Abstract
Purpose. To analyze 251 patients (101 males and 150 females) diagnosed with ano-rectal malignant melanoma (ARMM) reported to the Swedish National Cancer Registry during 1960–1999. Methods. Incidence, gender and age profiles, primary anatomical sites and density of the melanomas along with geographic distribution, and prognosis were investigated. Results. The age-standardized incidence of ARMM was significantly higher for females (1.0 per 106 females) than for males (0.7 per 106 males) throughout the 40-year-period. The incidence increased with age peaking at 75–84 years in both genders. 54% of the tumours were primary in the anal canal, 24% engaged the whole ano-rectal unit and 10% were located at the anal verge (11% unknown primary site). Although ARMM were rare in absolute numbers, their density (number of tumours/square unit) was higher than that of cutaneous malignant melanomas (CMM) on average. No linkage between the geographic distribution of ARMM and population density was found. The prognosis was very poor albeit with a significant gender difference with a five-year survival rate of 10.6% for males and 15.7% for females. The survival rates for both genders improved during the 40-year-period but significantly more for females than males. Conclusion. The reason(s) for the difference in incidence and prognosis according to gender is unknown. The majority of ARMM emerged primary in the anal canal and a primary location exclusively in the colonic mucosa of the rectum is questionable. The higher density of ARMM as compared to the average density of CMM tallies with the result of our previous studies on vulvar melanoma and might be instrumental in exploring non-UV light associated factors in melanoma genesis. The concentration of patients with anal squamous cell carcinoma to population-dense urban areas, as previously reported, was not found in cases of ARMM.
Although UV radiation is the best-recognised cause of cutaneous melanoma (CMM), several other risk factors or co-factors have been identified, e.g., hereditary/familial predisposition, a large number of nevi, skin phenotype, hair and eye colour. Additionally, melanomas can also emerge in sun-protected areas of the body, e.g., mucous membranes. Primary melanomas of the mucous membranes are, in absolute numbers, rare and, at least in Sweden, seem to have a predisposition for the vulva. However, the density of vulvar melanomas (i.e., the number of tumours per square unit of body surface) is, on average, higher than the density of CMM on the body surface Citation[1].
Ano-rectal melanomas (ARMM), which were originally described in 1857 Citation[2], are the second most common group of mucosal melanomas in Sweden. However, data on their incidence is sparse, both in population-based national series Citation[3] and in large regional databases Citation[4–6]. Patients afflicted with ARMM have a low five-year survival rate of only 6 to 22% Citation[4], Citation[6–9].
For this serial study, we examined the number and courses of ARMM in patients listed in the Swedish National Cancer Registry during the period 1960–1999. Based on our review of each case we tried to analyze trends in incidence and gender differences, to localize the melanomas to specific anatomical sites of the ano-rectal unit, to estimate the tumour density and the geographic distribution of patients with ARMM along with survival. For that purpose, archival material was collected throughout Sweden.
Patients and methods
Patient population
This series comprised 253 patients with a primary ARMM, as registered consecutively in the Swedish National Cancer Registry during a 40-year-period, 1960–1999. In each case, the diagnosis was based on histopathologic examination. The present study was approved by the Human Ethical Committee at the Karolinska Institute.
Since 1958, Swedish law has decreed that all malignant tumours must be reported to the population-based Swedish National Cancer Registry. Consequently, almost all (95%) cancer patients in Sweden are registered in this way by both clinicians and pathologists/cytologists Citation[10]. The Cancer Registry is linked to the Swedish National Causes of Death Registry. Dates and causes of death are copied into the Cancer Registry.
Melanoma sites
The sites of the melanomas were first delineated according to the International Classification of Diseases, version 7 (ICD-7), throughout the whole period 1960–1999, and the histological types were classified according to the old histology code 176 for malignant melanoma (WHO/HS/CANC/24.1 histology code). ICD-7 has a four-digit code identifying the exact location of the tumour, i.e., 1540 for rectum, 1541 for anus and 1548 for tumours engaging both anus and rectum. The three-digit code, i.e., 154, was also frequently used for melanomas in the ano-rectal area without any further specification. According to the classifications 1540 and 1541, as many as 85 melanomas were located in the rectum and 115 in the anus. We re-evaluated the primary tumour sites. Data from the clinical records and pathology reports for each patient were weighed together, including digital palpation and endoscopical findings along with the macroscopical and histological orientation of surgical tumour specimens to various segments of the anal canal. Each case was reviewed by an oncologist (BRO), a surgeon who specialized in coloproctology (PN) and a pathologist (LO), first independently and then together to reach a consensus.
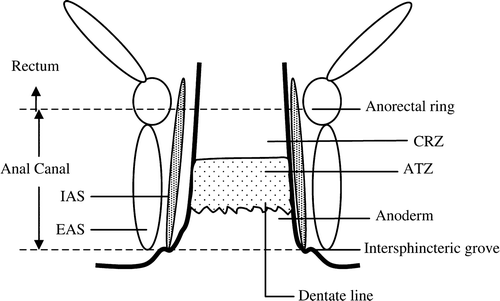
Although some confusion regarding definitions of the anal anatomy exists, the clear-cut terminology proposed by Wendell-Smith Citation[11] has been used in this study. The anatomy is schematically illustrated in . The anal canal, here referring to the surgical anal canal, varies considerably in length among different individuals, but an average length of 4.2 cm (range 3.0–5.3) has been calculated in both genders combined Citation[12]. The canal extends from the palpable intersphincter groove at the anal verge to the anal ring. It encompasses the anoderm covered by squamous cells, the dentate line, the transitional zone, and a zone of colonic mucosa called the “colo-rectal zone” Citation[13]. By means of endoscopy the different zones can usually be recognized. Melanocytes have been found in the perianal skin and in the anal canal except for the colo-rectal zone Citation[12], Citation[13].
Melanoma density
For this study, an attempt was made to compare the average density of ARMM, i.e., the number of tumours per square unit of the mucosa in the anal canal, with the average density of all CMM during the same time period. The anal canal's area was estimated here by considering the canal as a cylinder measuring 4.2 cm in average length Citation[12] with two alternative diameters of 1.9 and 2.9 cm, respectively, during the anal canal's “basal” or “strained” condition in normal individuals, as determined by simultaneous endosonography and manometry Citation[14]. Our calculations yielded two alternative areas of 25 and 38 cm2, respectively. The average body surface of Swedish males is 1.9 m2 and of Swedish females 1.7 m2 Citation[15]. During the 40-year-period of investigation, 17 923 males and 19 176 females with a primary CMM were reported. Thus, the ratios of ARMM versus CMM per square area unit could be calculated.
Statistical methods
All patients included here were divided into eight age groups, each spanning ten years, except for the youngest (0–24 years) and oldest (>85 years). The calendar period was divided into five-year-segments from 1960 through 1999. Incidence was analyzed by date, age-standardisation and gender so that the absolute incidence was represented during each calendar year. The age distribution 1980–1984 was used as a standard. The relative change in incidence over the 40-year-period was calculated by linear regression after logarithmic transformation.
The incidence of ARMM per million inhabitants and the population density in the 24 counties of Sweden were analyzed by linear regression with the method of least squares. After 1998, some of these 24 counties merged into 21 larger regional units, but for the sake of uniformity in calculations the subdivision into 24 counties has been maintained throughout the 40 years of observation. The population 1980–1984 in each county was used as a background population. The definition of population density, as given by Statistics Sweden Citation[16], is based on population centres. Each centre is a community with at least 200 inhabitants and a maximum distance between houses of 200 m. The whole country is divided into densely and sparsely populated communities. Statistics Sweden then divides the total number of inhabitants of densely populated communities in any given county by the total number of inhabitants in that county.
Survival rates were estimated with the “life table method”, taking censored observations into account. However, patients diagnosed after 1997 were observed less than 10 years, since the cut-off date for follow-up was December 31, 2006. Relative survival was analysed using a computer program package for cancer survival studies Citation[17]. The relative survival rate is the ratio between the observed survival in the patient group and the expected survival in a general population with the same characteristics as the patient group regarding age, gender and calendar year of observation. The excess mortality rate is one minus the relative survival rate. Regression analysis of the hazard functions (excess mortality functions) as developed by Hakulinen and Tenkanen, 1987 Citation[18], was performed using a GLIM software package Citation[19]. Categorical factors studied were: calendar year (1960 through 1999) at diagnosis, age in years (0–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, 85–) at diagnosis and gender.
Results
Incidence
During 1960 through 1999, 253 patients in Sweden were diagnosed with ARMM and reported to the (compulsory) Swedish National Cancer Registry. One of those 253 cases turned out to be coded wrongly as having an ARMM, and another case was registered twice. Of the 251 remaining patients, 101 were males and 150 females. The males′ ages ranged from 37 to 90 years (mean age 71 years, median age 74 years), and the females from 40 to 91 years (mean age 69 years, median age 71 years). Most patients (200 of 251–79%) with an ARMM were more than 60 years of age.
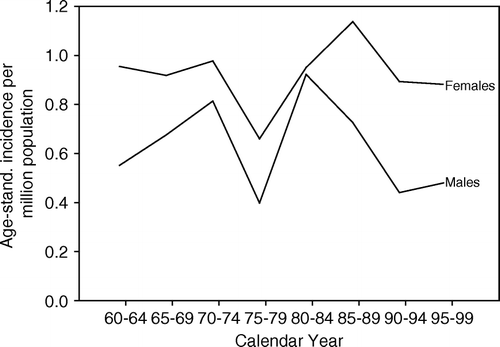
The age-standardised incidence was higher for Swedish females (1.0 per million) than for males (0.7 per million), and the difference reached the level of statistical significance (mean difference = 0.30, SD = 0.14, p < 0.001). No change in the age-standardised incidence for either gender occurred during the time period specified (). Although the age-specific incidence increased with rising age, peaking at 75–84 years in both genders, females had a slightly higher but not significant rate.
Primary tumour sites
As displayed in , the vast majority, 136 or 54%, of all ARMM emerged in the anal canal. Sixty, or 24%, of the tumours grew both in the anal canal and the rectum and were computed as belonging to the “ano-rectal unit”. Twenty-six, or 10%, were located at the anal verge/perianal area and 28, or 11% of the tumours had unknown site in the anorectal unit. No melanoma was proven to arise initially and exclusively in the rectum.
Table I. Anatomical sites of primary ano-rectal melanomas (1960–1999) according to retrospective clinical and pathological scrutiny.
Melanoma density
Only 137 melanomas within the anal canal (55 males and 82 females) were included. When calculating the area of the anal canal's surface for comparison to that of a human's external skin surface, we used an average anal canal area of 25 cm2 as detailed in the Methods section. Accordingly, the ratio of the ARMM versus the CMMs per surface unit was 2.40 for men and 2.98 for women. When an area of 38 cm2 was calculated the corresponding ratios were 1.60 and 1.96, respectively. Consequently, the density of melanomas (average number per square unit) was higher in the anal canal than the average density at cutaneous sites.
Geographic distribution
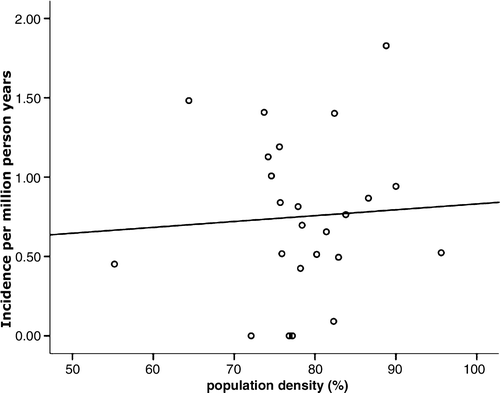
No correlation between population density (by county) and incidence of ARMM could be detected (p = 0.775) (). Similarly, age-standardised incidence and population density showed no correlation (p = 0.540).
Follow-up
Within the entire 40-year-period 1960–1999 of this study, with the cut-off date for follow-up on December 31, 2006, none of the subjects was lost to follow-up because of emigration, and none was listed as “non-existent in register.” For four individuals, survival time could not be calculated, because the melanoma was not apparent until autopsy, and data for another nine who lived less than one month after diagnosis were not included in calculations of survival.
The median survival times for females and males were only 15 and 8 months, respectively.
The observed and relative survival rates after five years‘ follow-up for all patients were 14 and 18%, respectively, (for females 16 and 20%, and for males 11 and 14% (). About half the patients, with a slight predominance of males, died during the first year of the follow-up period (). Excess mortality (one minus relative survival time), a term that here refers to patients whose cause of death was ARMM, was high among these patients; in fact, the disease accounted for almost all their deaths. Observed mortality at five years was 86%, of which 83% was caused by the melanomas, meaning that only 4% of these patients died from other causes.
Figure 4. Observed and relative survival rates in Swedish patients with primary ano-rectal melanomas 1960 through 1999.
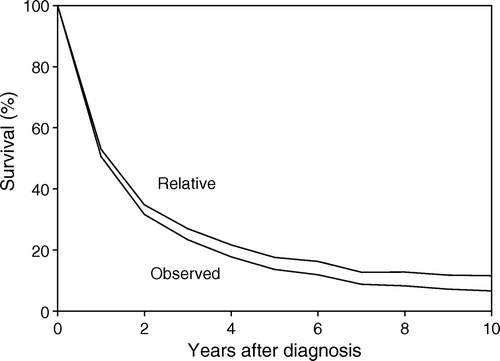
Table II. Observed and relative survival (%) by follow-up year and gender.
The Cox multivariate regression analysis was performed to measure the simultaneous effect of calendar period, patient age at diagnosis and gender on the observed survival interval (). Survival rates improved during the 40-year period reported. Furthermore, the 5-year survival increased by 5.8% during 1990–1999 as compared to 1960–1969. Again, females survived for a significantly longer period than males. Furthermore, patients′ ages correlated inversely with survival time.
Table III. Cox multivariate regression analysis of observed survival. Relative hazards (RH) and 95% confidence intervals (CI). All clinical stages.
Discussion
To the best of our knowledge, this is the largest nation-wide, population-based report on ARMM so far published and only the second one of its kind Citation[3]. However, the earlier report included only anal melanomas. The strength of our study is the inclusion of virtually all primary ARMM diagnosed in Sweden from 1960 through 1999. Each diagnosis was confirmed primarily by histopathology, and the follow-up was complete through the end of 2006.
In our experience, exclusions because of erroneous diagnosis and/or incorrect coding are rare within series reported to the National Swedish Cancer Registry. For example, in our previous report on all primary vulvar melanomas in Sweden 1960–1984 Citation[1], only eight cases (4%) were excluded because of errors in histopathological diagnosis and/or statistical coding.
Our present results showed that the majority of ARMM at the sites included here emerged from the anal canal and to a smaller extent from the ano-rectal unit and the perianal zone. No melanomas convincingly emerged exclusively in the rectum, which is in accordance with a previous report that the colonic mucosa does not contain melanocytes Citation[13], but at variance with two case reports claiming that melanocytes and ARMM can be found primarily in the rectum Citation[20], Citation[21]. However, we noted a discrepancy between the localization of these tumours to specific parts of the ano-rectal unit compared to those registered according to the ICD-7 coding. The reason(s) is not fully understood, but clinicians apparently assumed, in many instances, that the tumours were distal rectal adenocarcinomas. Therefore, histopathology reports describing an ARMM were unexpected and may have led to misclassification, since the clinicians’ original description contained the word “rectal”.
In our investigation, the melanomas in the anal canal were more common than CMMs when the spatial relationship between surfaces of the anal canal and the body's exterior covering is taken into consideration. The same phenomenon has previously been reported for vulvar Citation[1] and ocular Citation[22] melanomas. The similarity in density between melanomas in cutaneous, relatively sun-exposed areas and in sun-shielded areas emphasizes the need to search for factors that underlie the genesis of melanomas not associated with UV radiation, e.g., anatomical and local tissue factors.
As recently shown, marked differences exist at the molecular level between melanomas of the skin and of mucous membranes. A significantly larger incidence of Nras mutations occur in cutaneous than in mucosal melanomas Citation[23], genetic alterations differ in the two tumour types Citation[24], and BRAF mutations are absent from mucosal melanomas but present in CMM Citation[25].
In this series, the age-standardized incidence of ARMM was stable during the four decades surveyed, in contrast to the decreasing incidence of vulvar melanomas Citation[26] and the increasing incidence of CMM Citation[27] in Sweden. The stable age-standardised incidence of ARMM is in accordance with a previous American report (1973–1987) covering approximately 10% of that population Citation[4] but at variance with another report from the USA for the years 1973–1992 covering 9.5% of the population Citation[5]. Interestingly, the latter report cited an increasing incidence of ARMM among young men in the San Francisco area simultaneously with a reported increase of anal carcinomas in both genders but with a dramatic increase among men Citation[28]. Although the incidence of anal squamous cell carcinomas has been linked to densely populated geographic areas Citation[28], we found no connection between the incidence of ARMM in either sparsely or densely populated regions of Sweden. However, the small number of melanomas as compared to carcinomas makes an adequate comparison difficult.
The increase in anal squamous cell carcinomas has been linked with sexual practices and the transmittance of human papilloma viruses (HPV) in large population-based studies, e.g. in USA Citation[28], Citation[29] and in Denmark and Sweden Citation[30]. However, we were unable to find a linkage between the presence of HPV DNA representing 36 subtypes in mucosal melanoma cells and the advent of melanomas Citation[31].
The incidence of ARMM reported here was significantly higher among women than men, whereas the incidence of CMM in Sweden did not differ notably by gender Citation[27]. Yet, others have reported that females are more often afflicted with ARMM than males Citation[3–6]. The reason(s) is unknown.
The ARMM surveyed here occurred with great frequency in elderly people, as do the vulvar melanomas Citation[26]. In contrast, CMM, except for the lentigo maligna type, do not predominate in aged individuals.
ARMM are rare tumours, for which little experience exists in the registration process. Therefore, such melanomas, especially those located at the verge of the anus or engaging the perianal skin, may be miscoded as CMM or as unspecified entities. Accordingly, one can speculate that the incidence of ARMM might have been higher than registered.
The prognosis for patients with ARMM is dismal, the five-year survival rate being only 18%. However, women with ARMM displayed a significantly greater longevity than men. This is in accordance with a previous report Citation[7] but at variance with two other reports Citation[5], Citation[6]. Interestingly, patients with CMM in Sweden Citation[32] and other countries Citation[33], Citation[34] display a significantly longer survival among females than males. Multifactorial analyses have shown that gender is an independent prognostic factor for CMM Citation[32], Citation[34]. Melanoma cells often display oestrogen receptors but there is no obvious relationship between the amount of sex hormones and survival in individuals with CMM Citation[35]. Accordingly, the biological differences between melanomas in males and females are presently not understood.
With respect to the significantly extended survival time we documented during the 40-year-time period, this observation also appeared in other studies Citation[4], Citation[6]. The most likely explanation is earlier detection in recent years. The benefit of early detection of ARMM has not been proven but seems likely because surgical excision is apparently the only cure, because melanomas in general are highly resistant to chemo- and radiotherapy. Accordingly, an improved survival with such a combined therapy that has recently been reported in cases of anal squamous carcinoma Citation[36] seems unlikely in ano-rectal melanoma. Besides, our series does not allow any significant analysis of the effect of such a therapy.
In conclusion, this investigation shows that the majority of ARMM emerge from the anal canal and, to a lesser extent, from the ano-rectal unit, and anal verge/perianal area. Furthermore, the ARMM, although rare in absolute numbers, have a density higher than the average density of the CMM in more-or-less sun-exposed body areas. This is an incentive for further studies on non-UV light-associated factors in the melanoma genesis. The incidence of ARMM in Sweden has been stable over a 40-year-period (1960–1999), and the incidence among women is significantly higher than among men. The prognosis for these patients is, in general, disheartening, although somewhat improved over the 40-year-time period reported. Women survived significantly longer than men, indicating a gender difference still to be explored.
Acknowledgements
This investigation was supported by Radiumhemmets Research Fund; King Gustav V′s Jubilee Fund. The authors sincerely thank Mrs Phyllis Minick for editorial assistance.
References
- Ragnarsson-Olding BK, Kanter-Lewensohn LR, Lagerlöf B, Nilsson BR, Ringborg UK. Malignant melanoma of the vulva in a nationwide, 25-year study of 219 Swedish female: Clinical observations and histopathological features. Cancer 1999; 86: 1273–84
- Moore Recurrent melanomas of the rectum, after previous removal from the verge of the anus, in a man age sixty-five. Lancet 1857;1:290.
- Goldman S, Glimelius B, Påhlman L. Anorectal malignant melanoma in Sweden. Dis Colon Rectum 1990; 33: 874–7
- Weinstock MA. Epidemiology and prognosis of anorectal melanoma. Gastroenterology 1993; 104: 174–8
- Cagir B, Whiteford MH, Topham AB, Rakinic J, Fry RD. Changing epidemiology of anorectal melanoma. Dis Colon Rectum 1999; 42: 1203–8
- Podnos Y, Tsai N-C, Smith D, Ellenhorn J. Factors affecting survival in patients with anal melanoma. Am Surg 2006; 72: 917–20
- Brady MS, Kavolius JP, Quan SH. Anorectal melanoma. A 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum 1995; 38: 146–51
- Roumen RMH. Anorectal melanoma in the Netherlands: A report of 63 patients. Eur J Surg Oncol 1996; 22: 598–601
- Thibault C, Sagar P, Nivatvongs S, Ilstrup DM, Wolf BG. Anorectal melanoma – an incurable disease?. Dis Colon Rectum 1997; 40: 661–8
- Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register: Non-notified cancer causes recorded on death certificates in 1978. Acta Radiol (Oncol) 1984; 23: 305–13
- Wendell-Smith CP. Anorectal nomenclature. Fundamental terminology. Dis Colon Rectum 2000; 43: 1349–58
- Nivatvongs S, Stern HS, Fryd DS. The length of the anal canal. Dis Colon Rectum 1981; 24: 600–1
- Clemmensen OJ, Fenger C. Melanocytes in the anal canal epithelium. Histopathology 1991; 18: 237–41
- Marzio L, Ciccaglione FA, Falcucci M, Malatesta MG, Grossi L, Travaglini N, et al. Relationship between anal diameter and pressure evaluated simultaneously by endosonography and manometry in normal human subjects. Int J Colorect Dis ;:21 1998; 13: 6
- Swedish Survey of Living Conditions (ULF investigation) In: Official Statistics of Sweden: The National Swedish Central Bureau of Statistics. Stockholm 1999.
- Statistical Yearbook of Sweden Publication Services Statistics, Sweden, Annual Publications. Örebro 1999.
- Hakulinen T, Abeywickrama K. A computer program package for relative survival analysis. Comput Prog Biomed 1985; 19: 197–207
- Hakulinen T, Tenkanen L. Regression analysis of relative survival rates. Appl Stat 1987; 36: 309–17
- Francis B, Gree M, Payne C. The GLIM.System. Generalized Linear Interactive Modelling System Manual. Clarendon Press, OxfordUK 1993
- Werdin C, Limas C, Knodell RG. Primary malignant melanoma of the rectum. Evidence for origination from rectal mucosal melanocytes. Cancer 1988; 61: 1364–70
- Nicholson AG, Cox PM, Marks CG, Cook MG. Primary malignant melanoma of the rectum. Histopathology 1993; 22: 261–4
- Bergman L, Seregard S, Nilsson B, Ringborg U, Lundell G, Ragnarsson-Olding B. Incidence of uveal melanoma in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci 2002; 43: 2579–83
- Jiveskog S, Ragnarsson-Olding B, Platz A, Ringborg U. N-ras mutations are common in melanomas from sun-exposed skin of humans but rare in mucosal membranes or unexposed skin. J Invest Dermatol 1998; 111: 757–61
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2004; 353: 2135–47
- Edwards RH, Ward MR, Wu H, Medina CA, Brose MS, Volpe P, et al. Absence of BRAF mutations in UV-protected mucosal melanomas. J Med Genet 2004; 41: 270–2
- Ragnarsson-Olding B, Johansson H, Rutqvist LE, Ringborg U. Malignant melanoma of the vulva and vagina. Trends in incidence, age distribution and long-term survival among 245 consecutive cases in Sweden 1960–1984. Cancer 1993; 71: 1893–7
- Månsson-Brahme E, Johansson H, Larsson O, Rutqvist L, Ringborg U. Trends in incidence of cutaneous malignant melanoma in a Swedish population 1976–1994. Acta Oncol 2002; 41: 138–46
- Melbye M, Rabkin C, Frisch M, Biggar R. Changing patterns of anal cancer incidence in the United States, 1940–1989. Am J Epidemiol 1994; 139: 772–80
- Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004; 101: 270–80
- Frisch M, Glimelius B, van den Brule AJ, Wohlfahrt J, Meijer CJ, Walboomers JM, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med 1997; 337: 1350–8
- Dahlgren L, Schedvins K, Kanter-Lewensohn L, Dalianis T, Ragnarsson-Olding B. Human papilloma virus (HPV) is rarely detected in malignant melanomas of sun sheltered mucosal membranes. Acta Oncol 2005; 44: 694–9
- Måsbäck A, Olsson H, Westerdahl J, Ingvar C, Jonsson K. Prognostic factors in invasive cutaneous malignant melanoma: A population-based study and review. Melanoma Res 2001; 11: 435–45
- Stidham K, Johnson J, Seigler H. Survival superiority of females with melanoma. A multivariate analysis of 6383 patients exploring the significance of gender in prognostic outcome. Arch Surg 1994; 129: 316–24
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli NS, et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001; 16: 3622–34
- Grin CM, Driscoll MS, Grant-Kels JM. The relationship of pregnancy, hormones and melanoma. Sem Cutaneous Med Surg 1998; 17: 167–71
- Lund JA, Wibe A, Sundström SH, Haaverstad R, Kaasa S, Myrvold HE. Anal carcinoma in mid-Norway 1970–2000. Acta Oncol 2007; 46: 1019–26