Abstract
The sentinel node biopsy (SNB) procedure is a multidisciplinary technique, invented to gain prognostic information in different malignant tumors. The aim of the present study was to study the cohort of patients with malignant melanoma, operated with SNB, from the introduction of the technique in Sweden, concerning the prognostic information retrieved and the outcome of the procedures. In Sweden all patients with malignant melanoma are registered at regional Oncological Centers. From these databases ten centers were identified, treating malignant melanoma and performing sentinel node biopsy. Consecutive data concerning tumor characteristics, outcome of the procedure and disease related events during the follow-up time were collected from these ten centers. All cases from the very first in each centre were included. The SNB procedure was performed in 422 patients with a sentinel node (SN) detection rate of 97%, the mean Breslow thickness of the primary tumors was 3.2 mm (median 2.4 mm) and the proportion of ulcerated melanomas 38%. Metastasis in the SN was found in 19% of the patients but there was a wide range in the proportion of SN metastases between the different centers (5–52%). After a follow-up of median 12 months of 361 patients, SN negative patients had better disease-free survival than SN positive (p<0.0001). A false negative rate of 14% was found during the follow-up time. In this study the surgical technique seemed acceptable, but the non-centralized pathology work-up sub-optimal. However, SNB was still found to be a significant prognostic indicator, concerning disease free survival.
The treatment of regional lymph node basins in patients with primary cutaneous malignant melanoma is controversial Citation[1–3]. Before the introduction of the sentinel node biopsy (SNB) technique, only two treatment options were available: immediate elective lymph node dissection (ELND) or observation, with clinical lymph node dissection (CLND), if node metastases became evident. Before 2000 the Swedish guidelines recommended observation only as previous randomized clinical trials had failed to show that elective lymph node dissection improved patient survival compared to wide excision alone Citation[4–6]. Besides that, the side effects of ELND is known to be substantial Citation[7].
In 2000 the Swedish Melanoma Study Group (SMSG) recommended sentinel lymph node biopsy in patients with cutaneous malignant melanoma of =1.5 mm Breslow thickness and radical lymphadenectomy if the sentinel node (SN) was metastatic. This was based on the observation in a nation wide Swedish case control study by Thörn et al., that a subgroup of patients with intermediate thickness melanoma (1.50–2.49 mm) had a significantly reduced risk of late mortality after ELND Citation[8]. In 2004 SMSG changed the recommendations to Breslow thickness >1.0 mm, as used in the MSLT-I trial Citation[9], and the group also decided to perform a follow-up of the introduction of the SNB technique in Sweden. The aims were to study the detection rates, possible differences between centres, and disease free survival of the patients after the procedures.
Material and methods
In Sweden all patients with malignant melanoma are mandatorily registered at regional Oncological Centres. These registers are quality assurance registers in the Swedish health care. From these databases ten different centres were identified, treating malignant melanoma and performing sentinel node biopsy. Data from all consecutive patients operated with SNB in relation to the primary surgery for malignant melanoma between January 1, 1997 and December 31, 2003 were collected from these centres. Also included in the analysis were consecutive data from procedures performed up to December 31, 2004 from eight of the centres and from three of the centres data from procedures peformed up to September 30, 2005. All cases from the very first in each centre were included.
Patients were followed according to Swedish guidelines, every sixth month up to the fifth year and once a year thereafter with some local variations.
Sentinel node biopsy technique
In all centres preoperative lymphoscintigraphy was performed using 40 MBq 99mTc labelled human albumin colloid, injected intradermally near the scar, in 2 or 4 sites. One centre initially used 99mTc labelled Albu-Res but changed to 99mTc labelled Nanocoll during 2004, all other centres only used 99mTc labelled Nanocoll. The scintigraphy was performed either the day before surgery or a few hours before the procedure. Intraoperative identification of the sentinel node was obtained with a handheld gamma probe. All centres also injected Patent Blue Violet (Guerbet) intradermally near the scar 5 to 10 minutes before the biopsy incision.
Pathology work-up
The different centres did not use a common standard protocol for the histopathological examination of the sentinel nodes. According to a survey, multiple sections was done in most centres but serial sectioning was not performed. Some centres used immunohistochemistry as an adjunct in doubtful cases.
Statistical analysis
Differences between groups of patients were evaluated using Student's t-test or χ2 tests as appropriate. Correlation between different variables were tested with simple linear regression analysis. Survival was illustrated using Kaplan-Meier plots and differences in survival between groups were tested using the log-rank test. For the survival analysis, patients with head and neck melanoma were excluded, as were all 16 cases from one centre, in which half of the patients were lost to follow-up. Patients with head and neck melanomas were not intended to be included from the start, but those performed are included in the descriptive part of the study.
Results
Four hundred and twenty two SNB procedures were performed in the ten centres. The number of procedures at each centre ranges from 7–112, (median 36.5). The cumulative number of SNB per year in the ten centres up to December 31, 2003 is shown in . Patient characteristics are shown in . The median age of the patients was 60 years. Median and mean tumour thickness (Breslow) were 2.4 and 3.2 mm respectively and only 2% of the tumours were =1 mm thick. The thickness according to Breslow was slightly greater among melanoma on the leg, compared to other locations, however, the difference was not statistically significant. There was a correlation between patient age and tumor thickness. Mean tumor thickness was 2.3 mm, 3.0 mm and 3.5 mm for patients <35 years, 35–60 years and >60 years respectively (p < 0.001). Ulceration was present in 156 of the tumors (38%). SN positive tumors had a median thickness of 3.2 mm and SN negative 2.2 mm (p < 0.001). In this series, 3% of the tumors were located in the head and neck region.
Figure 1. Annual number of sentinel node biopsies for Melanoma in Sweden, from January 1, 1997 to December 31, 2003.
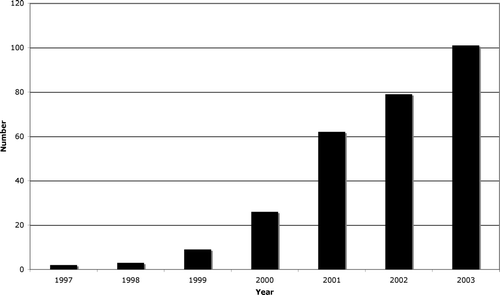
Table I. Characteristics of 422 melanoma patients undergoing sentinel lymph-node biopsy.
In 3% of the procedures no sentinel node was found.
Mean 2.1 SN were removed from each patient, range 1–9. Significantly more nodes were found in SN positive patients (mean 2.5) than in SN negative (mean 2.0) (p = 0.002)
Eighty three percent of the patients had their SN in one nodal basin while 13% had SN in two basins and 2% in three nodal basins, all with truncal melanoma.
Sentinel node positivity
The SN was positive in 79 (19%) and negative in 330 (78%) of the 422 patients. No SN was found in 12 patients and the status is unknown in one. The relation between some histopathological characteristics and sentinel node status is shown in . Among the 79 SN positive patients, mean 1.3 nodes were positive (range 1–5). Seventy eight percent had 1 node positive and 22% 2 or more nodes positive. Ulcerated melanoma tended to be SN positive more often (24%) than non-ulcerated (17%), however the difference was not statistically significant (χ2 test, p = 0.13).
Table II. Age group, location, depth and ulceration in 79 SN positive patients and proportion of SN positivity.
There was a correlation between tumor thickness and SN positivity. No patient with AJCC T1 tumors had a positive SN, while 12% percent of T2 tumors, 19% of T3 tumors and 33% of T4 tumors had a positive SN.
Number of positive basins
Of the 79 SN positive patients 75 (95%) had 1 nodal basin positive and only 4 had 2 nodal basins positive. Of the 4 with 2 basins positive 3 were trunk melanoma and one an arm melanoma.
Lymphadenectomy
For unknown reasons 12 (15%) patients did not undergo a lymphadenectomy despite they were SN positive. Among the 67 SN positive patients who were operated with radical lymphadenectomy, 18 had positive non sentinel nodes (27%).
Two SN negative patients were operated with radical lymphadenectomy. One of them had a positive non sentinel node and was thus falsely negative.
Incidence of sentinel node positivity in different centres
The rate of SN positivity varied between 5% and 57% between the different centers, .This could be partly explained by the fact that some of the centers had performed few SNB-procedures. There was no statistically significant correlation between the mean tumor thickness in the different centres and the proportion of positive SN.
Table III. Incidence of sentinel lymph node metastases and meantumor thickness, according to investigating center. Patients with known SN status (n = 409).
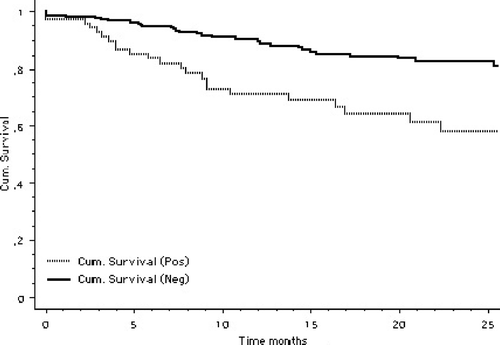
Survival analysis
After exclusion of head and neck melanoma and one centre with poor follow-up data, disease free survival (DFS) analysis was performed on 361 patients, 73 of which were SN positive (20%) The follow-up time was median 12 months (range 0–72 months). There was a statistically significant (p < 0.0001) difference in disease free survival between sentinel node positive and negative patients as shown in .
Recurrences
During the follow-up time 27 (37%) of the 73 sentinel node positive patients, experienced a recurrence. The localisation of the first recurrence is shown in .
Table IV. Localisation of first recurrence according to sentinel node status In 361 patients, 73 sentinel node positive [No (%)]
Seven of the recurrences were in the explored regional basin (10%). One of these patients had not been operated with lymphadenectomy in spite of the positive SN. Thirty nine (14%) of the 288 SN negative patients had a recurrence. Twelve of them (4%) had the recurrence in the explored regional node basin representing a false negative outcome. In-transit metastases were rare events during this short follow-up period, but more common in the SN positive (4%) than in the SN negative group (1%). The most common first recurrence localization in the SN positive group was distant metastases (14%) while for 10% it was a local recurrence.
Discussion
The sentinel node biopsy technique was first described for malignant melanoma by Morton et al. 1992 Citation[10] who showed that the sentinel node status was representative of the nodal status of the investigated nodal basin. This has later been confirmed and sentinel node status has been found to be the most significant prognostic factor besides Breslow thickness and ulcerationCitation[11], Citation[12].
The technique was introduced in Sweden 1997.
The follow-up of this introductive SNB series in Sweden shows a SN detection rate of 97%, which is in accordance with other reports Citation[9], Citation[13–16]. The high detection rate could be due to the fact that the procedures were mainly performed by surgeons with experience of sentinel node biopsy in breast cancer.
The follow-up also shows that the inclusion of sentinel node biopsy in the standard care of patients with cutaenous malignant melanoma has been a slow process in Sweden, where the AJCC tumor staging Manual has been used since 2003.
During 2003 around 100 SNB were performed in Sweden. It can, however, be estimated from the Swedish Cancer Register and the distribution of the Breslow thickness of primary malignant melanoma analysed in the West- Swedish region Citation[17], that around 750 procedures could have been performed, if thin melanomas with ulceration had been included, as recommendations now stands. According to the old recommendations valid during 2003, it can be calculated that around 550 procedures could have been performed. The time-lag before new recommendations are carried out seems quite considerable. In a population based study in North Carolina Stitzenberg et.al. Citation[18] found that only 48% of intermediate thickness melanoma underwent SNB even though it was the recommended standard of care.
Tumor thickness and ulceration have been shown to be the dominant independent predictors of SN metastases in patients with clinically negative regional basins Citation[14], Citation[19], Citation[20].
In this study with a median tumor thickness of 2.4 mm, 19% of the patients had positive SN which is in accordance with the study by Rutkowski et al. with a median tumor thickness of 2.5 mm, using serial sectioning of the SN Citation[21]. In routine pathologic analysis of melanoma with a median thickness of 3.0 mm, Spanknebel et al. found that 20% of the patients were SN positive, Citation[22]. However, after enhanced patholocic analysis 61% of the patients had positive SN. Our findings are lower than expected when comparing the report from van Akooi et al. Citation[13] who reported that 29.4% of 262 patients with a mean Breslow thickness of 2.8 mm, had positive SN, when using the EORTC MG guidlines for the handling of the SN. Our population had a mean Breslow thickness of 3.2 mm and 37% of the melanoma were ulcerated, which makes the expected rate of SN positivity still higher compared to the findings in the van Akkooi report where 27% of the tumors were ulcerated. Rousseau et al. Citation[19] and van Akkooi et al. Citation[13] found that 35% and 41% of patients with ulceration were sentinel node positive respectively, while Rutkowski Citation[21] found that 69% of the ulcerated melanomas were SN positive, as compared to our finding that 24% of the ulcerated melanoma were sentinel node positive.
We found no positive sentinel node in the few patients with thin melanomas (< 1.0 mm), which is in accordance with other published studies Citation[14], Citation[19], Citation[23] supporting the view that there is no indication for performing SNB in thin melanoma.
In this collective study, without a central pathologic review work-up of SN, we found a wide difference between the proportion of SN positivity between the different centers, from 5 to 57%. This could partly be explained by the difference in mean Breslow thickness, and small numbers, between centres. Of further importance is the different handling of the sentinel nodes. There is, no generally accepted method to evaluate sentinel lymph node specimens for detection of relevant occult metastases. This research area must be given high priority to make comparisons possible, between different reports Citation[22], Citation[24].
The finding that 83% of the patients had drainage to one, 13% to two and 2% to three nodal basins is in accordance with other authors Citation[15], Citation[20]. For truncal melanoma we found that 29% had SN in more than one lymph node basin which is approximately the same proportion as reported by Porter et al. Citation[25].
This study includes the clinical follow-up of 361 of the patients with primary melanoma, representing the first experiences of SNB in Sweden. Seventy three (20%) were SN positive. Even in the short follow-up of median 12 months, the sentinel node biopsy turned out to be a significant prognostic factor.
During the observation time 37% of the 73 SN positive experienced a recurrence, which is in accordance with Clary et al. Citation[23] but less than the 46% reported by Berk et al. Citation[14] with longer observation. Seven (10%) of the recurrences were in the explored regional lymph basin. One of these patients belonged to the group that had not been operated with lymphadenectomy in spite of the positive SN.
Thirty nine (14%) of the 288 SN negative patients had a recurrence which is the same proportion as reported by Berk et al. Citation[14], which means that the recurrence rate in our study will be higher with further observation time, probably reflecting different sensitivity for node positivity in the two reports. The incidence of lymph node metastases in the explored regional nodal basin, was twelve (4%) in the SN negative group (false negative). This is in accordance with Berk et al. Citation[14] and Rutkowski et al. Citation[21] with median follow-up times of 29 resp. 37.5 months. As 73 patients were SN positive, in our study, the false negative rate for the technique is 14%, which is lower than what could be calculated from Berk et al. (21%) and Rutkowski et al. (17%). However, the true false negative rate is expected to be higher in our study, as routine pathologic analysis, mostly used, means a single or some permanent hematoxyline and eosine sections from the faces of the bivalved SN. Both Berk et al. and Rutkowski et al. used serial sectioning in their pathologic analysis of the SN. Spanknebel et al. reports that if “routine” pathologic analysis is used instead of enhanced pathologic analysis up to 12% of positive SN are reported as negative Citation[22].
A comparison with other published series of the metastatic pattern after sentinel node biopsy Citation[13–15], Citation[23] shows that the proportion of local recurrence in sentinel node positive patients is higher in this study than in the compared studies. This is probably due to more advanced primary tumors in this study. The low proportion of patients with distant metastases is probably due to the relatively short observation time ().
Table V. Proportion of patients with recurrence in different localizations, mean tumor thickness and follow-up time reported in some recent publications.
Meaningful stratification of patients in clinical trials of adjuvant therapy of course requires accurate knowledge of regional node status. As the SN is the first site of metastasis, in a high proportion of patients, it provides a unique opportunity to study the early phases of tumor-lymph node immune interaction and might prove an important diagnostic tool for the evaluation of interactions between the tumor and the host immune system, helping to select patients who might benefit from adjuvant immunotherapy.
References
- Russell-Jones R, Acland K. Sentinel node biopsy in the management of malignant melanoma. Clin Exp Dermatol 2001; 26: 463–8
- Morton DL, Hoon DS, Cochran AJ, Turner RR, Essner R, Takeuchi H, et al. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: Therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg 2003;238:538–49; Discussion 49–50.
- Thomas JM. Caution with sentinel node biopsy in cutaneous melanoma. Br J Surg 2006; 93: 129–30
- Veronesi U, Adamus J, Bandiera DC, Brennhovd IO, Caceres E, Cascinelli N, et al. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med 1977; 297: 627–30
- Sim FH, Taylor WF, Ivins JC, Pritchard DJ, Soule EH. A prospective randomized study of the efficacy of routine elective lymphadenectomy in management of malignant melanoma. Preliminary results. Cancer 1978; 41: 948–56
- Balch CM, Soong S, Ross MI, Urist MM, Karakousis CP, Temple WJ, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol 2000; 7: 87–97
- Pearlman NW, Robinson WA, Dreiling LK, McIntyre RC, Jr, Gonzales R. Modified ilioinguinal node dissection for metastatic melanoma. Am J Surg 1995;170:647–9; Discussion 9–50.
- Thorn M, Bergstrom R, Hedblad M, Lagerlof B, Ringborg U, Adami HO. Predictors of late mortality in cutaneous malignant melanoma–a population-based study in Sweden. Int J Cancer 1996; 67: 38–44
- Morton DL, Cochran AJ, Thompson JF, Elashoff R, Essner R, , Glass ECet al. Sentinel node biopsy for early-stage melanoma: Accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg 2005;242:302–11; Discussion 11–3.
- Morton D, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early-stage melanoma. Arch Surg 1992; 127: 392–9
- Cascinelli N, Belli F, Santinami M, Fait V, Testori A, Ruka W, et al. Sentinel lymph node biopsy in cutaneous melanoma: The WHO melanoma program experience. Ann Surg Oncol 2000; 7: 469–74
- Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng C-h, et al. Multi-institutional melanoma lymphatic mapping experience: The prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol 1999; 17: 976–83
- van Akkooi AC, de Wilt JH, Verhoef C, Graveland WJ, van Geel AN, Kliffen M, et al. High positive sentinel node identification rate by EORTC melanoma group protocol. Prognostic indicators of metastatic patterns after sentinel node biopsy in melanoma. Eur J Cancer 2006; 42: 372–80
- Berk DR, Johnson DL, Uzieblo A, Kiernan M, Swetter SM. Sentinel lymph node biopsy for cutaneous melanoma; The Stanford Experience, 1997–2004. Arch Dermatol 2005; 141: 1016–22
- Tiffet O, Perrot JL, Gentil-Perret A, Prevot N, Dubois F, Alamartine E, et al. Sentinel lymph node detection in primary melanoma with preoperative dynamic lymphoscintigraphy and intraoperative gamma probe guidance. Br J Surg 2004; 91: 886–92
- Nowecki ZI, Rutkowski P, Nasierowska-Guttmejer A, Ruka W. Sentinel lymph node biopsy in melanoma patients with clinically negative regional lymph nodes–one institution's experience. Melanoma Res 2003; 13: 35–43
- Dahllöf K, Stierner U. Regionala Kvalitetsregister/Rapport 2006. Gothenburg: Onkologiskt Centrum, Västra sjukvårdsregionen; 2006.
- Stitzenberg KB, Thomas NE, Beskow LM, Ollila DW. Population-based analysis of lymphatic mapping and sentinel lymphadenectomy utilization for intermediate thickness melanoma. J Surg Oncol 2006; 93: 100–8
- Rousseau DL, Ross MI, Johnson MM, Prieto VG, Lee JE, Mansfield P, et al. Revised American Joint Committee on cancer staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. Ann Surg Oncol 2003; 10: 569–74
- McMasters KM, Wong SL, Edwards MJ, Ross MI, Chao C, Noyes RD, et al. Factors that predict the presence of sentinel node metastasis in patients with melanoma. Surgery 2001; 130: 151–6
- Rutkowski P, Nowecki ZI, Zurawski Z, Dziewirski W, Nasierowska-Guttmejer A, Switaj T, et al. In transit/local recurrences in melanoma patients after sentinel node biopsy and therapeutic lymph node dissection. Eur J Cancer 2006; 42: 159–64
- Spanknebel K, Coit DG, Bieligk SC, Gonen M, Rosai J, Klimstra DS. Characterization of micrometastatic disease in melanoma sentinel lymph nodes by enhanced pathology: Recommendations for standardizing pathologic analysis. Am J Surg Pathol 2005; 29: 305–17
- Clary BM, Brady MS, Lewis JJ, Coit DG. Sentinel lymph node biopsy in the management of patients with primary cutaneous melanoma: Review of a large single–institutional experience with an emphasis on recurrence. Ann Surg 2001; 233: 250–8
- Kammula US, Ghossein R, Bhattacharya S, Coit DG. Serial follow-up and the prognostic significance of reverse transcriptase-polymerase chain reaction–staged sentinel lymph nodes from melanoma patients. J Clin Oncol 2004; 22: 3989–96
- Porter GA, Ross MI, Berman RS, Lee JE, Mansfield PF, Gershenwald JE. Significance of multiple nodal basin drainage in truncal melanoma patients undergoing sentinel lymph node biopsy. Ann Surg Oncol 2000; 7: 256–61