Abstract
Introduction. Population-based cancer survival analysis constitutes valuable reference material for the clinical field of oncology. The objectives of this study were to assess the quality of the Hamburg Cancer Registry's (HCR) database in this respect, to perform survival analyses by means of selected sites, and to evaluate the results in relation to prevalent opinions and external estimates. Methods. Data quality was assessed by the proportion of cases documented as diagnosed at death, external estimates of completeness, reliability of follow-up, histological verification and information on stage. Included were first primary malignancies of the colon (ICD10 C18, n=4 544), female breast (C50, n=9 259), prostate (C61, n=5 707) and urinary bladder (C67, D09.0, n=3 148), diagnosed in Hamburg residents 1995–2003. Observed and relative survival (OS, RS) were estimated by site, sex, time, age and stage. Results. Regarding female breast cancer in Hamburg, high levels of data quality and completeness exist while the explanatory power concerning malignancies of the colon, prostate and urinary bladder is limited. Age-standardised 5-year relative cancer survival estimates amounted for female breast to 81%, for colon to 49% (male) and 52% (female), for prostate to 81% and for urinary bladder to 71% (male) and 62% (female). Conclusion. The study demonstrates the capacities and limitations of an epidemiological cancer registry to produce convincing survival estimates for clinical use, under the terms of a voluntary case reporting system.
Population-based cancer survival analysis can give useful indications regarding the effectiveness of cancer related activities within a healthcare system. Recently published survival estimates referring to malignancies diagnosed in Germany since 1995 predominantly have been derived from the cancer registries of Saarland and Munich in the South of Germany or from the New Laender in the East of Germany Citation[1–7]. However, a substantial data stock has been collected by the Hamburg Cancer Registry (HCR) in Northern Germany, which started operation in 1929 Citation[8], Citation[9]. Since 1985, when the corresponding federal state law ‘Hamburgisches Krebsregistergesetz’ became effective, epidemiological cancer registration in the 1.7 million metropolitan area of Hamburg has been based on the voluntary right of physicians to notify cases dependent on the patient's informed consent. By 1995 the overall proportion of those reported by death certificates only (DCO-cases) could be reduced to below 20%, and since then varied from 8 to 14% per year with a decreasing trend. Information on actual deaths is continuously derived from matching all death certificates issued in Hamburg with the entire HCR's database. Additionally in 2004 the continuous update of documented personal data concerning changes of name, sex, place of residence and vital status, started by a cooperation with the residential registry office, which was introduced by a total cross-validation of all patients kept as ‘alive’ at that time. Thus for some years before 2004 0.6% registered cancer patients are documented as migrated from Hamburg annually, compared to constantly 4% on average thereafter. Correspondingly an unpublished active follow-up of 2 962 registered colorectal cancer cases in Hamburg residents diagnosed in 1990–1998 proved information concerning vital status to be more than 95% correct (R. Fertmann, unpublished observation, Hamburg 2005). The remaining 5% consisted of <1% unknown deaths, <1% lost to follow-ups and 4% migrants.
Comprehensive cancer survival analysis based on epidemiological registry data from Hamburg has not yet been performed, and comparable results from Northern Germany are scarcely published Citation[6], Citation[7]. Meanwhile several neighbouring European cancer registries have established survival estimates as part of their periodic reports providing a sound basis for quality assessment within clinical oncology Citation[10–14].
The aim of this study is to assess the HCR's data quality concerning survival analysis and to perform analyses within selected malignancies. The results are evaluated in relation to estimates of neighbouring cancer registries. It is intended to appraise the databases clinical usability with respect to cancer survival estimation.
Material and methods
The study was limited to first primary malignancies diagnosed in Hamburg residents. With regard to an exemplary and explorative analysis, four cancer sites and an appropriate period of diagnosis were selected according to the following criteria: Entities were considered among the ten most frequent malignancies in Hamburg, with at least 100 notified cases per sex and year, representing gender-specific occurrence vs. similar manifestation in both sexes and diverse degrees of completeness. A maximum allowance of 20% cases annually documented as diagnosed at death (DCO-notifications and clinical reports omitting date of diagnosis) was set to restrict the possible bias of survival estimates to a tolerable level Citation[15]. Estimates of completeness by the Central Cancer Surveillance Programme at the Robert Koch-Institute (RKI) were consulted as an external rating of data quality, although they refer to notified cases regardless whether first and second primaries. This indicator is derived by an indirect method which estimates the disease's frequency in the catchment area concerned, based on the data of a cancer registry where case registration is complete. Under the assumption that no major differences exist with regard to the case fatality of cancers among German states and that different regional cancer risks are reflected by differences in mortality, the age-specific proportions of incidence to mortality are used to estimate the frequency of cancer indirectly. An iterative procedure is used to model the quotients in a log-linear approach with polynomial time trends. Initially, the reference registry has been represented by the Saarland Cancer Registry Citation[16], Citation[17].
The selection resulted in malignancies of the colon (ICD10 C18), female breast (ICD10 C50), prostate (ICD10 61) and urinary bladder (ICD10 C67 and D09.0) of which were diagnosed in 1995–2003 (). Excluded in each site were second primary tumours, DCO-cases and other cases with date of diagnosis documented as equal to death. Sporadically dubious case documentation was clarified individually by comparing originally reported data and processed best-of information. Urinary bladder invasive and in situ neoplasms (ICD10 C67 and D09.0) were grouped together because of changes in the coding of non-invasive papillary transitional cell carcinoma in 2000 Citation[18], Citation[19]. The selection of 15–99-year-old patients resulted in an actual age range of 18–99 years at diagnosis in the present study. Ninety-one percent of the included cases were histologically verified. For each site the trend of case numbers, indicators of completeness and the proportion of cases documented with stage (local, regional, distant metastases) and T-category according to the TNM scheme was identified. The mortality-to-incidence (M/I) ratio trends are commented in respect of each site. The overall values are listed in .
Table I. Selection of database for exemplary survival analysis from Hamburg Cancer Registry (HCR)
Information on survival was derived from routine matching with the Hamburg death certificates and with the Hamburg residential registration office's data up to the end of 2004. Survival time was calculated using the difference in quarters between the date of diagnosis and date of moving outside of Hamburg, death or December 31, 2004, whichever was earliest. Measures of observed and relative survival (OS, RS) were calculated for multiple-year cohorts. The observed survival, defined as the proportion of patients surviving a specific amount of time after cancer diagnosis, was computed according to the life-table method Citation[20–22]. Relative survival, defined as the ratio between observed and expected survival in a comparable group of the regional population, was estimated according to Hakulinen Citation[22–24]. Reference data for expected survival estimates originated from sex- and calendar year-specific life tables for Hamburg by the statistical state office. The life tables’ ranges of age (0–90 years) were extended up to the age of 99, according to the Gompertz distribution by means of logistic regression Citation[25]. Standard errors (SE) of the estimated relative survival were obtained using Greenwood's formula; the 95%-confidence interval (95%CI) was approximated by addition and subtraction of twice the standard error Citation[22], Citation[26]. Crude relative survival ratios were estimated by site, sex, age-group and consecutive triennial calendar periods of diagnosis. In addition, for the purpose of comparison, age standardized 3- and 5-year relative survival ratios were calculated by the direct method using European standard population for cancer-survival analysis Citation[27].
The acceptance of calculated values into graphs was limited to the condition that at the beginning of the respective interval at least 30 patients were alive and SE ≤25%. Descriptive data analysis was conducted by means of SPSS version 12.0 and Microsoft Excel 2003. Survival analysis was performed by using SURV3, a shareware package from the Finnish Cancer Registry Citation[28], Citation[29].
Results
Colon
The ratio of female (f) to male (m) colon cancer patients was 1.4:1. From 1995 to 2003 the annual numbers of included cases slowly dropped from 263 to 203 (m) and from 320 to 247 (f), i.e. by approximately a fifth in both sexes without obvious changes in the distribution of age and stage. Sixty-nine point eight years represented the median age at diagnosis in men compared to 75.6 years in women. Sixty-eight percent of the included cases were staged, 59% were provided with T-categories. Sixteen percent (m) and 20% (f) of exclusions, fluctuating M/I ratios between 0.47–0.62 (m) and 0.57–0.78 (f), and an estimated completeness of 77% (m) and 72% (f) indicate a certain amount of underregistration ().
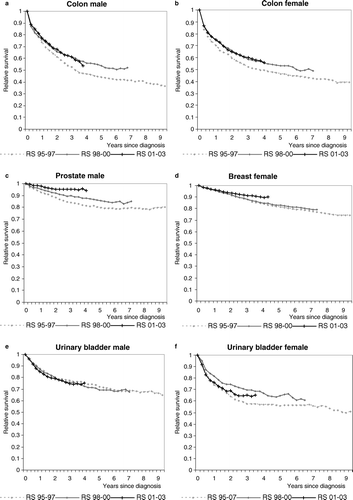
The survival analysis shows almost equal crude estimates for both sexes (5 years RS 50% (m) and, 51% (f)) except minor differences in the ratios by age-group (). An obvious increase in long-term relative survival curves is seen between the 1995–1997- and the 1998–2000-cohort, yet no further improvement for those diagnosed in 2001–2003 (a, 1b).
Table II. One- and five-year observed and relative survival (OS and RS in %) for selected tumour sites diagnosed in 1995–2003, by sex and age-group.
Breast
The included cases of female breast cancer ranged on a fluctuating level of approximately 1 000–1 200 per year. The median age at diagnosis gradually rose from 60 (1995) to 62 years (2003). The low proportion of exclusions (7%), a steadily from 0.40 to 0.34 declining M/I ratio, and an estimated completeness of 95%, indicate a high degree of coverage (). A good data quality is confirmed by 94% histologically verified diagnoses and 83% specified stages. T-categories were available for 67% of included cases.
The relative survival curves for female breast cancer are almost lineally and slowly declining on a high level (5 years RS 83%). Regarding age-specific survival only patients above 74 years have distinctly inferior values (5 years RS 75%) compared to the other age-groups (d, ). Crude relative survival curves relating to three consecutive triennials of diagnosis show constant increases beyond the first year after diagnosis. Stratification by stage applied to 5-years RS of moving diagnosis periods from 1995–1997 to 1998–2000 supports this finding for local and regional stages only ().
Prostate
Prostate cancer case numbers increased constantly from less than 500 cases per year (1995) to more than 900 (2003), simultaneously the median age at diagnosis dropped from 70 to 67 years. 14% of excluded cases correspond reasonably to overall 77% estimated completeness. The annual M/I ratio declined from 0.39 to 0.23 (). Of the included, 61% are staged and 52% provided with T-categories.
Long-term observed and relative survival diverges more distinctly compared to other sites investigated in this study (). The descent of the relative survival curve is constantly slow for the whole cohort (5 years RS 86%). The 55–74-year-old patients present the best estimates (5 years RS 92%) in contrast to significantly subjacent rates of those aged 75 years and more (5 years RS 66% for 75–84 and 39% for 85 + ) (). Three successive periods of diagnosis show clear increases for crude relative survival curves of prostate cancer (c).
Urinary bladder
The cohort shows rather constant annual case numbers of approximately 250 (m) and 100 (f), men being affected 2.4 times as often and at a younger age than women (median age at diagnosis 70.7 (m) and 73.9 (f) years). Sixty-eight percent of the cases got staged, 64% were equipped with T-categories. Concerning indicators of completeness, comparatively minor proportions of DCO and equivalent cases (8% (m) and 15% (f)) diverge from the RKI-estimates (64% (m) and 63% (f)). M/I ratios fluctuated particularly in respect of women in a range of 0.43–0.61, compared to 0.30–0.41 in men ().
Female urinary bladder cancer patients exhibit significantly worse observed and relative survival than male patients (5 years RS 56% (f) vs. 70% (m)) (). Relative survival of the age-groups 75 years and more is inferior to that of younger patients with a major difference in women. Crude relative survival curves of consecutive triennials of diagnosis show scant alterations in men. In women, an apparent increase between the 1995–1997 cohort (5 years RS 56%) and the 1998–2000 cohort (5 years RS 64%) is followed by a decrease (e, f). Female urinary bladder cancer patients diagnosed 2001–2003 appear to have survived similarly to those from 1995–1997 during the first two years, but then show a moderately improved relative survival remaining below that of the 1998–2000 cohort.
Discussion
The HCR's data stock is heterogeneous in respect of periods and sites. Generally data quality concerning follow-up information is maintained on a high level. According to an active trace back study in 2005, the vital status has been documented accurately in more than 95% of the cases, and the current migration ascertainment of registered patients will improve this aspect even more. With regard to the influence of vital status misclassification on survival estimates, a magnitude of 5% at the most might result in a deviation of approximately 1% in 5-year relative survival ratios Citation[15]. A major proportion of histologically verified diagnoses and declining trends of DCO cases since the mid-nineties indicate a substantial and increasing degree of registration. In the future further improvement will be achieved by the currently established pathologist's legal obligation to report cancer cases to the HCR, which is in force since 2007 April 24. Yet the proportion of excluded cases in this study, which are documented as diagnosed at death either as DCO, autopsy or incomplete case report, still exceeds that of many publications on population-based cancer survival analysis Citation[30–33]. In addition the ascertained two-thirds amount of staged cases is in need of enhancement, not to mention merely 52–67% of T-categories available at present.
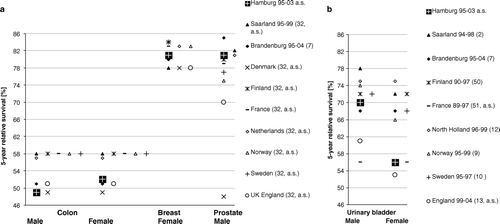
Population based survival analysis referring to malignancies of the colon, female breast, prostate and urinary bladder in Hamburg however revealed a differentiated picture. In the following common statements and currently available data on screening activities, measures of early detection and therapeutical developments are taken into account. A comparison with five-year relative survival ratios, issued in the context of the EUROCARE-4 study and by neighbouring cancer registries for population-based patient cohorts diagnosed during similar periods, supplements the site-specific reflection Citation[33]. Though age-standardized values are included as far as possible, the comparison between regions has to be regarded with utmost caution given the differences concerning populations, conditions of registration, inclusion criteria and methodological details ( a), 3 b), detailed references there).
Figure 3. Population-based 5-year relative survival estimates for colon, breast and prostate cancer patient cohorts in neighbouring regions, diagnosed in the stated period and age-standardised if labelled ‘a.s’(references in legend)
Colon malignancies in Hamburg residents were registered as having been diagnosed prior to death at a rate of 70–80% according to the surrogate markers of completeness. Fluctuating M/I-ratios with an average exceeding most of those from neighbouring cancer registries (as published for the years 1998–2002 in Cancer in Five Continents IX) add to the suspicion of underregistration Citation[34]. Furthermore the almost 20% drop of case numbers over time is inconsistent with the constant trend estimated for the whole of Germany Citation[2]. There is no lucid explanation for this decline apart from growing obligatory medical documentation which may effect voluntary requirements like cancer reports to the HCR Citation[35].
The similarity of relative survival among male and female patients, a decline in higher age-groups and the improvement during the nineties correspond to observations within other population-based survival studies Citation[2], Citation[6], Citation[30], Citation[32]. Still the absence of further increase for the most recent patient cohorts and an altogether 5-year relative survival ratio of 50%, which ranks among the lower estimates of comparative registries, remain difficult to be interpreted (a). The assumption of up to a fourth of non-reported cases and missing information on stages for roughly half of all presumably incident colon malignancies in Hamburg during the study period impedes further findings.
The collection of data on survival relevant therapeutical care is beyond the scope of this study. Concerning early detection colorectal cancer screening activities during the observation period were limited to optional faecal occult blood test and digital rectal examination from the age of 45, with an estimated attendance of less than half of the entitled women and below seventh of entitled men in Germany Citation[36], Citation[37]. In October 2002 screening colonoscopy was introduced into the National Cancer Prevention Programme in Germany. Less than 3% of the eligible population aged 55 and above responded to this subsequently Citation[36], Citation[38], Citation[39].
Female breast cancer in Hamburg has been documented at a 93% level of completeness, and approximately 80% of staged cases permit differentiated survival-analysis. The relative survival curves show the characteristic shape lacking a plateau within the first decade after diagnosis Citation[40], Citation[41]. A continuous increase during the period of observation corresponds to a slow decline of M/I ratios and to the presumption of earlier diagnoses due to opportunistic screening in Germany Citation[42], Citation[43]. According to available data in 1996 5.2 mill mammographies have been performed, averaging out to one in 6.5 female Germans above the age of 20 Citation[42]. A similar calculation for Hamburg in 2000 results in a numerical proportion of 1:4.7, suggesting an increasing use of mammograms during the period of observation (Hamburg Association of Statutory Health Insurance Physicians (KVH), unpublished communication, Hamburg 2005).
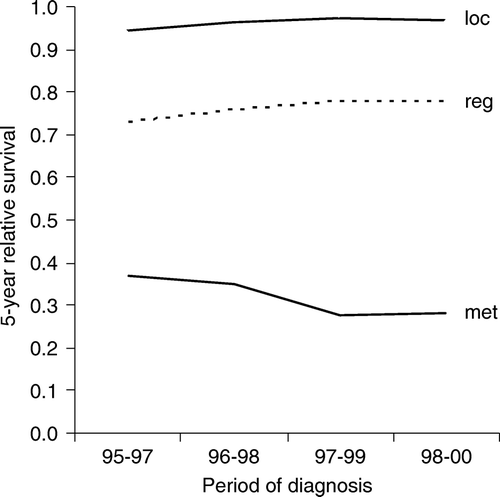
In the presence of distant malignancies the survival trend of breast cancer patients did not improve, thus consistent with recently published results () Citation[5], Citation[44]. Whether the positive tendency found both for local and regional stages could be explained by stage migration cannot convincingly be elucidated by the available database: Crude stage-specific incidence for the included metastasized breast cancer cases remained stable on a level of 7/100 000 over the period of observation while that for local tumours slightly fell from 50 to 46/100 000, and that for regional spreading rose from 24 to 32/100 000. An overall age-standardised 5-year relative survival estimate for female breast cancer of 81% ranges near the upper equivalent figures issued by neighbouring European regions (a), all of which have been performing mammography screening schemes during the period of observation Citation[45].
The rising prostate cancer incidence figures have to be cautiously interpreted in the presence of presumably spreading analyses of prostate-specific antigen (PSA) Citation[46]. A considerable increase of PSA-testing may be assumed according to accessible information: In 1995 1.6 mill PSA-analyses have been billed within the German statutory health insurance system, i.e. approximately one test in 25 members of the male population Citation[47]. In 2000 the same ratio in Hamburg amounted to 1:6 (KVH, unpublished communication, Hamburg 2005). Further the proportion of under-70-year-old patients increased from 46% in 1995 to 65% in 2003, and within the T-staged fraction T1-tumors advanced from 18% to 28%. These observations support an incidence elevating effect of the suspected opportunistic PSA-screening. Regarding data quality rising estimates of up to 96% completeness in 2003 and simultaneously growing proportions of staged cases (above 70% in 2003) hint at an improving, yet not throughout satisfactory condition.
Survival analysis resulted in a putatively pronounced increase of prognosis since the mid-nineties. This observation must be interpreted in connection with spreading PSA-testing and its effects of advancing lead-time and overdetection of prostate cancer Citation[48]. As in other studies, the youngest patients do not have the best outcome Citation[49]. The overall 5-year relative survival of prostate cancer affected men in Hamburg ranges among the upper half of comparative external estimates (a).
Regarding urinary bladder cancer in this study, the degree of coverage remains difficult to assess because of contrasting indicators of completeness. This may be interpreted partially as a consequence of classification changes during the relevant period and their heterogeneous realization among German cancer registries. The applicable RKI estimates of completeness depend on the definition of ICD10 C67 as including non-invasive papillary tumours or not by the contributing German registries during the relevant period.
Concerning survival estimates for Hamburg, male bladder cancer patients have significantly better probabilities than female ones, a finding that had been confirmed by others before lacking conclusive explanation Citation[50]. The time trends of relative survival curves do show almost unchanged shapes in men while female bladder cancer patients seem to improve until 2000 and worsen thereafter. The latter observation has to be relativized regarding the largely overlapping confidence intervals of second and third diagnosis periods’ 5-year relative survival: 62–75% for 1998–2000 vs. 57–73% for 2001–2003.
As survival for bladder cancer patient cohorts has not been estimated within the EUROCARE-4 study, alternative publications of neighbouring cancer registries are supplied and shown in a separate diagram ( b) Citation[2], Citation[7], Citation[10], Citation[11], Citation[13], Citation[14], Citation[51], Citation[52]. The more or less comparable European 5-year relative survival estimates exhibit a considerable range of variation of up to 22%, partially due to lack of stratification by sex and too diverse classification of non-invasive malignancies (). The overall rate for male bladder cancer patients from Hamburg ranges near a mean estimate of neighbouring regions, while the female counterpart marks the lower end.
In conclusion the present study demonstrates the capacities and limitations of an epidemiological cancer registry to produce convincing survival estimates for clinical use, under the terms of a voluntary case reporting system. The situation is determined by a high standard of data maintenance on the part of the HCR, by the capability of improvement on the part of those generating notifications, and by an obvious necessity of numerical data on oncological care. Nevertheless, the basis of clinical usability is formed in terms of comparing survival estimates of selected oncological patients with those of Hamburg. Evaluations like the present one, with a timely feedback to the clinical field, reveal the assessment of oncological performance quality, and have the potential to set off a productive dialogue among those who are engaged in prevention and therapy of cancer.
Acknowledgements
We are grateful to Ms. Nolene Sheppard for the textual revision of this paper.
References
- Brenner H, Stegmaier C, Ziegler H. Long-term survival of cancer patients in Germany achieved by the beginning of the third millennium. Ann Oncol 2005; 16: 981–6
- Association of Population-Based Cancer Registries in Germany in cooperation with the Robert Koch Institute. In: Cancer in Germany. 5th ed. Saarbrücken, Germany: 2006. Available from: http://www.ekr.med.uni-erlangen.de/GEKID/Doc/kid2006_englisch.pdf.
- Engel J, Eckel R, Aydemir U, Aydemir S, Kerr J, Schlesinger-Raab A, et al. Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys 2003; 55: 1186–95
- Kerr J, Engel J, Eckel R, Hölzel D. Survival for rectal cancer patients and international comparisons. Ann Oncol 2005; 16: 664–72
- Schlesinger-Raab A, Eckel R, Engel J, Sauer H, Löhrs U, Molls M, et al. Metastasiertes mammakarzinom: Keine lebensverlängerung seit 20 Jahren. Dt Ärztebl 2005; 102: A2706–A2714
- Fietkau R, Zettl H, Klocking S, Kundt G. Incidence, therapy and prognosis of colorectal cancer in different age groups. A population-based cohort study of the Rostock Cancer Registry. Strahlenther Onkol 2004; 180: 478–87
- Tumorzentrum Land Brandenburg e.V. und Qualitätskonferenz Onkologie. Qualitätssicherung durch klinische Krebsregister: Sachbericht Onkologie 2004/2005. Frankfurt (Oder) 2006. Available from: http://www.tumorzentrum-brandenburg.de/pwp/(S(urgnipzt5jvjch55qslofra0))/uploads/Sachbericht_2004–2005.pdf.
- Sieveking GH. Das Krebsproblem in der öffentlichen Gesundheitsfürsorge. Zeitschrift für Gesundheitsverwaltung und Gesundheitsfürsorge. 1930; 1: 23–30
- Estève J, Kricker A, Ferlay J, Parkin DM, Facts and figures of cancer in the European Community. International Agency for Research on Cancer 1993. Available from: http://telescan.nki.nl/iarc.html.
- Cancer Registry of Norway, Institute of Population-based Cancer Research. Cancer in Norway 2004. Oslo. Available from: http://www.kreftregisteret.no/forekomst_og_overlevelse_2004/kreft_i_norge_2004web.pdf.
- Centre for Epidemiology, National Board of Health and Welfare. Cancer patient survival in Sweden 1980–2002. 2004. Published at and available from: www.sos.se/epc/publikationer/nyartikel/cancersurvival/cancerpatientsurvival.htm.
- Finnish Cancer Registry, Institute for Statistical and Epidemiological Cancer Research. Cancer in Finland 2002 and 2003. Helsinki 2005. Available from:, , http://www.cancerregistry.fi/eng/statistics/index_7.pdf.
- Integraal Kankercentrum Amsterdam. Overlevingscijfers IKA-regio (Noord-Holland and Flevoland) Last update 16-04-2007. Available from: http://www.ikcnet.nl/IKA/index.php?id=1735&nav_id=160®io_id=117.
- National Statistics. Cancer Survival, England, 1999–2004. Last update 21-08-2007. Available from: http://www.statistics.gov.uk/downloads/theme_health/Survival_21_sites_1999_2003_by_age_group_v6.xls.
- Brenner H, Hakulinen T. Population-based monitoring of cancer patient survival in situations with imperfect completeness of cancer registration. Br J Cancer 2005; 92: 576–9
- Colonna M, Grosclaude P, Faivre J, Revzani A, Arveux P, Chaplain G, et al. Cancer registry data based estimation of regional cancer incidence: Application to breast and colorectal cancer in French administrative regions. J Epidemiol Community Health 1999; 53: 558–64
- Haberland J, Schön D, Bertz J, Görsch B. Vollzähligkeitsschätzungen von Krebsregisterdaten in Deutschland. Bundesgesundheitsbl-Gesundheitsforsch-Gesundheitsschutz 2003; 46: 770–4
- Percy C, van Holten V, Muir C, International classification of diseases for oncology. 2nd ed. Geneva: World Health Organization; 1990.
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology. Geneva: World Health Organization; 2000.
- Berkson J, Gage RP. Calculation of survival rates for cancer. Proceedings of Staff Meetings of the Mayo Clinic 1950; 25: 270–86
- Cutler SJ, Ederer F. Maximum utilisation of the life table method in analyzing survival. J Chron Dis 1958; 8: 699–712
- Voutilainen ET, Dickman PW, Hakulinen T. SURV2: Relative survival analysis program. Software Manual Program version 2.02ß. Helsinki 1998, Manual updated August 9, 2000. Available from: http://www.cancerregistry.fi/surv2/manual.pdf.
- Ederer F, Axtell LM, Cutler SJ. The relative survival rate: A statistical methodology. Natl Cancer Inst Monogr 1961; 6: 101–121
- Hakulinen T. Cancer survival corrected for heterogeneity in patient withdrawal. Biometrics 1982; 38: 933–42
- Manton KG, Stallard E, Woodbury MA, Dowd JE. Time-varying covariates in models of human mortality and aging: Multidimensional generalizations of the Gompertz. J Gerontol A Biol Sci Med Sci 1994; 49: B169–B190
- Greenwood M. The errors of sampling of the survivorship tables. reports on public health and medical subjects. 1926; 33: 1–30
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004; 40: 2307–16
- Hakulinen T, Gibberd R, Abeywickrama K, Söderman B. A computer program package for cancer survival studies. Cancer Society of Finland Publication No. 39, Helsinki: Finnish Cancer Registry, 1988.
- Finnish Cancer Registry. SURV3: Windows software for relative survival analysis (version 3.01), last update December 20, 2002. Available from: http://www.cancerregistry.fi/surv3/.
- Teppo L, Dickman PW, Hakulinen T, Luostarinen T, Pukkala E, Sankila R, et al. Cancer patient survival—patterns, comparisons, trends–a population-based Cancer Registry study in Finland. Acta Oncol 1999; 38: 283–94
- Levi F, Randimbison L, Te VC, Franceschi S, La Vecchia C. Trends in survival for patients diagnosed with cancer in Vaud, Switzerland, between 1974 and 1993. Ann Oncol 2000; 11: 957–63
- Capocaccia R, Gatta G, Roazzi P, Carrani E, Santaquilani M, De Angelis R, et al. The EUROCARE-3 database: Methodology of data collection, standardisation, quality control and statistical analysis. Ann Oncol 2003;14(Suppl 5):v14–v27.
- Berrino F, De Angelis R, Sant M, Rosso S, Lasota MB, Coebergh JW, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in –1999: Results of the EUROCARE-4 study. Lancet Oncol ;8 1995; 2007: 773–83
- Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al., Cancer incidence in five continents Vol. IX. IARC Scientific Publications No 160, Lyon, IARC.
- Blum K, Müller U. Dokumentationsaufwand im ärztlichen Dienst der Krankenhäuser Bestandsaufnahme und Verbesserungsvorschläge. Düsseldorf: Deutsche Krankenhaus Verlagsgesellschaft; 2003.
- Becker N, Brenner H, Klug SJ, Schilling FH, Spix C. Beiträge der Epidemiologie zur Krebsfrüherkennung DOI 10.1007/s00761-006-1127-2; Onkologe 2006 online.
- Neuhaus H. Vorsorge zur Prävention oder Früherkennung des Kolorektalen Karzinoms. Dt Ärztebl 1998; 95: A530–A537
- Altenhofen L, Knöpnadel J, Brenner G. Das Früherkennungsprogramm Kolonkarzinom-Screening in Deutschland—ein Zwischenbericht. Lecture at a symposium of the Epidemiological Cancer Registry Lower Saxony (Krebsregister Niedersachsen) 30th Oct 2004. Available from: http://www.krebsregister-niedersachsen.de/registerstelle/dateien/Vortrag3.pdf.
- Merzenich H, Giersiepen K, Kieschke J, Zeeb H. Früherkennung kolorektaler tumoren: Gibt es die optimale Screeningmaßnahme?. Gesundheitswesen 2005; 67: 803–8
- Joensuu H, Toikkanen S. Cured of breast cancer?. J Clin Oncol 1995; 13: 62–9
- Stenbeck M, Rosén M, Holm LE. Cancer survival in Sweden during three decade,1961–1991. Acta Oncol 1995; 34: 881–91
- Gibis B, Busse R, Reese E, Richter K, Schwartz FW, Köbberling J. Mammography screening as a method for the early detection of breast cancer. Schriftenreihe HTA des DIMDI 3, 1st ed. Baden-Baden: Nomos Verlagsgesellschaft; 1998. Available from: http://gripsdb.dimdi.de/de/hta/hta_berichte/hta003_bericht_de.pdf.
- Nekolla EA, Griebel J, Brix G. Introduction of a mammography screening program in Germany—Benefit-risk considerations. Der Radiologe 2005; 45: 245–54
- Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: Surveillance, epidemiology, and end results data. Cancer 2001; 92: 2211–9
- Lynge E, Olsen AH, Fracheboud J, Patnick J. Reporting of performance indicators of mammography screening in Europe. Eur J Cancer Prevent 2003; 12: 213–22
- Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int 2002; 90: 162–73
- Siebert U, Mühlberger N, Behrend C, Wasem J. PSA- Screening beim prostatakarzinom. Systematischer gesundheitsökonomischer Review. Berichte aus der Schriftenreihe HTA des DIMDI Band 19, 2001, ISBN 3-7890-7165-X. Available from: http://gripsdb.dimdi.de/de/hta/hta_berichte/hta019_bericht_de.pdf.
- Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, et al. Cancer surveillance series: Interpreting trends in prostate cancer. Part I: Evidence of the effect of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst 1999; 91: 1017
- Merrill RM, Bird JS. Effect of young age on prostate cancer survival: A population-based assessment (United States). Cancer Causes Control 2002; 13: 435–43
- Mungan NA, Aben KK, Schoenberg MP, Visser O, Coebergh JW, Witjes JA, et al. Gender differences in stage-adjusted bladder cancer survival. Urology 2000; 55: 876–80
- Brenner H, Hakulinen T. Long-term cancer patient survival achieved by the end of the 20th century: Most up-to-date estimates from the nationwide Finnish cancer registry. Br J Cancer 2001; 85: 367–71
- Bossard N, Velten M, Remontet L, Belot A, Maarouf N, Bouvier AM, et al. Survival of cancer patients in France: A population-based study from The Association of the French Cancer Registries (FRANGIM). Eur J Cancer 2007; 43: 149–60