Abstract
Background. This is the first study to explore the relationship between the expression of fragile histidine triad, FHIT and cyclin D1 proteins, and the clinicopathological significance of the two proteins in Chinese patients with cholangiocarcinoma. Material and methods. Immunohistochemistry was used to study 53 cases of cholangiocarcinoma, 30 para-neoplastic and 20 normal bile ducts for their expression status of FHIT and cyclin D1 and then the results were analyzed with the patient's age, sex, tumour site, histological grade and clinical stage as well as overall median survival time. Results. Compared with the para-neoplastic and normal cholangiocytes, the expression of FHIT was obviously decreased (p=0.0001), whereas that of cyclin D1 was significantly increased (p=0.0001) in carcinoma cells. The expression of FHIT was found to be correlated with the histological grade (p=0.007) and the clinical stage (p=0.004), but not with age (p=0.776), sex (p=0.246) or tumour site (p=0.347). The expression of cyclin D1 was also showed statistically associated with the histological grade (p=0.043) and clinical stage (p=0.047), but not with age (p=0.965), sex (p=0.751) or tumour site (p=0.948). Further, the expression of FHIT was found to be inversely correlated with the expression of cyclin D1 (p=0.0001). The loss of expression of FHIT and the expression of cyclin D1 were significantly related to the cancers with shorter median survival time (p=0.0001, p=0.0081). The expression of FHIT was an independent prognostic factor (p=0.005). Discussion. The expression of FHIT may be inversely correlated with the expression of cyclin D1. It is suggested that the loss of FHIT protein and overexpression of cyclin D1 protein may play an important role in carcinogenesis and prognosis of cholangiocarcinoma.
Cholangiocarcinoma is one of the most aggressive malignancies, with an extremely poor prognosis. This is because by the time they become clinically evident; most cancers of the biliary tract will have grown beyond the limits of curative resection. Complete resection seems to be the only potentially curative therapy for all types of biliary tract neoplasms. In prognosis, although 5-year survival rates for intrahepatic and distal cholangiocarcinomas are generally better than those for perihilar carcinomas (30–40%, 20–30%, and 9–18%, respectively) Citation[1], Citation[2], stage-related 5-year survival rates seem more comparable. The morbidity of bile duct cancer varies with country, area, and race Citation[3]. In China, the number of this kind of rare carcinoma in the west of the world is high with a tumor-free 5-year survival rate of 13.3%. Among the different causes for incidence, hepatitis virus infection, especially hepatitis C virus (HCV), chronic biliary infection of intestinal source, primary desmoplastic cholangitis, bile duct lithiasis, infection with Clonorchis sinensis, and the worst environmental carcinogens are possibly the pathogenetic factors for Chinese cholangiocarcinoma patients. Advances in biological research, especially those in mutation-independent activation of the Hedgehog pathway Citation[4], have provided interesting information on the carcinogenesis of this rare tumor. Furthermore, these results bring us the possibility of development of targeted therapies in biliary tract cancer. However, early pathologic diagnosis is still difficult in this kind of highly desmoplastic, submucosal and infiltrating cancers, and the sensitivity for the diagnosis of cholangiocarcinoma is only ∼30% for cytology to 40 to 70% for combined brush cytology and biopsy, making a negative result virtually useless Citation[1], Citation[2]. This reality has raised therapeutic problems, and new early diagnostic tools and therapeutic techniques in this disease are urgently needed.
A growing body of evidence suggests that certain human chromosomal fragile sites have roles to play in cancer Citation[5]. Research in this area focused on the fragile histidine triad gene (FHIT) located in the chromosome 3 at 3p14.2. FHIT is altered in many kinds of primary or cultured carcinomas in the form of deletion within both FHIT alleles, resulting in loss of exons and concomitant absence of full-length FHIT transcript and protein Citation[6]. Loss of expression of the FHIT gene, leading to loss of regulatory control, is common in epithelial malignancies. The FHIT gene and/or its expression have been found in primary tumors and cell lines of lung, breast, head and neck, esophagus, stomach, colon and rectum, pancreas, kidney, cervix, and liver Citation[5–11]. In cholangiocarcinoma, genetic alternations or decreased FHIT protein expression have been noted only in a small sample of nineteen patients from Germany Citation[12] and in hamsters from Japan Citation[13], but there is still no investigation in larger samples, nor for the patients from China. The relationship between the expression of FHIT and the clinicopathological features of cholangiocarcinoma has not been reported in the literature. Cyclin D1 is another frequent site of loss of cell cycle-regulatory control. As an upstream inhibitor of the Rb gene product, cyclin D1 overexpression has been identified in several malignancies, including cholangiocarcinoma Citation[14], Citation[15], but there is no investigation of its relationship with FHIT expression. In this study, we compare the expressions of FHIT and cyclin D1 with the clinicopathological features of cholangiocarcinoma and explore the possible relationship between the two proteins. It is hoped that the study will give useful information on the clinicopathology of cholangiocarcinoma.
Material and methods
Patients
Fifty-three cases of cholangiocelluar carcinoma tissue samples derived from a cohort of patients who had undergone surgery for cholangiocarcinoma, 30 cases of the corresponding para-neoplastic bile duct tissue and 20 cases of normal bile ducts with inflammation were retrieved from the archival file of the Department of Pathology, Chinese People's Liberation Army General Hospital. Each tissue specimen was histologically evaluated by at least two experienced pathologists. The carcinoma patients included 33 men and 20 women aged from 17–73 (mean = 53.6; median = 56.0) years old. In tumour location, 12 were intrahepatic, 27 perihilar, and 14 distal cholangiocarcinomas. Tumour grading and staging were performed by applying WHO (2000) and UICC (1997) criteria, and in grading, 16 were at grade 1, 15 at grade 2 and 22 at grade 3; in staging, 13 were at stage I, 14 at stage II, 18 at stage III and 8 at stage IV. This research work was approved and supported by the Chinese PLA General Hospital and without ethical conflicts.
Immunohistochemical analysis
Paraffin tissue sections, 4 µm in thickness, were cut, dewaxed in xylene and rehydrated in a graded ethanol series. Then sections were immersed in 3% hydrogen peroxide in methanol for 10 minutes to block endogenous peroxidase activity and rinsed in running water. They were then immersed in boiling 0.01 M citrate buffer (pH 6.0) in a pressure cooker. The pressure cooker was then sealed and brought to full pressure. The heating time was 2 minutes. After that, the pressure cooker was de-pressured and cooled under running water. The lid was then removed, and the hot buffer was flushed out with cold water from a running tap. The cooled sections were washed twice in phosphate buffered saline (PBS) before immunohistochemical staining. The primary antibodies were then added to the sections for 1 hour. The polyclonal rabbit antibody against human FHIT protein (Zymed, South San Franciso, CA, USA) and the mouse antibody against human cyclin D1 protein (Zymed, South San Franciso, CA, USA) were diluted 1 in 100. After exposure to primary antibody, the sections were allowed to react with the poly peroxidase-anti-mouse/rabbit IgG for 20 minutes using the standard non-biotin PV-6000 Polymer Detection System (Zymed). The sections were then washed in water, counter-stained with Mayer's haematoxylin for one minute at room temperature, dehydrated, cleared and finally mounted. Paraffin blocks of human liver, lung, spleen and breast ductal carcinoma tissues were used as positive controls. Negative controls were sections treated as above but with omission of the primary antibody which was replaced by 0.01M PBS. For immunohistochemical evaluation of FHIT or cyclin D1, cytoplasmic or nuclear labelling of tumour cells was classified as either negative (if no staining or positive staining was present in < 10% of tumour cells) or positive (if > 10% of tumour cells stained positively) Citation[12].
Statistical analysis
Fisher's exact test, Pearson χ2 test for trends in proportions and the Kaplan-Meier method with Log rank test or Cox Regression method for univariate or multivariate overall survival analysis were used to assess the associations between FHIT or cyclin D1 expression and pathological indices. A p < 0.05 was considered statistically significant.
Results
Expressions of FHIT and cyclin D1 in normal, para-neoplastic bile ducts and cholangiocelluar carcinoma
FHIT protein was expressed uniformly strongly in the cytoplasm of cholangiocytes of normal bile ducts, which served as an internal control (A). In carcinoma, FHIT expression was completely absent in 24 of 53 cases (45.3%) (B); in other tumours, the number of immunoreactive cells ranged from 10% to almost 100%. Most poorly-differentiated cancer cells were negative for FHIT protein. All 20 normal and 30 para-neoplastic bile ducts were stained strongly positive for FHIT protein in the cytoplasm of cholangiocytes. There was a statistical difference between cholangiocellular carcinomas and para-neoplastic or normal bile ducts (p = 0.0001).
Figure 1. The results of immunohistochemical staining in cholangiocarcinoma. It was showed a positive staining for FHIT in the normal bile ducts (A), but a negative staining in the infiltrating tumour cells (B). On the contrary it was showed a negative staining for cyclin D1 in normal bile ducts (C), but a strongly positive staining in the corresponding tumour cells at the serial slides (D)[A, B, C, D: 200×].
![Figure 1. The results of immunohistochemical staining in cholangiocarcinoma. It was showed a positive staining for FHIT in the normal bile ducts (A), but a negative staining in the infiltrating tumour cells (B). On the contrary it was showed a negative staining for cyclin D1 in normal bile ducts (C), but a strongly positive staining in the corresponding tumour cells at the serial slides (D)[A, B, C, D: 200×].](/cms/asset/69fc6209-3133-43e2-b2a1-52aa9629035d/ionc_a_321800_f0001_b.jpg)
Cyclin D1 was predominantly expressed in the nucleus of cholangiocellular carcinoma cells. Thirty-three of 53 cases (62.3%) of carcinoma were positive for cyclin D1 expression; only four of 30 (13.3%) cases of para-neoplastic bile ducts were partially positive for cyclin D1; all 20 cases of normal bile ducts were negative for cyclin D1 (C). There was a significant difference between carcinomas and para-neoplastic or normal bile ducts (p = 0.0001). On examination of serial sections it was obvious that poorly differentiated cancer cells that were negative for FHIT (B), were strongly positive for cyclin D1 (D).
Of the cholangiocarcinomas with FHIT expression, cyclin D1 expression was found only in 37.9% (11/29), whereas of the cancers with loss of FHIT, cyclin D1 presented up to 91.6% (22/24). There was a significant inverse relationship between expression of FHIT and cyclin D1 (γ = − 0.889, p = 0.0001).
Relationship between FHIT expression and age, sex, site, histological grade, clinical stage and prognosis
In this group of 53 cholangiocellular carcinomas, FHIT expression was not correlated with the patient's age (p = 0.776), sex (p = 0.246) or tumour site (p = 0.347) (). The percentage of the carcinomas with negative expression of FHIT increased from 25.0% (4 of 16) at grade 1 to 26.7% (4 of 15) at grade 2, and to 72.7% (16 of 22) at grade 3 thus the association between loss of FHIT expression and increased histological grade was statistically significant (p = 0.007, ). At stage I to II, 25.9% (7 of 27) carcinomas showed loss of FHIT expression, but 65.4% (17 of 26) at stage III to IV. Statistically, lack of expression of FHIT was significantly correlated with the more advanced clinical stage. (p = 0.004, ). Follow-up data showed that there was a significant difference in overall median survival time between the carcinomas with FHIT expression (28 months) and those without (13 months) (Log rank = 26.48; p = 0.0001) (A). These data suggest that the loss of FHIT expression is significantly related to the cancers with shorter overall median survival time (p = 0.0001). In the result of multivariate analysis by Cox Regression, FHIT expression was an independent prognostic factor (p = 0.005).
Figure 2. Survival curves in patients with cholangiocellular carcinoma. (A) Survival curves of patients with FHIT positive expression (pink) and negative expression (green). (B) Survival curves of patients with cyclin D1 overexpression (pink) and negative expression (green).
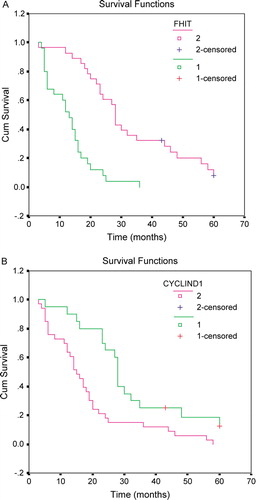
Table I. Relationship between expression of FHIT or cyclin D1 and clinicopathological features in cholangiocellular carcinoma.
Relationship between cyclin D1 expression and age, sex, site, histological grade, clinical stage and prognosis
In this study of 53 cholangiocarcinomas, cyclin D1 expression did not correspond to the patient's age (p = 0.965), sex (p = 0.751) or carcinoma site (p = 0.948) (). The percentage of carcinomas with expression of cyclin D1 increased from 43.8% (7 of 16) at grade 1 to 53.3% (8 of 15) at grade 2 and to 81.8% (18 of 22) at grade 3 thus this association of higher histological grade with cyclin D1 expression was statistically significant (p = 0.043, ). In this group of cholangiocarcinomas, the expression of cyclin D1 was 48.1% (13 of 27) at stage I to II, but 76.9% (20 of 26) at stage III to IV. Statistically, the expression of cyclin D1 was significantly correlated with the more advanced clinical stage. (p = 0.047, ). Follow-up data showed that there was a significant difference in overall median survival time between the carcinomas with cyclin D1 expression (16 months) and those without (28 months) (Log rank = 7.01; p = 0.0081) (B), implying that the expression of cyclin D1 is statistically related to the cancers with shorter overall median survival time (p = 0.0081). But in the result of multivariate analysis by Cox Regression, cyclin D1 expression was not an independent prognostic factor (p = 0.108).
Discussion
Cholangiocarcinoma still remains a lethal malignancy. There are only a few patients for whom aggressive surgical resection should be considered. The high rate of distant metastases with this cancer suggests that systemic chemotherapy could be useful. However, no standard adjuvant chemotherapy can be recommended at this moment. Improvements in the knowledge of carcinogenesis of this carcinoma and in the diagnostic tools or surgical procedures suggest that a higher proportion of patients could be cured in the future. Thus, biomarkers for molecular targeting therapy and related diagnosis in cholangiocarcinoma are urgently needed. FHIT protein is expressed in most types of normal human tissues but has been found to be frequently reduced or lost in a variety of human tumors due to alterations in its gene transcription or gene deletion Citation[5–7]. It has thus been suggested that the FHIT gene is a candidate tumor suppressor gene for multiple carcinomas. FHIT protein is a member of the histidine triad family and to date, the mechanism of its suppression on tumor cells still remains largely obscure Citation[5–7]. The following possible mechanisms have been considered for FHIT protein as a tumor suppressor Citation[15]: catabolizing ApppA (Ap3A) or related substrates, decapping function on mRNA cap analogs, signaling by FHIT-substrate complexes or compounds and a nucleotide-independent role Citation[16]. Elucidation of the protein effectors of FHIT signaling may lead to identification of targets for cancer therapy. Recently, it was found that loss of FHIT expression resulted in increased cyclophilin A expression and restoration of FHIT expression led to down-regulated cyclophilin A protein expression, which subsequently prevented up-regulation of cyclin D1, Cdk4, and resultant cell cycle progression (G1-S transition) Citation[17]. Thus, cyclophilin A may be a downstream target in FHIT-mediated cessation of cell cycle progression at late G1 phase. FHIT protein could also inhibit cell growth by attenuating the signaling mediated by nuclear factor-kappaB in colon cancer cell lines Citation[18]. The results of Hu et al. Citation[19] indicated that FHIT and CHK1 had opposing effects on homologous recombination repair. Synergistic tumor suppression by coexpression of FHIT and p53 may coincide with FHIT-mediated MDM2 inactivation and p53 stabilization in human non-small cell lung cancer cells Citation[20]. In this research, we also found that expression of FHIT was inversely correlated with that of cyclin D1, which might be partly explained by the cyclophilin A pathway mentioned above Citation[17].
Previous analysis based on a small sample of nineteen cases from Koch et al. Citation[12] found that there was a loss of FHIT protein in 52.6% cases of primary intrahepatic cholangiocellular carcinoma, but there was no study the cancer occurring in perihilar and distal bile ducts. In our study of 53 cases, loss of FHIT expression was present in six of 12 (50.0%) cases of intrahepatic cholangiocarcinoma, coinciding with the result of Koch et al. Furthermore, we report the loss of FHIT was 51.9% (14/27) in perihilar and 28.6% (4/14) in distal cholangiocarcinomas; all together, the loss ratio of FHIT was 45.3% (24/53) in cholangiocarcinoma throughout the bile duct, whereas the para-neoplastic and normal bile ducts showed uniformly strong FHIT expression, suggesting that the loss of FHIT might play an important role in the carcinogenesis of cholangiocytes (p = 0.0001). Our results show that FHIT protein was gradually decreased with the poorer differentiated carcinomas, suggesting that the higher the grading, the lower the expression (p = 0.007). There was also a trend that the loss of FHIT protein was more frequently observed in the most advanced clinical stage III to IV (65.4%) than in the earlier clinical stage I to II (25.9%) (p = 0.004), in cancers with shorter median survival time (p = 0.0001). Further, Loss of FHIT protein seemed to be an independent prognostic factor, implying that FHIT protein may play an important role as a tumor suppressor in the evolution of cholangiocellular carcinoma.
The expression of cyclin D1 in cholangiocarcinoma has been reported by several groups outside China Citation[14], Citation[15], Citation[21], Citation[22]. Cyclin D1 is found to be correlated with the tumorigenesis of cholangiocytes by decreasing the growth inhibitory effect of TGF-beta1 Citation[23] and by activating beta-catenin in the Wnt signaling pathway Citation[24]. However, the relationship between cyclin D1 and FHIT has not been investigated before this study. There is no report on cyclin D1 expression in Chinese cholangiocarcinoma patients to date. In our research, the expression of cyclin D1 is significantly higher in cholangiocarcinoma than in para-neoplastic or normal bile ducts (p = 0.0001), not only confirming that expression of cyclin D1 is related to carcinogenesis, but also suggesting that it might be a useful marker to differentiate cholangiocellular carcinoma from some diagnostically difficult lesions, such as chronic desmoplastic cholangitis. The ratio of cyclin D1 expression was previously reported from 41.7 to 61.9% of intrahepatic cholangiocarcinoma Citation[21], Citation[22], Citation[24] and that of our result in intrahepatic cancer (41.7%) from China agrees with that of Sugimachi Citation[22] and Tokumoto et al. Citation[24] from Japan. In the current study, the expression of cyclin D1 was more frequently observed in poorly differentiated carcinomas (p=0.043), in aggressive clinical stage (p=0.047) and in shorter median survival (p = 0.0081). This corresponds to reports from Japan Citation[14], Citation[22], also indicating that the expression of cyclin D1 could be related to poorer differentiation, more advanced progression, metastasis of tumor, and shorter median survival, and thus to the prognosis of cholangiocarcinoma.
In summary, the expression of FHIT was inversely related to that of cyclin D1 and the loss of FHIT or the overexpression of cyclin D1 was significantly associated with carcinogenesis, poor differentiation, late stage of tumor and shorter median survival in cholangiocarcinoma. The combined alteration of two markers, FHIT negative and/or cyclin D1 positive seems to provide the worst diagnostic and prognostic information. Now that FHIT and cyclin D1 proteins can be conveniently detected by immunohistochemistry in routine clinical pathology, they may become not only new adjunct diagnostic and prognostic markers, but also potential biomarkers for molecular targeting therapy in cholangiocarcinoma.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper.
References
- Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, et al Guidelines for the diagnosis and treatment of cholangiocarcinoma: Consensus document. Gut 2002;51(Suppl 6):VI1–VI9.
- Gores GJ. A spotlight on cholangiocarcinoma. Gastroenterology 2003; 125: 1536–8
- Heron DE, Stein DE, Eschelman DJ, Topham AK, Waterman FM, Rosato EL, et al. Cholangiocarcinoma: The impact of tumor location and treatment strategy on outcome. Am J Clin Oncol 2003; 26: 422–8
- Berman DM, Karhadkar SS, Maitra A, Montes-De-Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumors. Nature 2003; 425: 846–51
- Croce CM, Sozzi G, Huebner K. Role of FHIT in human cancer. J Clin Oncol 1999; 17: 1618–24
- Huebner K, Croce CM. Cancer and the FRA3B/FHIT fragile locus: It's a HIT. Br J Cancer 2003; 88: 1501–6
- Ishii H, Ozawa K, Furukawa Y. Alteration of the fragile histidine triad gene early in carcinogenesis: An update. J Exp Ther Oncol 2003; 3: 291–6
- Zhao P, Song X, Nin YY, Lu YL, Li XH. Loss of fragile histidine triad protein in human hepatocellular carcinoma. World J Gastroenterol 2003; 9: 1216–9
- Yuan BZ, Keck-Waggoner C, Zimonjic DB, Thorgeirsson SS, Popescu NC. Alterations of the FHIT gene in human hepatocellular cell carcinoma. Cancer Res 2000; 60: 1049–53
- Hao X.P, Willis JE, Pretlow TG, Rao JS, MacLennan GT, Talbot IC, et al. Loss of Fragile Histidine Triad Expression in colorectal carcinomas and premalignant lesions. Cancer Res 2000; 60: 18–21
- Pekarsky Y, Zanesi N, Palamarchuk A, Huebner K, Croce CM. FHIT: From gene discovery to cancer treatment and prevention. Lancet Oncol 2002; 3: 748–54
- Koch E, Fiedler W, Tannapfel A, Ballhausen WG. Alteration of the fragile histidine triad gene in intrahepatic cholangiocarcinoma. Eur J Gastroenterol Hepatol 2003; 15: 907–13
- Kitahashi T, Tsujiuchi T, Satoh K. Aberrant transcription of FHIT gene in intrahepatic cholangiocellular carcinomas induced by N-nitrosobis(2-oxopropyl)amine in hamsters. Exp Toxicol Pathol 2004; 56: 153–7
- Ito Y, Takeda T, Sasaki Y, Sakon M, Yamada T, Ishiguro S, et al. Expression and clinical significance of the G1-S modulators in intrahepatic cholangiocellular carcinoma. Oncology 2001; 60: 242–51
- Jarnagin WR, Klimstra DS, Hezel M, Gonen M, Fong Y, Roggin K, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: Correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol 2006; 24: 1152–60
- Pace HC, Garrison PN, Robinson AK, Barnes LD, Draganescu A, Rosler A, et al. Genetic, biochemical, and crystallographic characterization of Fhit-substrate complexes as the active signaling form of Fhit. Proc Natl Acad Sci USA 1998; 95: 5484–9
- Semba S, Huebner K. Protein expression profiling identifies cyclophilin A as a molecular target in Fhit-mediated tumor suppression. Mol Cancer Res 2006; 4: 529–38
- Nakagawa Y, Akao Y. Fhit protein inhibits cell growth by attenuating the signaling mediated by nuclear factor-kappaB in colon cancer cell lines. Exp Cell Res 2006; 312: 2433–42
- Hu B, Wang H, Wang X, Lu HR, Huang C, Powell SN, et al. Fhit and CHK1 have opposing effects on homologous recombination repair. Cancer Res 2005; 65: 8613–6
- Nishizaki M, Sasaki J, Fang B, Atkinson EN, Minna JD, Roth JA, et al. Synergistic tumor suppression by coexpression of FHIT and p53 coincides with FHIT-mediated MDM2 inactivation and p53 stabilization in human non-small cell lung cancer cells. Cancer Res 2004; 64: 5745–52
- Kang YK, Kim WH, Jang JJ. Expression of G1-S modulators (p53, p16, p27, cyclin D1, Rb) and Smad4/Dpc4 in intrahepatic cholangiocarcinoma. Hum Pathol 2002; 33: 877–83
- Sugimachi K, Aishima S, Taguchi K, Tanaka S, Shimada M, Kajiyama K, et al. The role of overexpression and gene amplification of cyclin D1 in intrahepatic cholangiocarcinoma. J Hepatol 2001; 35: 74–9
- Zen Y, Harada K, Sasaki M, Chen TC, Chen MF, Yeh TS, et al. Intrahepatic cholangiocarcinoma escapes from growth inhibitory effect of transforming growth factor-beta1 by overexpression of cyclin D1. Lab Invest 2005; 85: 572–81
- Tokumoto N, Ikeda S, Ishizaki Y, Kurihara T, Ozaki S, Iseki M, et al. Immunohistochemical and mutational analyses of Wnt signaling components and target genes in intrahepatic cholangiocarcinomas. Int J Oncol 2005; 27: 973–80
