2006 (). Those who stayed in the study did not differ from the patients who discontinued with regard to age, sex, educational, marital status, type of cancer, type of treatment, time post-treatment, past physical activity and baseline values of graded exercise testing.
Baseline characteristics
shows the baseline characteristics of the study participants. Groups were not different in socio-demographic, medical and past physical activity variables (all p-values > 0.05). The majority of our participants were female, middle-aged, married, educated, employed and overweight. Pre-intervention, subjects’ physical activity levels were decreased compared to their physical activity levels before the diagnosis of cancer (p < 0.0001).
Table II. Baseline characteristics*.
Adherence to the intervention
Both intervention groups completed 83.5% of 24 physical training sessions (PT + CBT 20±4.7 sessions; PT 20±5.2 sessions, p > 0.05) and the PT + CBT group completed 82.4% of 12 cognitive-behavioural training sessions (9.9±2.4 sessions).
One participant, assigned to PT, collapsed during the intervention and deceased at the first-aid station. After autopsy, physicians judged this death to be unrelated to the intervention. No further adverse events were reported.
Physical fitness outcome variables
Changes in exercise capacity, muscle strength and physical activity from baseline to post-intervention were not significantly different between PT and PT + CBT (see & ).
Table III. Maximal exercise performance and physical activity at baseline and post-intervention; and intra- and inter-group changes pre-intervention to post-interventiona.
Table IV. Muscle strength at baseline and intra-group and inter-group changes from pre-intervention to post-interventiona
VO2peak, Wpeak, exercise time, muscle strength of upper and lower limbs and physical activity improved significantly in PT + CBT and PT from baseline to post-intervention.
Additional analysis
Complete case analyses showed similar results for all outcome variables indicating that missing data had no impact on the results (data not shown).
Adding the covariate breast cancer yes/no and its interaction with treatment allocation to the model showed that results were not different between patients with breast cancer and patients with other types of cancer (data not shown).
Discussion
The present randomised controlled trial showed that physical fitness improved following physical training consisting of aerobic training, resistance exercise, and group sports in survivors from different types of cancer. In the present study participants received an intensive supervised physical training programme that incorporated principles of self-management. In addition, participants trained within exercise classes. This peer contact provided ample opportunities for social interaction, social comparison, group support and education that might improve self-efficacy and through that physical activity behaviour Citation[30]. We showed that adding structured cognitive-behavioural training to our exercise programme did not add to the beneficial effect of physical training on physical fitness. Apparently, our theory-based group exercise programme seemed to be sufficient to improve exercise capacity. However, this conclusion might be premature because long-term effects might be different. CBT did not enhance the effect of PT on physical fitness in the short-term, but PT + CBT might be superior in the long-term because CBT might possibly enhance long-term adherence to an active lifestyle. Hence, long-term follow-up measurements in our study population are needed. Furthermore, as our PT was an intensive, group-based supervised programme that also included social cognitive components a ceiling effect might partly explain that adding structured CBT did not offer additional benefits.
We showed that the effect of the intervention in breast cancer survivors were not different to the effect in survivors of other types of cancer, which is consistent with findings of others Citation[15]. Two pilot studies investigated the effect on physical fitness of a rehabilitation programme including aerobic training and resistance exercise in breast cancer survivors, and reported beneficial effects in the training group: Wpeak and VO2peak improved and the muscle strength of the lower limbs increased Citation[7], Citation[11]. Our results confirm these findings and extend them to survivors from all types of cancer.
At baseline, our population's VO2peak was 84.3±22.6% (mean±standard deviation) of predicted VO2peak based on height, weight, gender and age Citation[31] and below 30 mL.kg−1.min, which implies poor physical fitness. The enhancement of Wpeak and VO2peak found in the present study exceeds the day-to-day variability Citation[25] and could, therefore, be considered as clinically relevant. The magnitude of change of Wpeak and VO2peak in our study is comparable to results of other studies evaluating oncological rehabilitation in survivors, which reported improvements in Wpeak of almost 10% and increases of VO2peak from 6.2% to 18.6% Citation[3], Citation[11], Citation[32]. At pre-intervention, physical activity levels of our participants were decreased compared to pre-diagnosis as is also reported by others Citation[33], Citation[34]. At post-intervention, our participants reported an increase of physical activity levels compared to pre-intervention. According to Cohen Citation[35], this increase implied a small treatment effect.
Muscle strength of the lower and upper limb increased in both PT and PT + CBT participants from pre- to post-intervention. Increases of the knee extensor muscles were of moderate to large effect size, whereas effect sizes for increases of elbow flexor and extensor muscles were small Citation[35]. In contrast to upper limb muscles, lower limb muscles were not only trained during resistance training but also during bicycle exercise. Possibly, training of the upper limb muscles should be extended and, moreover, therapists should ensure careful implementation.
Strengths of the present study were the large sample size, the randomised controlled design with intention-to-treat analysis, the supervised, standardised and theory-based intervention, high attendance rates, and the validated measures of fitness. Our study did not include a control group. Cancer rehabilitation is presently offered to cancer patients in 60 centres throughout the Netherlands. It would have been unethical to ask patients to participate in a study and run the risk of being randomised to a control situation in which they had to wait for an intervention. In addition, recent reviews and meta-analyses Citation[4], Citation[5] reported that exercise is an effective intervention to improve cardiorespiratory fitness.
In conclusion, survivors of different types of cancer showed improved physical fitness following a supervised, self-management physical training programme which combines cardiorespiratory and resistance exercise with group sports. Adding a structured cognitive-behavioural intervention did not enhance the positive effects of physical training on physical fitness immediately following the intervention.
Conflict of interest
None declared.
Acknowledgements
This study was supported by grants from the Dutch Cancer Society (UU-2000-2585), Maastricht University and the Comprehensive Cancer Center Norht-Netherlands. The authors would like to acknowledge the contribution to our research project and the present paper of R.W. Trijsburg, Prof. (Department of Medical Psychology and Psychotherapy, Erasmus Medical Center Rotterdam) who passed away April 8th 2007. Furthermore, we would like to thank professional staff and all participants in all study centers, whose participation made this study possible. We also would like to acknowledge W. B. Busschers, MSc (Center for Biostatistics, Utrecht University) for his statistical support, and D. E. Grobbee, Prof., MD, PhD (Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht) for his useful comments on this manuscript.
References
- Bjordal K, Mastekaasa A, Kaasa S. Self-reported satisfaction with life and physical health in long-term cancer survivors and a matched control group. Eur J Cancer B Oral Oncol 1995; 31B: 340–5
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: A literature review. Ann Behav Med 1999; 21: 171–9
- van Weert E, Hoekstra-Weebers J, Grol B, Otter R, Arendzen HJ, Postema K, et al. A multidimensional cancer rehabilitation program for cancer survivors: Effectiveness on health-related quality of life. J Psychosom Res 2005; 58: 485–96
- Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2005; 14: 1588–95
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. Can Med Assoc J 2006; 175: 34–41
- Oldervoll LM, Kaasa S, Hjermstad MJ, Lund JA, Loge JH. Physical exercise results in the improved subjective well-being of a few or is effective rehabilitation for all cancer patients?. Eur J Cancer 2004; 40: 951–62
- Nieman DC, Cook VD, Henson DA, Suttles J, Rejeski WJ, Ribisl PM, et al. Moderate exercise training and natural killer cell cytotoxic activity in breast cancer patients. Int J Sports Med 1995; 16: 334–7
- Durak EP, Lilly PC. The application of an exercise and wellness program for cancer patients: A preliminary outcomes report. J Strength Cond Res 1998; 12: 3–6
- Durak EP, Lilly PC, Hackwordt JL. Physical and psychosocial responses to exercise in cancer patients. A two year follow-up survey with prostate, leukemia, and general carcinoma. J Exerc Phys online 1999; 2: 1
- Hayes SC, Davies PS, Parker TW, Bashford J, Green A. Role of a mixed type, moderate intensity exercise programme after peripheral blood stem cell transplantation. Br J Sports Med 2004; 38: 304–9
- Herrero F, San Juan AF, Fleck SJ, Balmer J, Perez M, Canete S, et al. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med 2006; 27: 573–80
- Damush TM, Perkins A, Miller K. The implementation of an oncologist referred, exercise self-management program for older breast cancer survivors. Psychooncology 2006; 15: 884–90
- Scholz U, Knoll N, Sniehotta FF, Schwarzer R. Physical activity and depressive symptoms in cardiac rehabilitation: Long-term effects of a self–management intervention. Soc Sci Med 2006; 62: 3109–20
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: A systematic review of randomized controlled trials. Diabetes Care 2001; 24: 561–87
- Courneya KS, Friedenreich CM, Sela RA, Quinney HA, Rhodes RE, Handman M. The group psychotherapy and home-based physical exercise (group-hope) trial in cancer survivors: Physical fitness and quality of life outcomes. Psycho-Oncology 2003; 12: 357–74
- Pickett M, Mock V, Ropka ME, Cameron L, Coleman M, Podewils L. Adherence to moderate-intensity exercise during breast cancer therapy. Cancer Pract 2002; 10: 284–92
- Scheier MF, Helgeson VS, Schulz R, Colvin S, Berga S, Bridges MW, et al. Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early-stage breast cancer. J Clin Oncol 2005; 23: 4298–311
- Creer TL. Self management of chronic illness. Handbook of self regulation. Academic Press, San Diego, CA, US 2002
- Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med 1988; 5: 303–11
- Persinger R, Foster C, Gibson M, Fater DC, Porcari JP. Consistency of the talk test for exercise prescription. Med Sci Sports Exerc 2004; 36: 1632–6
- Winningham ML. Walking program for people with cancer. Getting started. Cancer Nurs 1991; 14: 270–6
- Nezu AM, Nezu CM, Houts PS, Friedman SH, Faddis S. Helping cancer patients cope: A problem-solving approach. American Psychological Association, Washington, DC, US 1998
- van den Hout JH, Vlaeyen JW, Heuts PH, Zijlema JH, Wijnen JA. Secondary prevention of work-related disability in nonspecific low back pain: Does problem-solving therapy help? A randomized clinical trial. Clin J Pain 2003; 19: 87–96
- Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ. Principles of exercise testing and interpretation3rd ed. Lippincott Williams & Wilkins, Baltimore 1999; 129–132
- Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med 1985; 6: 197–201
- van der Ploeg RJ, Oosterhuis HJ. The “make/break test” as a diagnostic tool in functional weakness. J Neurol Neurosurg Psychiatry 1991; 54: 248–51
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J Clin Epidemiol 1993; 46: 153–62
- Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): According to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol 1997; 50: 541–6
- Fergusson D, Aaron SD, Guyatt G, Hebert P. Post-randomisation exclusions: The intention to treat principle and excluding patients from analysis. BMJ 2002; 325: 652–4
- Brassington GS, Atienza AA, Perczek RE, DiLorenzo TM, King AC. Intervention-related cognitive versus social mediators of exercise adherence in the elderly. Am J Prev Med 2002; 23: 80–6
- Jones NL. Clinical exercise testing. WB Saunders Company, Philadelphia 1988; 306
- Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol 2003; 21: 1660–8
- Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer 2003; 97: 1746–57
- Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med 1997; 3: 215–26
- Cohen J. Statistical power analysis for the behavioural sciences2nd ed. Lawrence Erlbaum Associates, Hillsdale, NJ 1988; 19–74
- Saltin B, Gollnick PD. Skeletal muscle adaptability: Significance for metabolism and performance. Handbook of Physiology. Skeletal Muscle, LD Peachey, PH Adrian, SR Geider. American Physiological Society, Bethesda, MD 1983; 555–631