Abstract
Introduction. Brain metastases develop in nearly half of the patients with advanced melanoma and in 15 to 20% of these patients CNS is the first site of relapse. Overall median survival is short, ranging from 2 to 4 months. Thalidomide has antiangiogenic and immunomodulatory effects. Results obtained in prior trials indicate that Thalidomide acts as a cytostatic agent in metastatic melanoma. We evaluated single agent antitumour activity and toxicity of Thalidomide in a phase II setting in patients with brain metastases associated with metastatic melanoma. Material and methods. Patients with measurable metastatic melanoma in progression and with PS≤2 were enrolled in the study. Thalidomide was given orally. Dose was escalated over 4 weeks from 100 mg/day to 400 mg/day. Primary objective of the study was to determine response rate, according to RECIST. Secondary objectives were to estimate time to progression, overall survival and to evaluate tolerability of the regimen. Results. Twenty five men and 11 women were enrolled in the study, median age 48 years. Among 36 eligible patients 35 were evaluable for response. None of the patients obtained a response in brain metastases. Three patients obtained a partial response in extracranial lesions. Toxicity was acceptable and manageably. Median time to progression and overall survival time was 1.7 and 3.1 months, respectively. Conclusion. There were no objective responses in the brain but single agent Thalidomide has some activity in melanoma patients with brain metastases. It has encouraged us to investigate Thalidomide in combination with Temozolomide, a very lipophilic agent, in this group of patients.
The prognosis for patients with metastatic melanoma is poor. Malignant melanoma is notorious for its propensity to metastasize and for its poor response to current medical therapeutic regimens with median survival in most studies ranging from 6 to 9 months and 5-year survival rates of approximately 10% Citation[1]. Central nervous system (CNS) metastases develop in nearly 50% of patients with metastatic melanoma and CNS metastases is the first failure site in approximately 40% among the 6–8% of patients with an initial complete response to IL-2 based therapy Citation[2]. Median survival in these patients is short, ranging from 2 to 4 months, and 1-year survival is 10 to 15% Citation[3]. Based on these data we find it important to develop a medical treatment strategy for metastatic melanoma in CNS.
Thalidomide was introduced in Europe and Canada in the late 1950s as a sedative and for the treatment of morning sickness in pregnant women. The drug was withdrawn from the market in the early 1960s because reports of teratogenecity associated with its use emerged Citation[4]. Thirty years later it was established that this complication was secondary to inhibition of blood vessel growth in the development of fetal limb buds. D′Amato et al. were the first to show the inhibitory activity of Thalidomide on angiogenesis in a rabbit model of corneal neovascularisation induced by basic fibroblast growth factor (bFGF) and/or vascular endothelial growth factor (VEGF) Citation[5]. Later on it was established that Thalidomide inhibits the inflammatory response by decreasing cyclo-oxygenase-2 activity and suppressing the synthesis of tumour necrosis factor-α (TNF-α) Citation[6], Citation[7] by inducing TNF-α mRNA degradation Citation[8]. Thalidomide enhances the production of IL-2 and IL-2 receptors, perhaps modulating the immune system to induce anticancer activity. The function of other cytokines, such as IL-6 and IL-12, are inhibited by Thalidomide, and it also modulates expression of cell adhesion molecules Citation[9–11].
Singhal et al. has reported that Thalidomide is able to induce partial or complete response in 32% of patients with relapsed and refractory myeloma Citation[12]. Several other studies have confirmed these results making Thalidomide the first new agent for myeloma in over three decades Citation[13], Citation[14].
The relationship between angiogenesis and metastasis and disease progression are well documented in solid tumours. Thalidomide has been investigated in a variety of solid tumours, but data suggest modest activity in patients with solid tumours, such as high-grade gliomas, renal cell carcinomas and prostate cancerCitation[15], Citation[16].
To evaluate the antitumour activity and toxicity of Thalidomide as single agent in patients with brain metastases associated with metastatic melanoma, a phase II study was conducted in two centers in Denmark, with referral of patients eligible for the study from all Oncology centers in Denmark.
Material and methods
Patients
Patients with histologically confirmed melanoma and unresectable brain metastases in progression, not requiring immediate radiotherapy for relief of symptoms, were included. Both patients with and without extracranial metastatic disease were considered eligible. Eligible patients also met the following criteria: WHO performance status (PS) of 0–2, age ≥18 years, WBC ≥3.0×109/l, platelets ≥100×109/l and bilirubin and creatinine ≤1.5 UNL. Eligible patients were required to have measurable target lesions according to RECIST Citation[17] established by computed tomography (CT) scan or magnetic resonance imaging (MRI) scan. Concomitant symptomatic treatment with steroids was allowed, provided the dose given was stable for at least 7 days prior to enrolment. Prior therapy for metastatic melanoma, including immunotherapy with IL-2, surgery for brain metastases, stereotactic or whole brain radiation was allowed, provided progressive disease was verified at study entry.
All eligible patients were obliged to comply with Pharmions Risk Management Program, conducted according to the System for Thalidomide Education and Prescribing Safety (STEPS) guidelines Citation[18]. Female patients were required to have negative urine pregnancy test, within 24 h prior to Thalidomide therapy. Any women of child-bearing potential had to use adequate contraceptive methods during the study and for 28 days after the last Thalidomide dose. Men were required to use condom in connection with sexual activity. Pregnant or nursing women were excluded. All patients provided written informed consent.
Treatment and patient evaluation
Starting dose of Thalidomide was 100 mg/d orally, taken in the evening to reduce drug-related daytime somnolence. In order to treat patients at maximal tolerated dose, dose was increased every 7 days to a maximum of 400 mg/d unless grade 3 or greater toxicity occurred. Dose >300 mg was divided into two daily doses.
Toxicity was assessed according to Common Toxicity Criteria (CTC) (version 3.0) Citation[19]. Dosing of Thalidomide was interrupted in case of grade 3–4 myelotoxicity, obstipation or somnolence. When toxicity was reduced to grade 1 the treatment could be restarted. In case of treatment interruption due to myelotoxicity or obstipation, dose was reduced with 100 mg/day. In case of somnolence (≥grade 3) or neuropathy (≥grade 2) treatment could be restarted with 50% dose reduction. No dose escalation was performed later on. In case of grade 4 neuropathy the treatment was stopped.
Evaluation of efficacy was performed every 3 months, according to RECIST assessed by physical examination and CT or MRI of all metastatic sites. In case of disease progression prior to first evaluation, treatment was stopped and the patient was designated as having disease progression (PD). In case of stable disease (NC) or an objective response (PR or CR), treatment was continued. Patients with objective benefit of the treatment after 6 months, was allowed to continue on treatment for a maximum of 12 months.
Clinical evaluation was performed after 2 and 4 weeks in order to perform proper dose escalation according to protocol. Thereafter patient had a clinical evaluation at 4 week intervals to assess toxicity and clinical benefit. During each visit a complete blood count was drawn, combined with evaluation of blood pressure, weight and performance status.
CT- or MRI-scan was performed every 3 months.
Statistics
The study was approved by the Ethics Committee of Funen and Vejle Counties.
The primary objective of the study was to determine efficacy of Thalidomide according to RECIST, whereas estimation of time to progression, overall survival and evaluation of tolerability of the regimen, was secondary objectives.
Descriptive statistics were used to describe baseline patient characteristics, response rate and toxicity. Time to progression and death were estimated using the Kaplan-Meier method, with overall survival measured from study entry until death from any cause.
Results
Patients
Between September 2002 and November 2004, 36 patients with multiple, measurable brain metastases in progression were enrolled in the study. None of the patients were eligible for surgery or stereotactic radiotherapy. No patients were considered ineligible for entry. The primary tumor site was cutaneous in 31 patients and in five patients a primary tumour was not found. Median age was 48 years (range 27–70) in 25 men and 11 women. WHO performance status varied: 12 patients with PS 0, 12 with PS 1 and 12 with PS 2. Prior therapy for metastatic melanoma, included palliative radiotherapy for brain metastases (22 patients), typically 25 Gy in 5 fractions or 30 Gy in 10 fractions, 5 fractions per week. Four patients developed brain metastases after initial IL-2 based immunotherapy. Twenty three patients received concomitant steroids, while on Thalidomide. Baseline patient characteristics are listed in .
Table I. Baseline patient characteristics (n = 36).
Toxicity
Treatment was generally well tolerated. Twenty one patients received the maximum daily dose of 400 mg, but only 14 patients continued on this dose without dose reduction. Four patients were not evaluable for toxicity. These patients did not show up for evaluation after initiation of treatment due to early disease progression and death. Toxicity among 32 evaluable patients is listed in . Fatigue, constipation, dry mouth and anorexia grade 1–2 occurred in 100%, 72%, 75% and 75% of the patients, respectively. Grade 1–2 nausea was reported in 16 patients. Eleven patients had disease related neurological symptoms (neuropathy-motor) at baseline. Fifteen patients developed treatment related sensory neuropathy. No grade 4 toxicity or treatment related deaths were registered and no haematological toxicity was seen.
Table II. Toxicity profile (n = 32).
Efficacy
Except for one patient all 36 patients enrolled in the study were evaluable for response. The patient not evaluable for response died on day 1 after treatment start due to progression in brain metastases. Eleven patients reached the first evaluation after 3 months without progression. No patient obtained an objective response in brain metastases. In two patients treatment was extended beyond 6 months. One PR in extracranial metastases was observed in a patient with metastases to the brain, the lungs, the liver and the mediastinal lymph nodes. After 3 months of treatment the patient had a CR in the lungs, a PR in the liver and stable disease in the other manifestations. After 9 months CNS progression was established, with continuous response in peripheral tumour manifestations. We chose to give whole brain radiation with concomitant Thalidomide therapy. After further 7 months of treatment PD in peripheral manifestations was established and Thalidomide was discontinued. The overall time to progression was 9 months with a treatment time of 16 months. This patient had received prior high-dose IL-2 based therapy and dendritic cell vaccination.
In four patients NC in brain metastases and in extracranial metastases was established, with duration of 4.1, 4.5, 4.8 and 8.3 months. Among the patients with stable disease, one patient had CR in lymph node and skin metastases, but NC overall, and one had a PR in the lungs but finally progressed in the brain. Summery of characteristics in patients obtaining PR or NC characteristics are listed in .
Table III. Patient characteristics of patients with clinical benefit (n = 5).
Among 30 patients with progressive disease, 19 patients did not reach the first evaluation. Among these 19 patients, 10 had a WHO performance status of 2 at study entry.
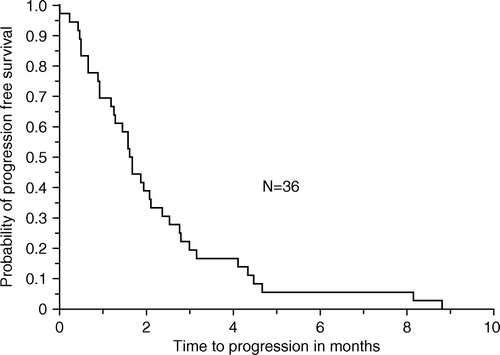
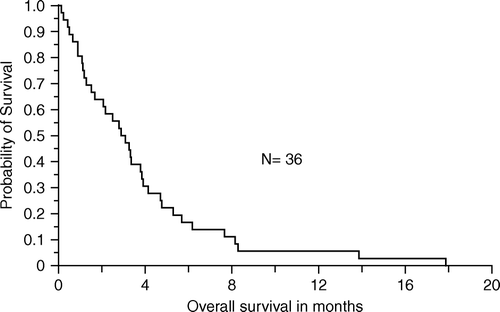
Median time to progression was 1.7 months (range 0–9) (), and the median overall survival was 3.1 months (range 0–18) ().
Discussion
This phase II study is the first published study investigating the activity of Thalidomide as a single agent in melanoma patients with brain metastases. Most of the patients included had received no prior medical therapy for metastatic melanoma, as no standard therapy for patients with metastatic melanoma to the brain exists. Patients with oligometastatic disease to the brain were offered local therapy (surgery or stereotactic radiotherapy). Patients with symptomatic brain metastases were offered initial palliative radiotherapy combined with steroids. All patients had brain metastases in progression at the time of inclusion.
Pawlak and Legha, published a study evaluating single-agent Thalidomide in metastatic malignant melanoma, showing no objective responses, but seven cases of stable disease Citation[20]. Other single agent phase II studies Citation[21], Citation[22] demonstrated stable disease in a few patients, with 30% of the patients included in these trials having ocular melanoma Citation[20], Citation[21]. This paper reports no objective response in brain metastases of patients with widely metastatic melanoma. Stable disease is very rare in patients with brain metastases with or without any treatment. Four patients (11%) had stable brain metastases for >4 months on Thalidomide, providing some indication of activity of Thalidomide in CNS. We demonstrated activity in peripheral tumour manifestations. The responding patient in our study progressed in the brain during treatment, with concomitant disease control in peripheral manifestations.
Thalidomide as a single agent in a daily dose up to 400 mg was well tolerated. Predominant toxicities observed were fatigue and constipation, and no grade 4 toxicity was seen. Twenty one patients (58%) received the maximum daily dose of 400 mg and 14 (39%) were able to continue on this dose after the 4 week escalation period. Seven patients needed dose reduction at the 400 mg dose level, but the toxicities were manageable and reversible.
The present study has shown that single agent Thalidomide has limited activity against metastatic melanoma in the CNS. However the minor effects we and other investigators have found on peripheral tumour manifestations, has led us to the conclusion that Thalidomide might be part of a future treatment strategy for these patients.
Temozolomide is a highly lipophilic oral alkylating agent with an excellent oral bioavailability and with an ability to penetrate the blood-brain barrier Citation[23].The schedule of Temozolomide could be of outmost importance Citation[24], Citation[25]. Based on these considerations, we chose to design a phase II protocol, testing the combination of Temozolomide (150 mg/m2 daily for 7 days, followed by 7 days off therapy) and Thalidomide, 200 mg/day, in patients with WHO performance status ≤1 and brain metastases from malignant melanoma.
References
- Lee ML, Tomsu K, Von Eschen KB. Duration of survival for disseminated malignant melanoma: Results of a meta-analysis. Melanoma Res 2000; 10: 81–92
- O'Day S, Atkins M, Weber J, Thompson J, Anderson C, Gonzalez R et al A phase II multi-center trial of maintenance biotherapy (MBT) after induction concurrent biochemotherapy (BCT) for patients (Pts) with metastatic melanoma (MM). ASCO 2005.
- Meier S. Survival and prognostic factors in patients with brain metastases from malignant melanoma. Onkologie 2004; 27: 145–9
- Thomas DA, Kantarjian HM. Current role of thalidomide in cancer treatment. Curr Opin Oncol 2000; 12: 573
- D‘Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci 1994; 91: 4082–5
- Quilitz R, Pharm D. Thalidomide in oncology. The peril and the promise. Cancer Control, JMCC 1999; 6: 483–95
- Rajkumar SV. Thalidomide in the treatment of cell malignancies. J Clin Oncol 2001; 19: 3593–5
- Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med 1993; 177: 1675–80
- Meierhofer C, Wiedermann CJ. New insights into the pharmacological and toxicological effects of thalidomide. Curr Opin Drug Discov Devel 2003; 6: 92–9
- Meierhofer C. Theoretical basis for the activity of thalidomide. BioDrugs 2001; 15: 681–703
- Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet 2004; 363: 1802–11
- Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Atitumor activity of thalidomide in refractory multiple myeloma. New Engl J Med 1999; 341: 1565–71
- Raje N, Anderson KC. Thalidomide and immunomodulatory drugs as cancer therapy. Curr Opin Oncol 2002; 14: 635–40
- Eleutherakis-Papaiakovou V. Thalidomide in cancer medicine. Ann Oncol 2004; 15: 1151–60
- Richardson P. Thalidomide: Emerging role in cancer medicine. Annu Rev Med 2002; 53: 629–57
- Kumar S. Thalidomid: Current role in the treatment of non-plasma cell malignancies. 12 2005;22:2477–88.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92: 205–16
- Zeldis J, Williams B, Thomas S, Elsayed M. S.T.E.P.S.: A comprehensive program for controlling and monitoring access to thalidomide. Clin Ther 1999; 21: 319–30
- Trotti A, Colevas A, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13: 176–81
- Pawlak WZ, Legha SS. Phase II study of thalidomide in patients with metastatic melanoma. Melanoma Res 2004; 14: 57–62
- Reiriz AB, Richter MF, Fernandes S, Cancela AI, Costa TD, DiLeone LP, et al. Phase II study of thalidomide in patients with metastatic malignant melanoma. Melanoma Res 2004; 14: 527–31
- Eisen T, Boshoff C, Mak I, Sapunar F, Vaughan MM, Pyle L, et al. Continuous low dose Thalidomide: A phase II study in advanced melanoma, renal cell, ovarian and breast cancer. Br J Cancer 2000; 82: 812–7
- Payne MJ, Pratap SE, Middleton MR. Temozolomide in the treatment of solid tumours: Current results and rationale for dosing/scheduling. Crit Rev Oncol/Hematol 2004; 53: 241–52
- Brock C, Newlands E, Wedge S, Bower M, Evans H, Colquhoun I, et al. Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res 1998; 58: 4363–7
- Tolcher A, Gerson S, Denis L, Geyer C, Hammond L, Patnaik A, et al. Marked inactivation of 06-alkylguanine-DNA alkyltransferase activity with protected temozolomide schedules. Br J Cancer 2003; 88: 1004–11