To the Editor
Desmoid tumors are benign growths of myofibroblasts that occur in musculoaponeurotic tissue. They occur in the general population with an incidence of approximately only 2 cases per million. However, 12 to 15% of patients with Familial Adenomatous Polyposis (FAP) develop a desmoid tumor. FAP is a dominantly inherited disorder due to an inactivating germ line mutation occurring within the Adenomatous Polyposis Coli (APC) gene. The majority of desmoid tumors, both sporadic and in FAP patients occur within the abdomen. We report a sporadic desmoid tumor in a patient determined to be homozygous for the APC*I1307K gene mutation.
Observation
The patient is a 28-year-old Ashkenazi female who developed the sudden onset of fever and right lower abdominal pain. Her past medical history was remarkable only for gastrointestinal reflux disease. One grandfather had colon carcinoma and one grandmother had breast carcinoma. She presented to an emergency room where a diagnosis of acute appendicitis led to a laparoscopic appendectomy. The pain persisted after surgery and a CT scan showed an 8 cm by 9 cm mesenteric mass. This was excised during an open procedure. Pathology revealed a mesenteric desmoid tumor ().
Figure 1. Hematoxylin and eosin stained tissue from abdominal desmoid showing a proliferation of bland spindle cells in a connective tissue background (×40).
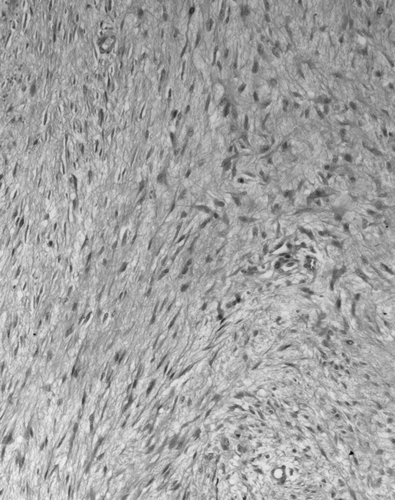
Subsequent colonoscopy revealed no adenomas or non-adenomatous polyps.
Methods
APC Gene Studies
DNA was available from peripheral blood white cells as well as normal colonic tissue and the desmoid tumor. The APC gene was sequenced between codons 1270 to 1492 using the overlapping primer sets 5′-CCA AGA AAC AAT ACA GAC TTA TTG TG-3′ (sense 1) plus 5′-ATG AGT GGG GTC TCC TGA AC-3′ (antisense 1) and 5′-TTC TTC AGG AGC GAA ATC TC-3′ (sense 2) plus 5′-TCC ATC TGG AGT ACT TTC TGT G-3′ (antisense 2). Multiplex ligation dependent probe amplification (MLPA) was performed on both peripheral blood lymphocyte DNA as well as normal tissue DNA. The probe mix included probes for each of the 15 coding exons of the APC gene, alternative exon, and three probes for the promotor region of the APC gene. Thirteen probes for other loci located on different chromosomes were used as control. Quantification was performed by SoftGenetics using Gene Mapper software. The protein truncation test (PTT) was also applied to the two normal DNA samples. The exon 3 region of the beta-catenin gene was amplified using PCR with the primers 5′-TTT CCA ATC TAC TAA TGC TAA TAC TG-3′ and 5′-CTG CAT TCT GAC TTT CAG TAA GG-3′.
Results
Normal tissue
Our patient is homozygous for a germ line mutation at codon 1307, a T to A transversion at nucleotide 3920 (). No other mutations were detected in this region. DNA from peripheral blood cells was homozygous for several APC markers but was heterozygous for the marker D5S492. Sequencing confirmed the presence of one or two copies of the I1307K mutation but no wild type sequence. No duplication or deletion was detected by MLPA, thereby indicating that the mutation at codon 1307 was indeed homozygous and not hemizygous. Normal tissue DNA analyzed by the protein truncation test revealed no small deletions, insertions or frameshifts. Additional testing indicated the patient was not a carrier of the MSH2* or MLH1*D132H germ line mutations.
Figure 2. DNA sequence analysis. A. Normal DNA. The arrow indicates the wild-type pattern with a single thymine peak at the second nucleotide position of codon 1307.B. Patient's desmoid DNA with germ line homozygous mutation. The arrow indicates only the presence of adenine at the second nucleotide position of codon 1307.
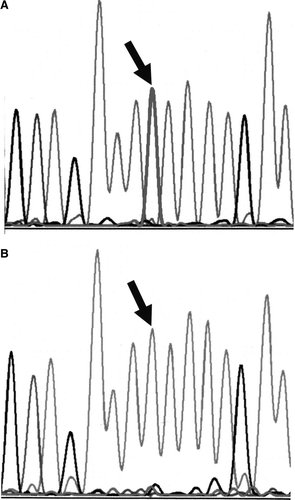
Desmoid tissue
The desmoid tumor DNA showed only the APC*I1307K mutation and no other mutation or loss in the region of exons 15G and 15H of the APC gene. The patient's tumor and normal colonic tissue DNA were sequenced for the two known ‘hot spots’, codons 41 and 45, of the beta-catenin gene. The desmoid tumor DNA revealed a transition in codon 41 of adenine to guanine in the first position, ACC to GCC (data not shown) that was not present in the normal colonic tissue.
Discussion
The APC*I1307K mutation is a T to A transversion at nucleotide 3920 that converts the sequence AAATAAAA to (A)8, resulting in a substitution of lysine for isoleucine at codon 1307. This mutation by itself is not associated with a stop codon and does not appear to affect the protein function. However, the (A)8 tract is unstable and may increase the rate of somatic mutations within this region, leading to frame shifts and APC gene inactivation Citation[1]. Studies have suggested the APC*I1307K mutation confers an increased risk for the development of colorectal neoplasms Citation[2]. Additionally, codon 1307 is within the region coding for the segment of the APC protein that binds to and exerts control over beta-catenin gene expression Citation[3]. Loss of APC protein control may result in accumulation of beta-catenin protein and increased expression of several proliferation genes controlling cell adhesion and cell signaling. Desmoid tumors are frequent in FAP patients with germ line APC gene mutations occurring beyond codon 1309, particularly in the region of codons 1445–1578 Citation[4], Citation[5]. Latchford et al. demonstrated both germ line and somatic APC mutations in 17 of 32 FAP patients with demoid tumors but did not report beta-catenin gene analyses on these tumors Citation[6]. They describe the loss of those areas of the APC protein that function as beta-catenin degradation sites resulting from a truncating APC mutation. These sites are coded by codons in the 1265–2000 region of the APC gene. Our data do not suggest a truncating mutation in this region.
Desmoids have been reported in non-FAP families with germ line APC gene mutations at codons 1924 and 1962 Citation[7], Citation[8]. Somatic mutations in the beta-catenin gene also have been identified as important in the development of desmoid tumors Citation[9], Citation[10]. Tejpar et al. performed mutational analysis of both the APC and beta-catenin genes in 42 sporadic desmoids, the largest reported study Citation[11]. Nine tumors had mutations in the APC gene, all between codons 1324 and 1567 and resulting in an early stop codon. In addition, ten tumors revealed a mutation in the beta-catenin gene, a codon 41 transition of adenine to guanine. This substitution has been shown to produce a stabilized beta-catenin protein product Citation[12]. Twelve additional tumors contained a point mutation in codon 45 of the beta-catenin gene.
It is possible that the APC gene mutation in our patient is uninvolved in the oncogenic process. However, the development of many tumors involves the accumulation of several genetic influences. Since the effects of beta-catenin and APC gene mutations can be additive, it is reasonable to postulate that there is an interactive oncogenic effect between the beta-catenin gene point mutation and the APC*I1307K mutation in our patient.
Our observation would be strengthened had we performed full mutational analysis of the APC and beta-catenin genes. However, the effect of the APC*I1307K mutation is limited to the area around codon 1307, which we did sequence. We also sequenced the major hot-spot for beta-catenin gene mutations. Immunohistochemical staining for beta-catenin has been shown to be sensitive but not a specific test for dermoids Citation[13] and therefore we did not use immunohistochemistry.
The mechanism by which homozygosity for the APC*I1307K mutation may have contributed to the development of a desmoid tumor is not yet clear. It is possible that homozygosity, similar to heterozygosity, fosters additional APC point mutation(s) that we did not detect, but this is unlikely given our detailed analyses of both the somatic and germ line APC genes. We suggest the possibility that homozygosity of the APC*I1307K mutation may have a qualitatively different effect than heterozygosity and may lead directly to abnormal APC function. Homozygosity for the APC*I1307K mutation is infrequent, just two of 5081 Ashkenazi Jews in one large study Citation[2] and two of 429 in another Citation[14]. A full description of the associated phenotype and genotype of APC*I1307K homozygosity has yet to be defined. In conclusion, we suggest the possibly that homozygosity of the germ line APC*I1307K gene mutation in our patient contributed, along with the observed somatic beta-catenin gene mutation, to the development of the desmoid tumor.
References
- Laken SJ, Peterson GM, Gruber SB, Oddoux C, Ostrer H, Giardiello FM, et al. Familial colorectal cancer in Ashkenazim due to a hypermutable tract in APC. Nat Genet 1997; 17: 79–83
- Woodage T, King SM, Wacholder S, Hartge P, Struewing JP, McAdams M, et al. The APC I1307K allele and cancer risk in a community-based study of Ashkenazi Jews. Nat Genet 1998; 20: 62–5
- Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol 2000; 18: 1967–79
- Bertario L, Russo A, Sala P, Eboli M, Giarola M, D'Amico F, et al. Genotype and phenotype factors as determinants of desmoid tumors in patients with familial adenomatous polyposis. Int J Cancer 2001; 95: 102–7
- Sturt NJH, Gallagher MC, Bassett P, Philip CR, Neale KF, Tomlinson IPM, et al. Evidence for genetic predisposition to desmoid tumours in familial adenomatous polyposis independent of the germline APC mutation. Gut 2004; 53: 1832–6
- Latchford A, Volikos E, Johnson V, Rogers P, Suraweera N, Tominson I, et al. APC mutations in FAP-associated desmoid tumours are non-random but not ‘just-right’. Hum Mol Genet 2007; 16: 78–82
- Eccles DM, Luijt R, Bruekel C, Bullman H, Bunyan D, Fisher A, et al. Hereditary desmoid disease due to a frameshift mutation at codon 1924 of the APC gene. Am J Hum Genet 1996; 59: 1193–201
- Scott RJ, Froggatt NJ, Trembath RC, Gareth D, Evans R, Hodgson SV, et al. Familial infiltrative fibromatosis (desmoid tumours) (MIM135290) caused by a recurrent 3′APC gene mutation. Hum Mol Genet 1996; 5: 1921–4
- Shitoh K, Konishi F, Iijima T, Ohdaira T, Sakai K, Kanazawa K, et al. A novel case of a sporadic desmoid tumour with mutation of the beta catenin gene. J Clin Pathol 1999; 52: 695–6
- Miyoshi Y, Iwao K, Nawa G, Yoshikawa H, Ochi T, Nakamura Y. Frequent mutations in the beta-catenin gene in desmoid tumors from patients without Familial Adenomatous Polyposis. Oncol Res 1998; 10: 591–4
- Tejpar S, Nollet F, Li C, Wunder JS, Michils G, Cin P, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 1999; 18: 6615–20
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science 1997; 275: 1790–2
- Carlson JW, Fletcher CDM. Immunohistochemistry for b-catenin in the differential diagnosis of spindle cell lesions: Analysis of a series and review of the literature. Histopathology 2007; 51: 509–14
- Zauber NP, Sabbath-Solitare M, Marotta SP, Zauber AZ, Foulkes W, Chan M, et al. Clinical and genetic findings in an Ashkenazi Jewish population with colorectal neoplasms. Cancer 2005; 104: 719–29